Tetrodotoxin Poisoning in Mainland France and French Overseas Territories: A Review of Published and Unpublished Cases
Abstract
:1. Introduction
2. Results
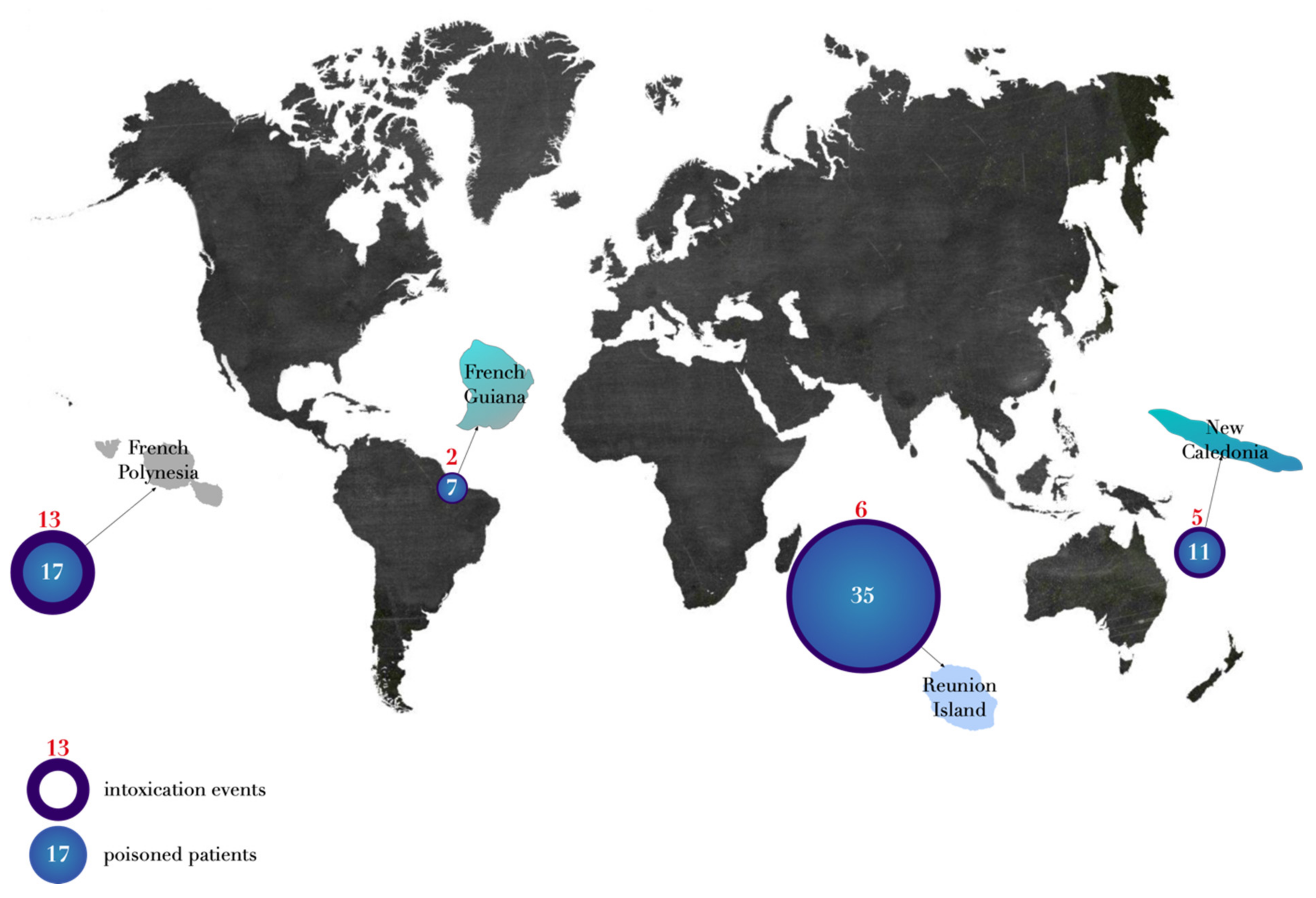
2.1. Demographic Characteristics
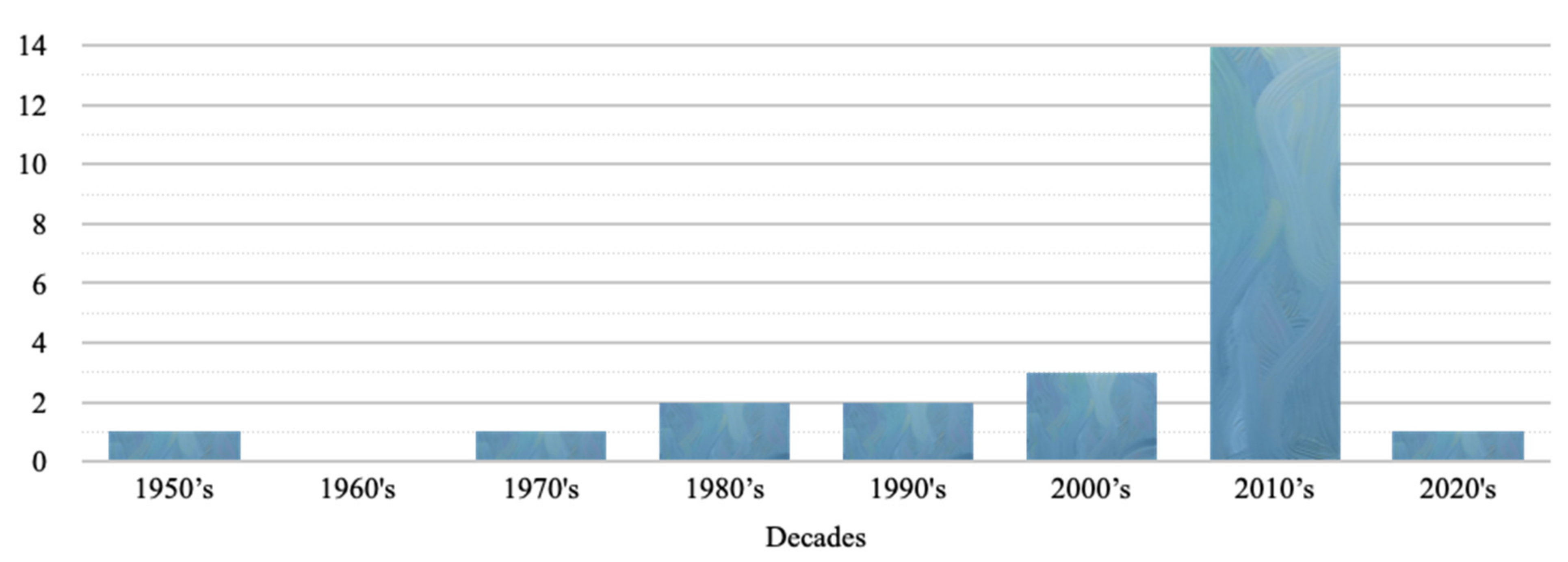
