Simultaneous Determination of Aflatoxins and Benzo(a)pyrene in Vegetable Oils Using Humic Acid-Bonded Silica SPE HPLC–PHRED–FLD
Abstract
:1. Introduction
2. Results and Discussion
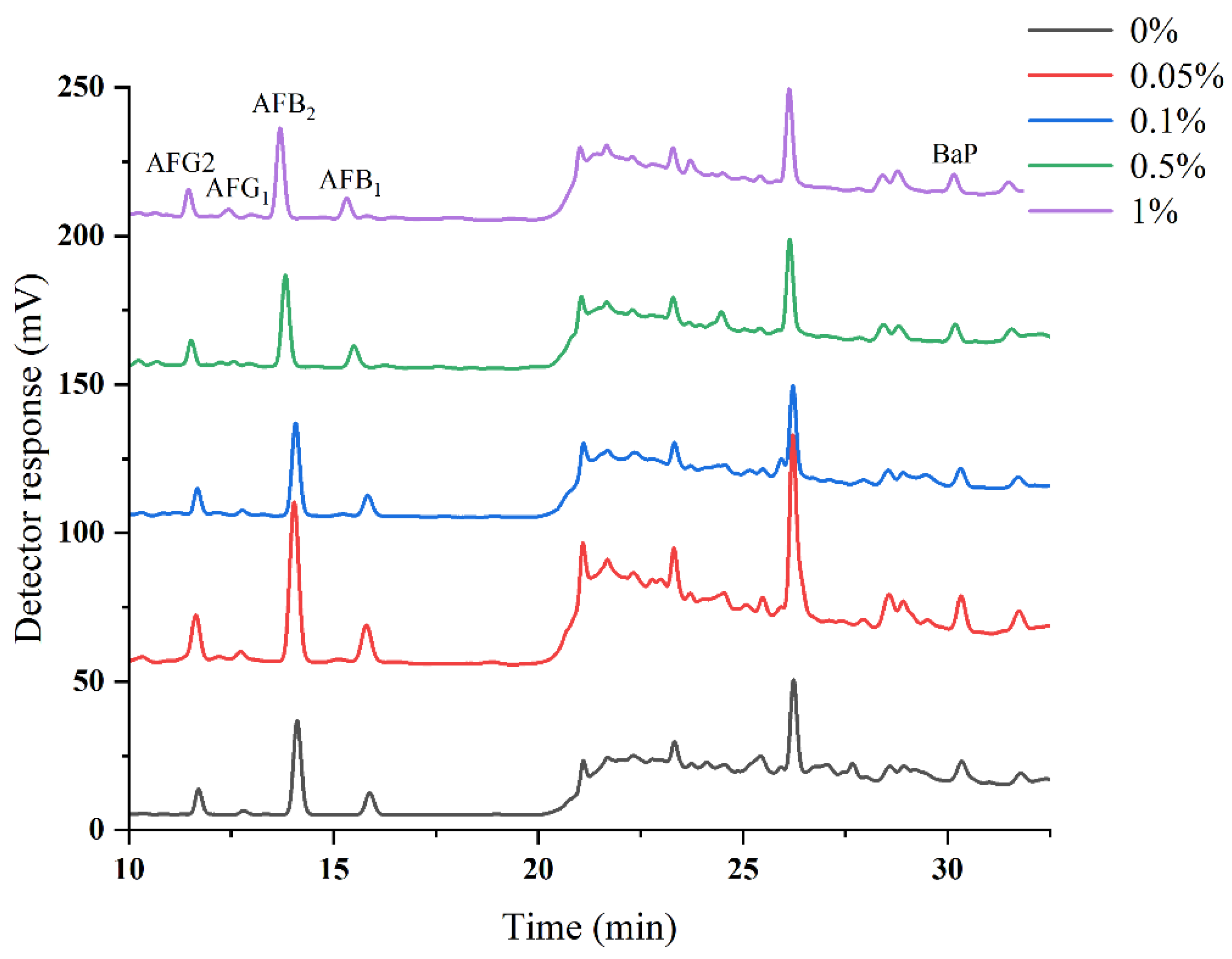
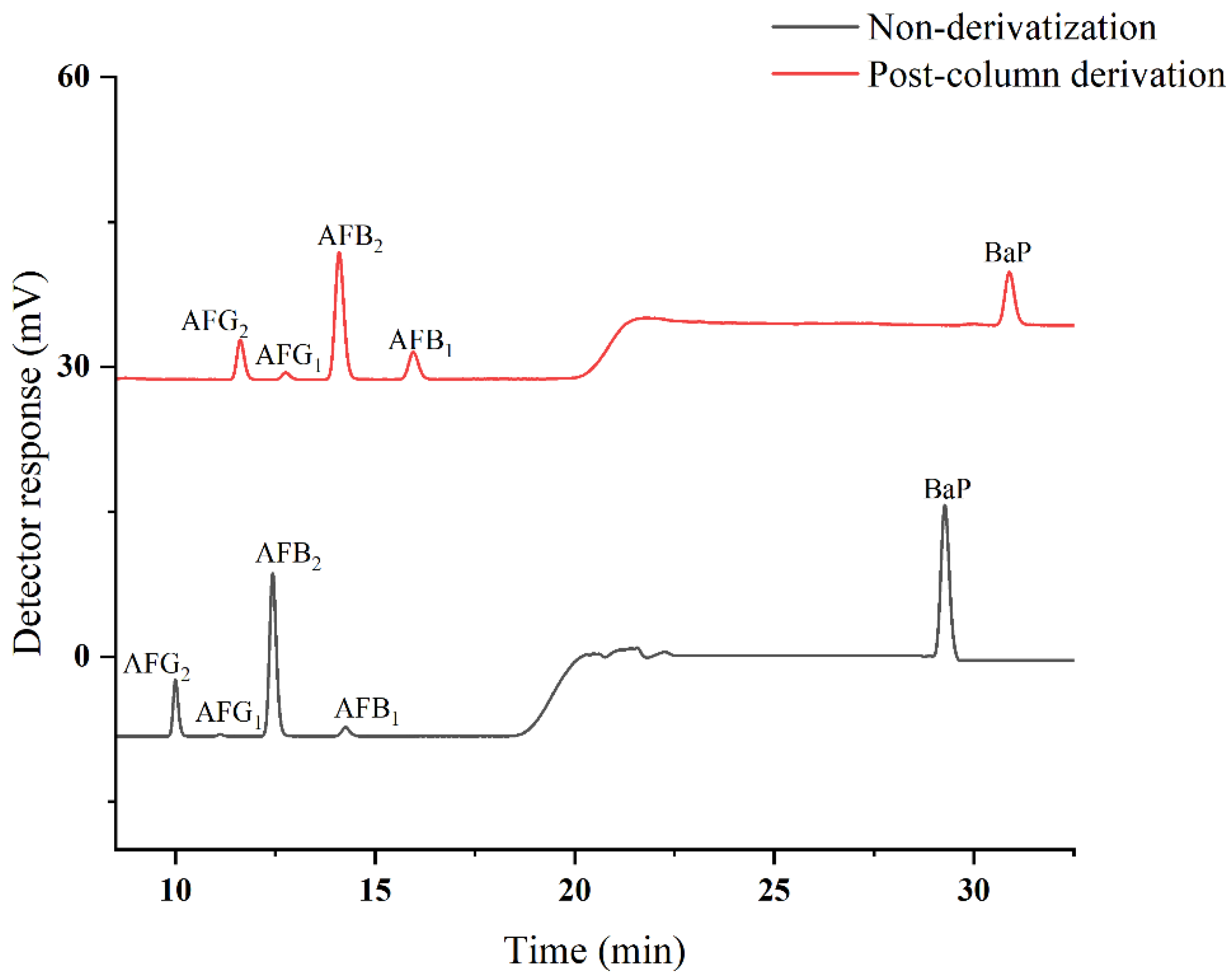
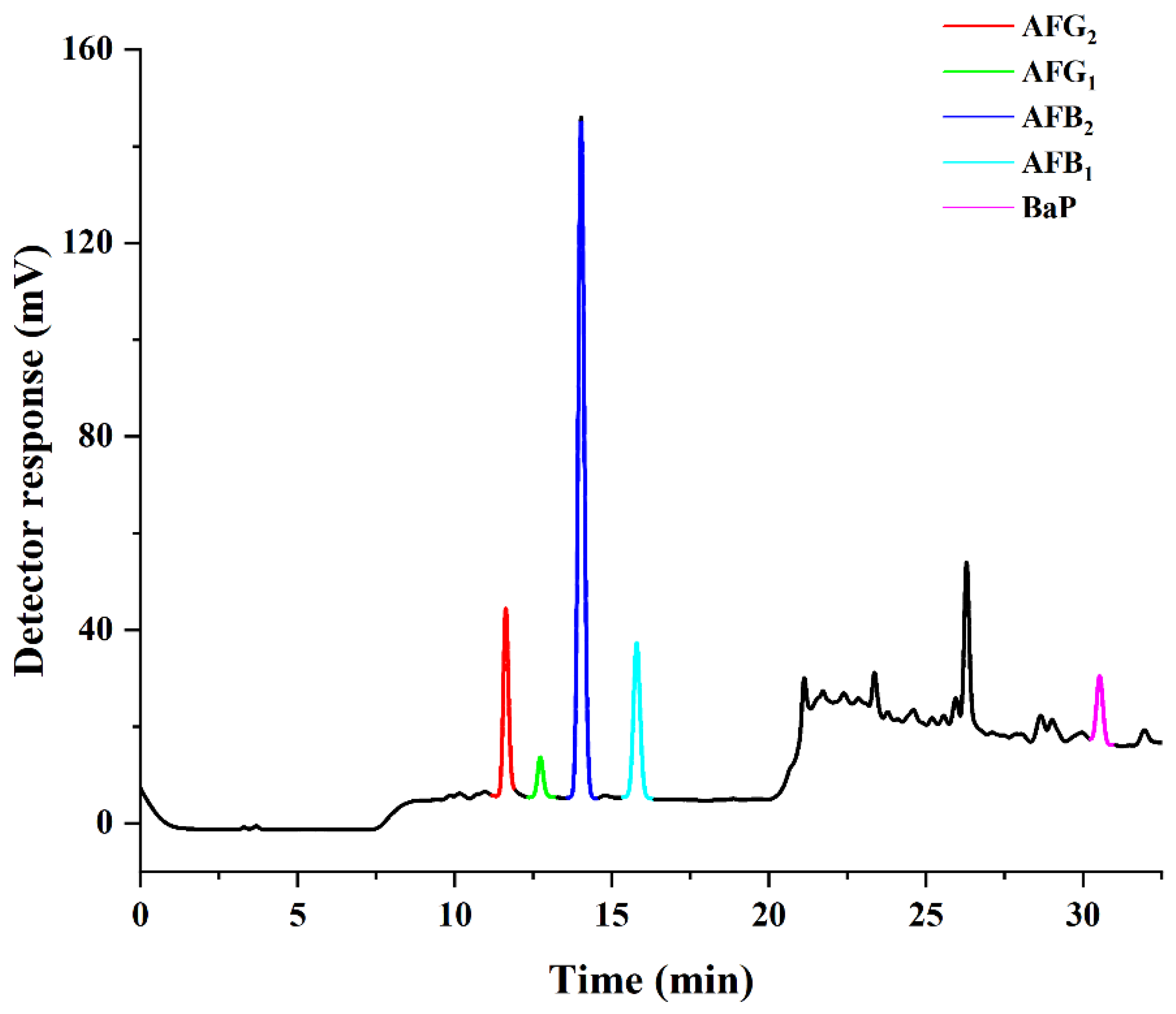
2.1. Optimization of HPLC–PHRED–FLD
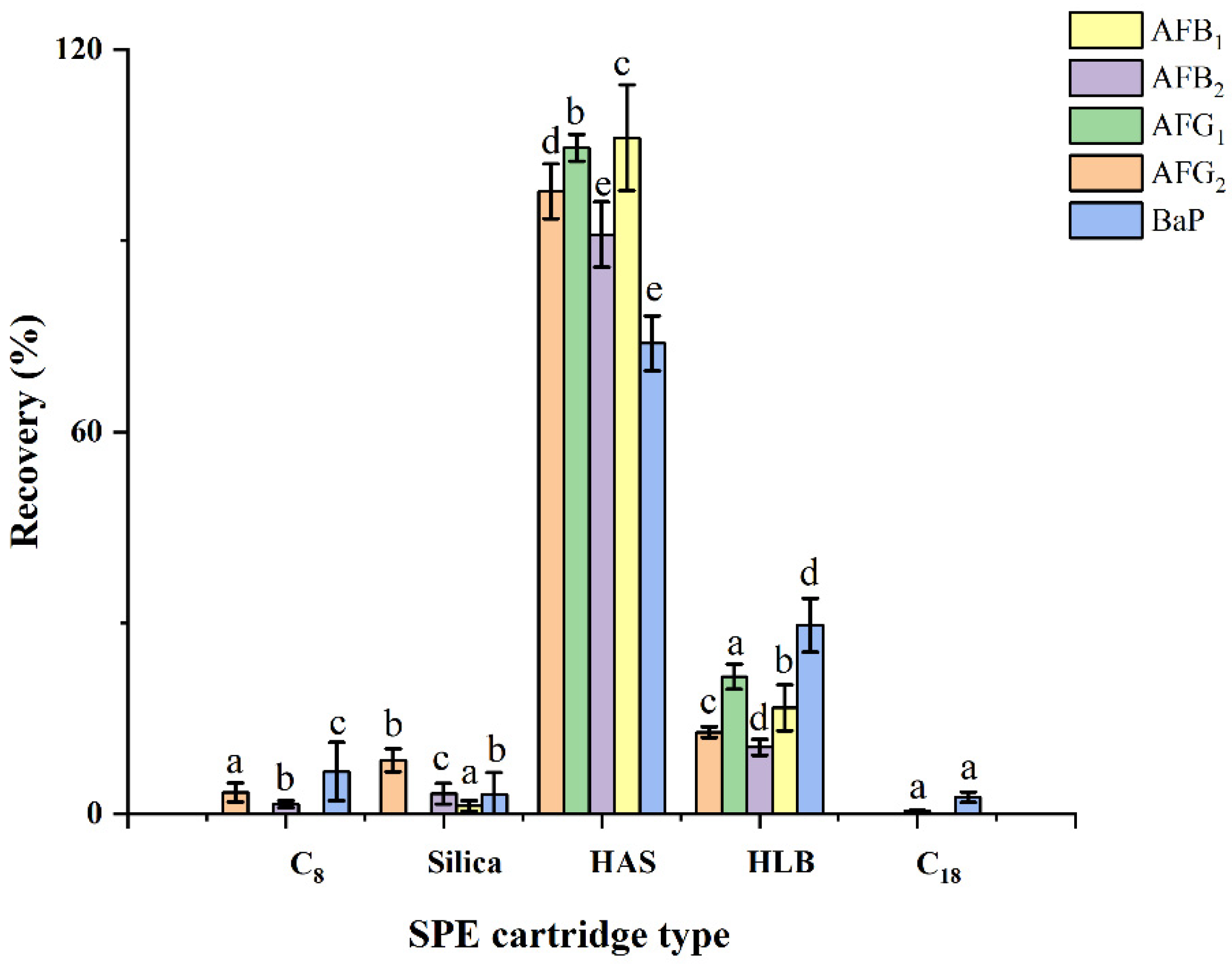
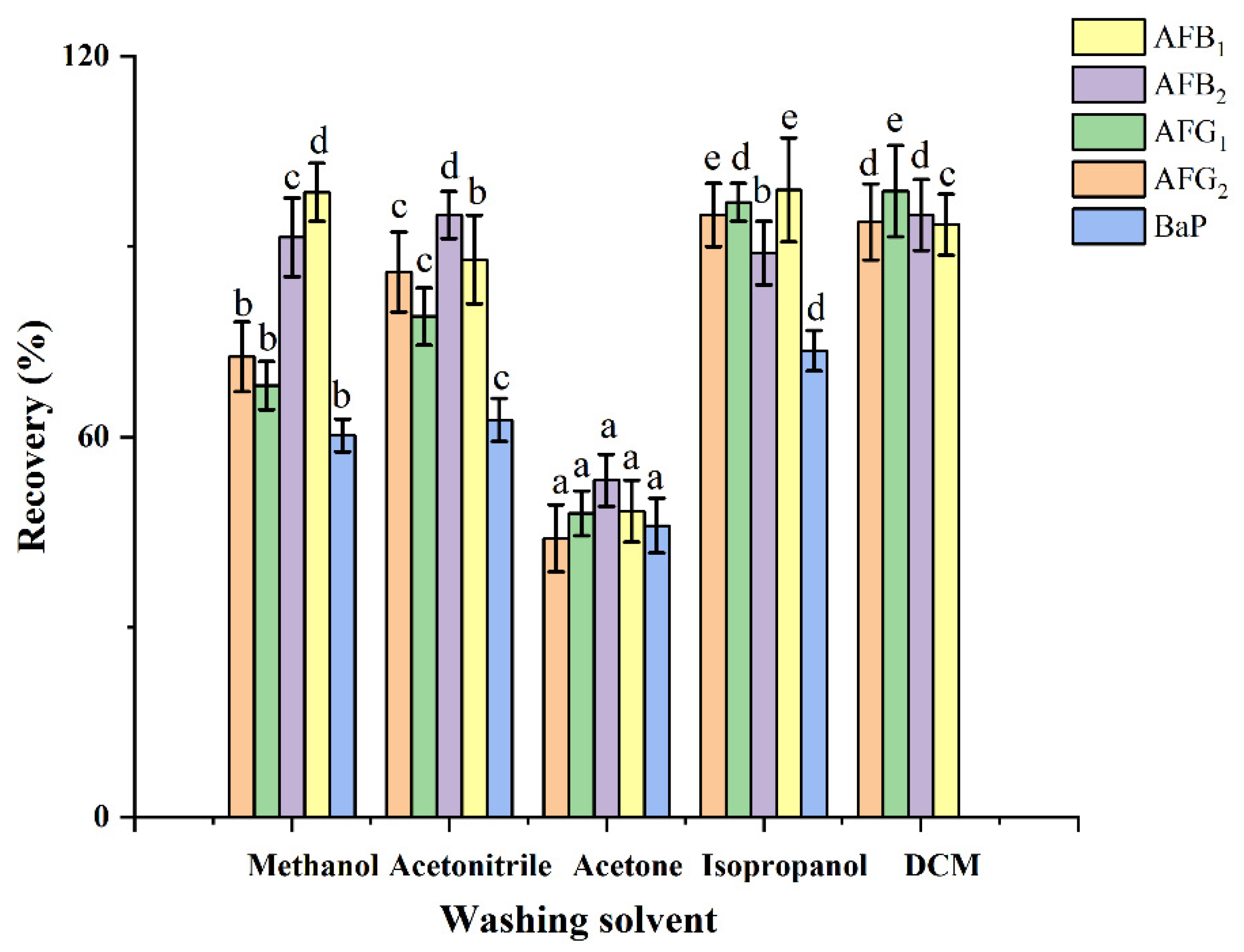
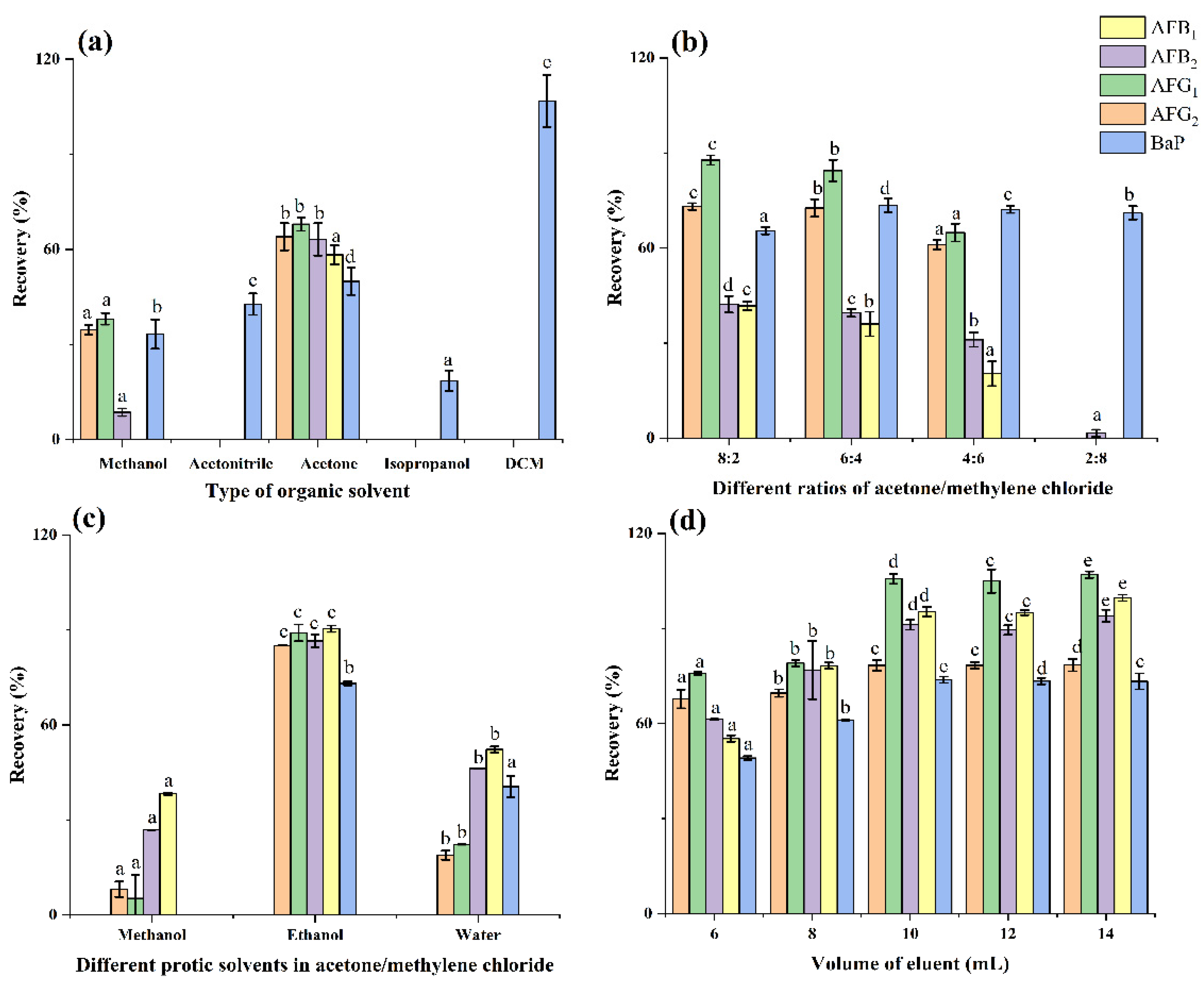
2.2. Optimization of HAS Solid-Phase Extraction
2.2.1. Loading Solvent
2.2.2. SPE Adsorbents
2.2.3. Washing Solvent
2.2.4. Eluting Solvent
2.3. Method Validation
2.3.1. Matrix Effect
2.3.2. Linearity and Sensitivity
2.3.3. Accuracy and Reproducibility
2.4. Analysis of AFs and BaP in Vegetable Oils
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Standards
4.3. Preparation of Vegetable Oils
4.4. HAS–SPE Adsorbents and Procedure
4.5. HPLC–PHRED–FLD Analysis
4.6. Method Validation
4.6.1. Matrix Effect
4.6.2. Sensitivity and Linearity
4.6.3. Accuracy and Precision
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadam, D.M.; Kumar, M.; Kasara, A. Application of high energy electromagnetic radiations in elimination of anti-nutritional factors from oilseeds. LWT 2021, 151, 112085. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Li, H.; Zhou, J.; Han, J.; Wei, C. Changes in the volatile profile, fatty acid composition and oxidative stability of flaxseed oil during heating at different temperatures. LWT 2021, 151, 112137. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, Z.; Tan, C.-P.; Liu, Y.; Xu, Y.-J. Biohazard and dynamic features of different polar compounds in vegetable oil during thermal oxidation. LWT 2021, 146, 111450. [Google Scholar] [CrossRef]
- Ren, X.; Branà, M.T.; Haidukowski, M.; Gallo, A.; Zhang, Q.; Logrieco, A.F.; Li, P.; Zhao, S.; Altomare, C. Potential of trichoderma spp. for biocontrol of aflatoxin-producing aspergillus flavus. Toxins 2022, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Shi, Q.; Lu, Q. Development of an indirect competitive ELISA kit for the rapid detection of benzopyrene residues. Food Anal. Methods 2016, 9, 966–973. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Luo, X.; Chen, Z.; Wang, L.; Zhou, Y.; Liu, W.; Cheng, M.; Zhang, C. Synthesis of rice husk-based MCM-41 for removal of aflatoxin B1 from peanut oil. Toxins 2022, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, F.-H.; Huang, Z.; Zhang, X. Elaidic acid enhanced the simultaneous neurotoxicity attributable to the cerebral pathological lesion resulted from oxidative damages induced by acrylamide and benzo(a)pyrene. Toxicol. Ind. Health 2011, 27, 661–672. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; International Agency for Research on Cancer. Aflatoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 1993; Volume 56, pp. 245–395. [Google Scholar]
- World Health Organization; International Agency for Research on Cancer. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 2010; Volume 92, pp. 754–759. [Google Scholar]
- Commission Regulation (EC) No. 835/2011 of 19 August 2011 Amending Regulation (EC) No. 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffs. Official Journal of European Union, OJ L 215. 20 August 2011. Available online: http://data.europa.eu/eli/reg/2011/835/oj (accessed on 12 February 2022).