2.2. Temporal Characteristics
2.3. Clinical Presentations
2.4. Neurologic Signs
2.4.1. Digestive Signs
2.4.2. General Signs
2.5. Cardio-Respiratory Signs
2.6. Cutaneous Signs
2.7. Severity Score
2.8. Treatments
2.9. Mortality
2.10. TTX Toxicological Analysis
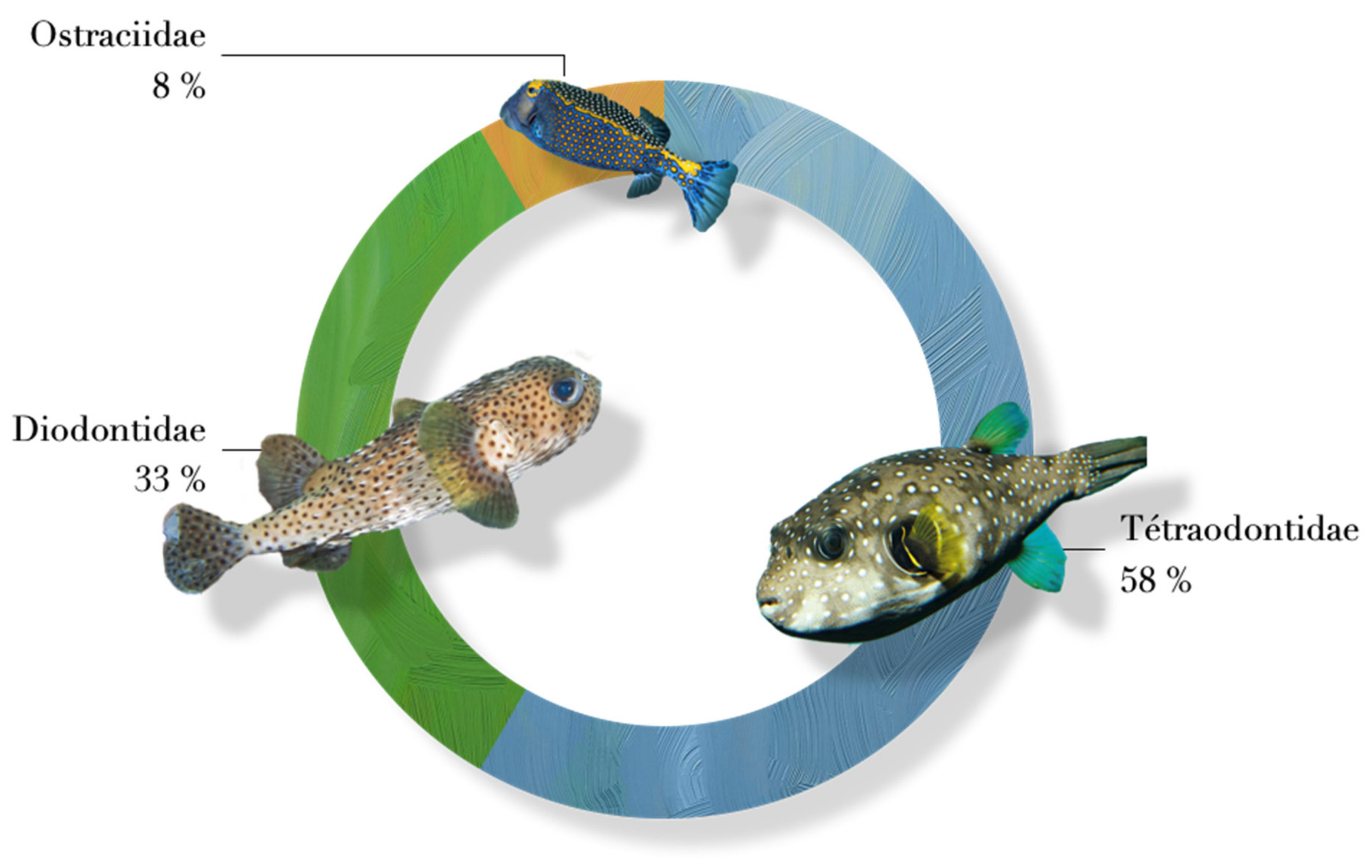
2.11. Incriminated Animals
3. Discussion
4. Conclusions
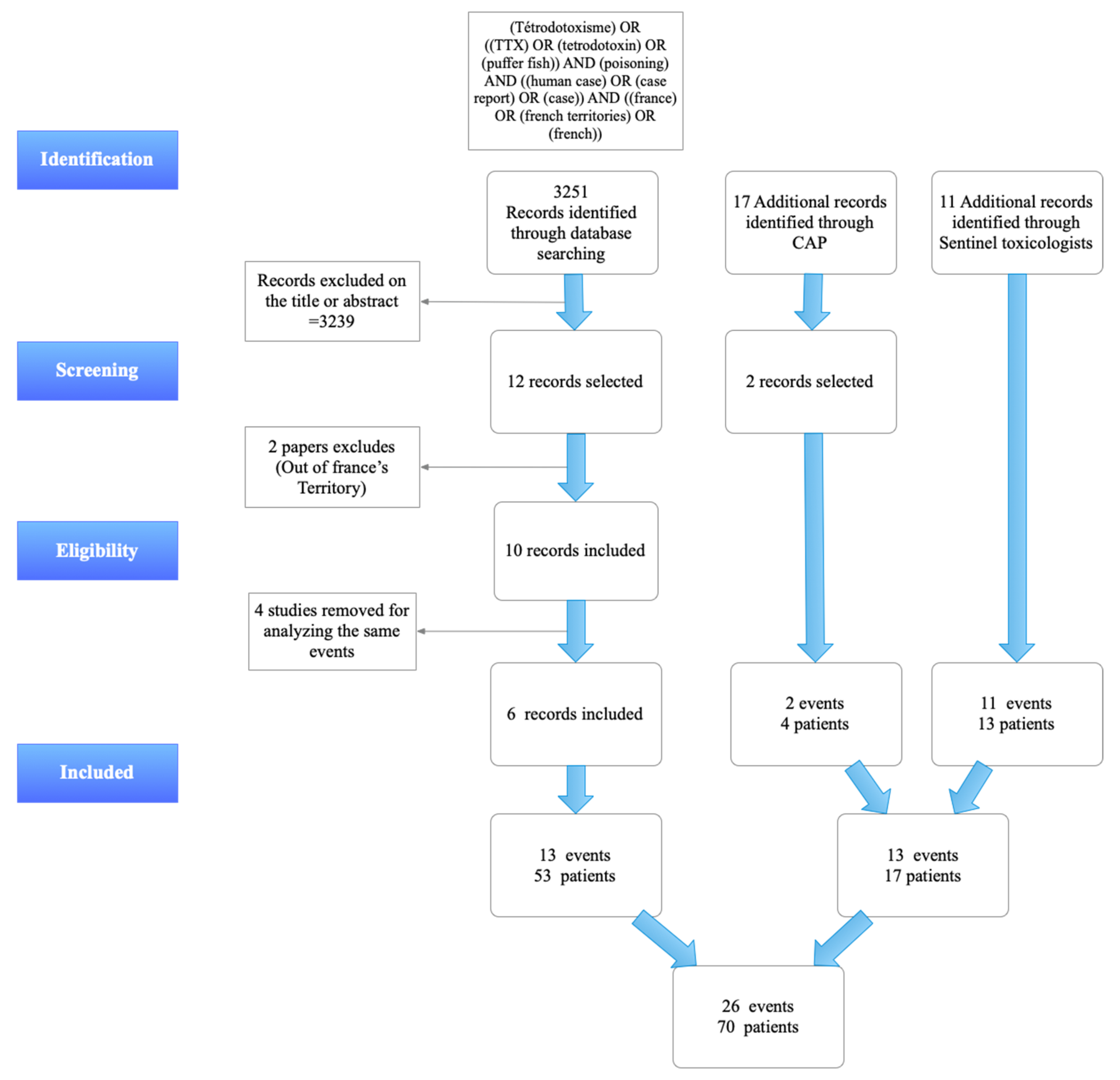
5. Materials and Methods
5.1. Search of Published Cases
5.2. Search of Non-Published Cases
5.3. Identification of the Cases
- The number of intoxicated patients, mortality;
- Location and date of intoxication;
- The species responsible for the poisoning and, if possible, the parts consumed;
- The symptoms, the severity according to the Poisoning Severity Score (PSS) [27] and the evolution;
- Tetrodotoxin toxicological analysis.