- National food Safety Standard for Contaminatant. Available online: http://www.nhc.gov.cn/sps/s7891/202103/0bdb6c4318724644b40e3f3f894aa88f.shtml (accessed on 12 February 2022).
- Pschenitza, M.; Hackenberg, R.; Niessner, R.; Knopp, D. Analysis of benzo[a]pyrene in vegetable oils using molecularly imprinted solid phase extraction (MISPE) coupled with enzyme-linked immunosorbent assay (ELISA). Sensors 2014, 14, 9720–9737. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.H.; Li, P.W.; Zhang, Q.; Zhang, W. Ultrasensitive nanogold probe-based immunochromatographic assay for simultaneous detection of total aflatoxins in peanuts. Biosens. Bioelectron. 2011, 26, 2877–2882. [Google Scholar] [CrossRef]
- Ouakhssase, A.; Chahid, A.; Choubbane, H.; Aitmazirt, A.; Addi, E.A. Optimization and validation of a liquid chromatography/tandem mass spectrometry (LC-MS/MS) method for the determination of aflatoxins in maize. Heliyon 2019, 5, e01565. [Google Scholar] [CrossRef] [Green Version]
- Abballe, C.; Gomes, F.M.L.; Lopes, B.D.; de Oliveira, A.P.F.; Berto, M.I.; Efraim, P.; Tfouni, S.A.V. Cocoa beans and derived products: Effect of processing on polycyclic aromatic hydrocarbons levels. LWT 2021, 135, 110019. [Google Scholar] [CrossRef]
- Mo, R.; Zhang, Y.; Ni, Z.; Tang, F. Determination of benzo[a]pyrene in camellia oil via vortex-assisted extraction using the UPLC-FLD method. Food Sci. Biotechnol. 2017, 26, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Yang, C.; Pang, Y.; Xie, W.; Shen, X. Dispersive solid-phase extraction using the metal–organic framework MIL-101(Cr) for determination of benzo(a)pyrene in edible oil. Anal. Methods 2019, 11, 3467–3473. [Google Scholar] [CrossRef]
- Corman, M.E.; Armutcu, C.; Uzun, L.; Denizli, A. Rapid, efficient and selective preconcentration of benzo[a]pyrene (BaP) by molecularly imprinted composite cartridge and HPLC. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 41–53. [Google Scholar] [CrossRef] [PubMed]
- McCullum, C.; Tchounwou, P.; Ding, L.-S.; Liao, X.; Liu, Y.-M. Extraction of aflatoxins from liquid foodstuff samples with polydopamine-coated superparamagnetic nanoparticles for HPLC-MS/MS analysis. J. Agri. Food Chem. 2014, 62, 4261–4267. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Peng, T.; He, J.L.; Shao, Y.; Fan, C.L.; Chen, Y.; Jiang, W.X.; Chen, M.; Wang, Q.; Pei, X.Y.; et al. Preparation and characterization of an immunoaffinity column for the selective extraction of aflatoxin B1 in 13 kinds of foodstuffs. J. Chromatogr. B 2015, 998–999, 50–56. [Google Scholar] [CrossRef]
- Speltini, A.; Merlo, F.; Maraschi, F.; Villani, L.; Profumo, A. HA-C@silica sorbent for simultaneous extraction and clean-up of steroids in human plasma followed by HPLC-MS/MS multiclass determination. Talanta 2021, 221, 121496. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Liu, Y.E.; Huang, L.Q.; Luo, X.J.; Tan, X.X.; Huang, C.C.; Corella, P.Z.; Mai, B.X. Determination of organophosphorus flame retardants in fish by freezing-lipid precipitation, solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2018, 1532, 68–73. [Google Scholar] [CrossRef]
- Muhamad, H.; Zainudin, B.H.; Abu Bakar, N.K. Comparative study of different clean-up techniques for the determination of λ-cyhalothrin and cypermethrin in palm oil matrices by gas chromatography with electron capture detection. Food Chem. 2012, 134, 2489–2496. [Google Scholar] [CrossRef]