5.4. Statistics Analysis
5.5. Ethics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Number of Cases | Percentage/Category | Percentage/Total | ||
|---|---|---|---|---|
| Total neurologic signs | 36 | 94.7% | ||
| Paresthesia | 33 | 91.7% | 86.8% | |
| Distal or of the extremities paresthesias | 21 | 58.3% | 55.3% | |
| Peribuccal paresthesia | 10 | 27.8% | 26.3% | |
| Labial paresthesia | 4 | 11.1% | 10.5% | |
| Buccal paresthesia | 3 | 8.3% | 7.9% | |
| Dermatologic paresthesia | 3 | 8.3% | 7.9% | |
| Sensitivity disorder | 15 | 41.7% | 39.5% | |
| “Touch disorder” | 9 | 25.0% | 23.7% | |
| Allodynia | 6 | 16.7% | 15.8% | |
| Superficial sensitivity disorder | 3 | 8.3% | 7.9% | |
| Deep sensitivity disorder | 2 | 5.6% | 5.3% | |
| Ataxia | 10 | 27.8% | 26.3% | |
| Walking disorder | 4 | 11.1% | 10.5% | |
| Coordination disorder | 3 | 8.3% | 7.9% | |
| Equilibrium disorder | 2 | 5.6% | 5.3% | |
| Reflex disorders | 6 | 16.7% | 15.8% | |
| Areflexia | 5 | 13.9% | 13.2% | |
| Corneal reflex abolition | 1 | 2.8% | 2.6% | |
| Mydriasis | 5 | 13.9% | 13.2% | |
| Anisocoria | 1 | 2.8% | 2.6% | |
| Vertigo | 11 | 30.6% | 28.9% | |
| Dysarthria | 8 | 22.2% | 21.1% | |
| Tétraparésie | 7 | 19.4% | 18.4% | |
| Headache | 7 | 19.4% | 18.4% | |
| Vision disorder | 3 | 8.3% | 7.9% | |
| Nystagmus | 2 | 5.6% | 5.3% | |
| Vestibular syndrom | 2 | 5.6% | 5.3% | |
| Coma | 2 | 5.6% | 5.3% | |
| Total digestive signs | 24 | 63.2% | ||
| Nausea | 16 | 66.7% | 42.1% | |
| Vomiting | 10 | 41.7% | 26.3% | |
| Diarrhea | 6 | 25.0% | 15.8% | |
| Hypersialorrhea | 3 | 12.5% | 7.9% | |
| Pain | 7 | 29.2% | 18.4% | |
| Epigastralgic | 4 | 16.7% | 10.5% | |
| Abdominal | 3 | 12.5% | 7.9% | |
| Total general signs | 23 | 60.5% | ||
| Muscular weakness | 16 | 69.6% | 42.1% | |
| Pain | 6 | 26.1% | 15.8% | |
| Arthralgias | 4 | 17.4% | 10.5% | |
| Myalgias | 2 | 8.7% | 5.3% | |
| Shiverings | 6 | 26.1% | 15.8% | |
| Faintness | 4 | 17.4% | 10.5% | |
| Hypothermia | 2 | 8.7% | 5.3% | |
| Drowsiness | 2 | 8.7% | 5.3% | |
| Anxiety | 1 | 4.3% | 2.6% | |
| Others | 9 | 39.1% | 23.7% | |
| Uro-genital discomfort | 3 | 13.0% | 7.9% | |
| Buccal/throat burns/dysgueusia | 8 | 34.8% | 21.1% | |
| Total cardiologic signs | 8 | 21.1% | ||
| Bradycardia | 6 | 75.0% | 15.8% | |
| Hypotension | 4 | 50.0% | 10.5% | |
| Tachycardia | 1 | 12.5% | 2.6% | |
| Total respiratory signs | 7 | 18.4% | ||
| Dyspnea | 4 | 57.1% | 10.5% | |
| Respiratory distress | 2 | 28.6% | 5.3% | |
| Desaturation | 2 | 28.6% | 5.3% | |
| Bradypnea | 2 | 28.6% | 5.3% | |
| Total dermatologic signs | 7 | 18.4% | ||
| Pruritus | 5 | 71.4% | 13.2% | |
| Sweating/excessive sweating | 3 | 42.9% | 7.9% | |
References
- De Haro, L. Animaux aquatiques dangereux et toxicologie marine. EMC 2011, 16, 078C-10. [Google Scholar] [CrossRef]
- Guardone, L.; Maneschi, A.; Meucci, V.; Gasperetti, L.; Nucera, D.; Armani, A. A Global Retrospective Study on Human Cases of Tetrodotoxin (TTX) Poisoning after Seafood Consumption. Food Rev. Int. 2019, 27, 1–23. [Google Scholar] [CrossRef]