- Boguta, P.; D’Orazio, V.; Senesi, N.; Sokołowska, Z.; Szewczuk-Karpisz, K. Insight into the interaction mechanism of iron ions with soil humic acids. The effect of the pH and chemical properties of humic acids. J. Environ. Manag. 2019, 245, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Zhao, Z.S.; Jiang, G.B. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Yu, Q.W.; Yin, H.R.; Feng, Y.Q. Humic acid-bonded silica as a novel sorbent for solid-phase extraction of benzo[a]pyrene in edible oils. Anal. Chim. Acta 2007, 588, 261–267. [Google Scholar] [CrossRef]
- Zhou, N.Z.; Liu, P.; Su, X.C.; Liao, Y.H.; Lei, N.S.; Liang, Y.H.; Zhou, S.H.; Lin, W.S.; Chen, J.; Feng, Y.Q.; et al. Low-cost humic acid-bonded silica as an effective solid-phase extraction sorbent for convenient determination of aflatoxins in edible oils. Anal. Chim. Acta 2017, 970, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, L.; Yang, H.; Guo, Q.; Shi, H.; Pan, H.; Zhao, L.; Qian, C. Determination of benzo(a)pyrene and aflatoxins (B1, B2, G1, G2) in vegetable oil by GPC-HPLC-FLD. Anal. Methods 2014, 6, 1545–1549. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, F.; Li, P.; Li, G.; Zhang, L.; Zhang, Q.; Zhang, W.; Wang, X. Simultaneous determination of isoflavones and resveratrols for adulteration detection of soybean and peanut oils by mixed-mode SPE LC-MS/MS. Food Chem. 2015, 176, 465–471. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Shi, L.; Yi, T.; Wen, Y.; Wang, J.; Liu, S. Determination of benzo[a]pyrene in edible oils using phase-transfer-catalyst-assisted saponification and supramolecular solvent microextraction coupled to HPLC with fluorescence detection. J. Sep. Sci. 2017, 40, 480–487. [Google Scholar] [CrossRef]
- Kong, W.J.; Li, J.Y.; Qiu, F.; Wei, J.H.; Xiao, X.H.; Zheng, Y.; Yang, M.H. Development of a sensitive and reliable high performance liquid chromatography method with fluorescence detection for high-throughput analysis of multi-class mycotoxins in Coix seed. Anal. Chim. Acta 2013, 799, 68–76. [Google Scholar] [CrossRef]
- Yu, L.; Ma, F.; Ding, X.; Wang, H.; Li, P. Silica/graphene oxide nanocomposites: Potential adsorbents for solid phase extraction of trace aflatoxins in cereal crops coupled with high performance liquid chromatography. Food Chem. 2018, 245, 1018–1024. [Google Scholar] [CrossRef]
- Commission Decision of 14 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results, Official Journal of the European Commission, No. OJ L221. 17 August 2002. Available online: http://data.europa.eu/eli/dec/2002/657/oj (accessed on 12 February 2022).
- Joint Research Center (European Commission); Robouch, P.; Stroka, J.; Haedrich, J.; Schaechtele, A.; Wenzl, T. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food; Publications Office of the European Union: Geel, Belgium, 2016. [Google Scholar]
| Analytes | Matrices | Linear Range (μg kg−1) | LOD (μg kg−1) | LOQ (μg kg−1) | Regression Equation | R2 | ME (%) | pKa a |
|---|---|---|---|---|---|---|---|---|
| AFB1 | Solvent | 0.10–50 | 0.03 | 0.10 | y = 17.505x − 2.0209 | 0.9982 | - | 17.79 |
| Soybean oil | 0.10–50 | 0.03 | 0.10 | y = 15.137x − 1.2543 | 0.9984 | −13.5 | ||
| AFB2 | Solvent | 0.10–50 | 0.03 | 0.10 | y = 15.754x − 1.233 | 0.9993 | - | 17.79 |
| Soybean oil | 0.10–50 | 0.03 | 0.10 | y = 14.448x − 2.628 | 0.9991 | −8.3 | ||
| AFG1 | Solvent | 0.30–50 | 0.09 | 0.30 | y = 4.294x − 0.5869 | 0.9998 | - | - |
| Soybean oil | 0.30–50 | 0.09 | 0.30 | y = 3.514x − 0.5094 | 0.9933 | −18.2 | ||
| AFG2 | Solvent | 0.10–50 | 0.03 | 0.10 | y = 18.165x − 3.2121 | 0.9981 | - | - |
| Soybean oil | 0.10–50 | 0.03 | 0.10 | y = 15.412x − 15.839 | 0.9976 | −15.2 | ||
| BaP | Solvent | 0.05–50 | 0.01 | 0.05 | y = 35.435x − 16.264 | 0.9977 | - | - |
| Soybean oil | 0.05–50 | 0.01 | 0.05 | y = 28.532x + 4.2851 | 0.9913 | −19.5 |
| Analytes | Recovery (%, n = 6) a | Precision (RSD, %) b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rapeseed Oil | Peanut Oil | Intra-Day (n = 6) | Intra-Day (n = 6) | |||||||
| 1 µg kg−1 | 5µg kg−1 | 10 µg kg−1 | 20 µg kg−1 | 1 µg kg−1 | 5 µg kg−1 | 10 µg kg−1 | 20 µg kg−1 | |||
| AFB1 | 76.5 | 85.3 | 101.6 | 96.9 | 83.3 | 98.1 | 98.8 | 100.2 | 1.7 | 2.5 |
| AFB2 | 102.2 | 96.4 | 100.1 | 97.7 | 116.3 | 104.7 | 99.8 | 99.6 | 2.4 | 3.4 |
| AFG1 | 114.8 | 89.4 | 106.3 | 97.7 | 102.8 | 95.3 | 101.0 | 99.9 | 3.8 | 7.2 |
| AFG2 | 118.4 | 87.9 | 102.3 | 96.8 | 109.4 | 94.8 | 100.6 | 99.6 | 6.6 | 5.9 |
| BaP | 74.1 | 66.9 | 74.1 | 74.8 | 75.8 | 70.9 | 69.3 | 70.6 | 3.4 | 3.0 |
| Vegetable Oils | Number of Samples | AFs Content (μg kg−1) | Detection Rate (%) | BaP Content (μg kg−1) | Detection Rate (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | Max | Min | Mean | ||||
| Peanut oil | 10 | 0.30 | ND b | ND | ND | 10% | 2.07 | ND | 0.99 | 90% |
| Corn oil | 10 | ND | ND | ND | ND | — | 0.80 | ND | 0.45 | 70% |
| Rapeseed oil | 10 | ND | ND | ND | ND | — | 0.88 | ND | 0.41 | 70% |
| Blended oil | 10 | ND | ND | ND | ND | — | 1.77 | ND | 0.99 | 90% |
| Camellia oil | 10 | ND | ND | ND | ND | — | 1.28 | ND | 0.69 | 80% |
| Soybean oil | 10 | ND | ND | ND | ND | — | 2.34 | 0.44 | 1.15 | 100% |
| Sesame oil | 10 | ND | ND | ND | ND | — | 1.33 | ND | 0.71 | 80% |
| Sunflower oil | 10 | ND | ND | ND | ND | — | 3.18 | ND | 1.82 | 80% |
| Sample | Analytes | Pretreatment | Determination Technique | LOQs (µg kg−1) | Advantages and Drawbacks | Ref. |
|---|---|---|---|---|---|---|
| Vegetable oil | BaP | HAS–SPE | HPLC–FLD | 0.2 | Simple, rapid, and high sensitivity but detects one class of analyte | [27] |
| Vegetable oil | BaP | Supramolecular solvent microextraction | HPLC–FLD | 0.19 | Uses less amount of organic solvent but requires tedious step including saponification and centrifugation | [31] |
| Coix seed | AFB1, AFB2, AFG1, AFG2, ZON, α-ZOL, β-ZOL | IAC | HPLC–PCD–FLD | 0.04~0.32 | Suitable and high-throughput but the immune adsorbents are expensive and not available for BaP | [32] |
| Cereal crop | AFB1, AFB2, AFG1, AFG2 | SPE | HPLC–FLD | 0.3~1 | Simple and rapid but involving complicated steps for synthetic adsorbents and pre-column derivatization | [33] |
| Vegetable oil | AFB1, AFB2, AFG1, AFG2 | HAS–SPE | LC–MS/MS | 0.039~0.12 | Rapid and high sensitivity but needs sophisticated apparatus | [28] |
| Vegetable oil | AFB1, AFB2, AFG1, AFG2, BaP | GPC | HPLC–FLD | 1.66~3.33 | Automated pre-processing but involving tedious steps and preparation equipment | [29] |
| Vegetable oil | AFB1, AFB2, AFG1, AFG2, BaP | HAS–SPE | HPLC–PHRED–FLD | 0.05~0.3 | Simple, rapid, high sensitivity, cost-effective and multi-residue determination | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, D.; Zhang, L.; Ma, F.; Li, P. Simultaneous Determination of Aflatoxins and Benzo(a)pyrene in Vegetable Oils Using Humic Acid-Bonded Silica SPE HPLC–PHRED–FLD. Toxins 2022, 14, 352. https://doi.org/10.3390/toxins14050352
Yuan D, Zhang L, Ma F, Li P. Simultaneous Determination of Aflatoxins and Benzo(a)pyrene in Vegetable Oils Using Humic Acid-Bonded Silica SPE HPLC–PHRED–FLD. Toxins. 2022; 14(5):352. https://doi.org/10.3390/toxins14050352
Chicago/Turabian StyleYuan, Di, Liangxiao Zhang, Fei Ma, and Peiwu Li. 2022. "Simultaneous Determination of Aflatoxins and Benzo(a)pyrene in Vegetable Oils Using Humic Acid-Bonded Silica SPE HPLC–PHRED–FLD" Toxins 14, no. 5: 352. https://doi.org/10.3390/toxins14050352
APA StyleYuan, D., Zhang, L., Ma, F., & Li, P. (2022). Simultaneous Determination of Aflatoxins and Benzo(a)pyrene in Vegetable Oils Using Humic Acid-Bonded Silica SPE HPLC–PHRED–FLD. Toxins, 14(5), 352. https://doi.org/10.3390/toxins14050352