- Watari, T.; Tachibana, T.; Okada, A.; Nishikawa, K.; Otsuki, K.; Nagai, N.; Abe, H.; Nakano, Y.; Takagi, S.; Amano, Y. A review of food poisoning caused by local food in Japan. J. Gen. Fam. Med. 2020, 22, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.F.; Noguchi, T. Tetrodotoxin poisoning. Adv. Food Nutr. Res. 2007, 52, 141–236. [Google Scholar]
- Fernández-Ortega, J.F.; Morales-de los Santos, J.M.; Herrera-Gutiérrez, M.E.; Fernández-Sánchez, V.; Rodríguez Loureo, P.; Rancaño, A.A.; Téllez-Andrade, A. Seafood poisoning by tetrodotoxin: First case in Europe. J. Emerg. Med. 2010, 39, 612–617. [Google Scholar] [CrossRef]
- Fukuda, A.; Tani, A. Records of puffer poisonings. Report 3. Nippon Igaku Oyobi Kenko Hoken 1941, 3528, 7–13. [Google Scholar]
- Rambla-Alegre, M.; Leonardo, S.; Barguil, Y.; Flores, C.; Caixach, J.; Campbell, K.; Elliott, C.T.; Maillaud, C.; Boundy, M.J.; Harwood, D.T.; et al. Rapid screening and multi-toxin profile confirmation of tetrodotoxins and analogues in human body fluids derived from a puffer fish poisoning incident in New Caledonia. Food Chem. Toxicol. 2018, 112, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.F.; Chataigner, D.; Arakawa, O.; Guegueniat, P.; Hommel, D.; de Haro, L.; Garnier, R. Familial Tetrodotoxin Poisoning in French Guiana. In Abstracts of the 2010 International Congress of the European Association of Poisons Centres and Clinical Toxicologists, Bordeaux, France, 11–14 May 2010; Taylor and Francis: Abingdon, UK, 2010; Volume 48, pp. 240–318. [Google Scholar]
- Puech, B.; Batsalle, B.; Roget, P. Family tetrodotoxin poisoning in Reunion Island (Southwest Indian Ocean) following the consumption of Lagocephalus sceleratus (Pufferfish). Bull. Soc. Pathol. Exot. 2014, 107, 79–84. [Google Scholar] [CrossRef]
- Quod, J.P. Reunion Tropical Fish Poisonings: Ciguatera Actual State, Epidemiologic and Bioecologic’ Phenomenom Characteristics, 1st ed.; Reunion Research and Technology Association: Saint-Denis, France, 1989; p. 82. [Google Scholar]
- Maillaud, C.; Barguil, Y.; Le Coq Saint-Gilles, H.; Mikulski, M.; Guittonneau-Leroy, A.-L.; Pérès, H. New caledonian tetrodotoxism. Clinical cases. Toxicol. Anal. Clin. 2016, 28, 57–63. [Google Scholar]
- Gatti, C.; Oelher, E.; Legrand, A.M. Severe seafood poisoning in French Polynesia: A retrospective analysis of 129 medical files. Toxicon 2008, 51, 746–753. [Google Scholar] [CrossRef]
- Hommel, D.; Hulin, A.; Saignavong, S.; Le Pelley, E.; Desbordes, J.M.; Fusciardi, J. Poisoning by the puffer fish (tetrodotoxin). Apropos of a case. Cah. Anesthesiol. 1992, 40, 424–426. [Google Scholar]
- Ababou, A.; Mosadik, A.; Squali, J.; Fikri, K.O.; Lazreq, C.; Sbihi, A. Puffer fish poisoning. Ann. Fr. Anesth. Reanim. 2000, 19, 188–190. [Google Scholar] [CrossRef]
- Awada, A.; Chalhoub, V.; Awada, L.; Yazbeck, P. Reversible deep areactive coma following mediterranean fish offals poisoning. Rev. Neurol. 2010, 166, 337–340. [Google Scholar] [CrossRef]
- Barguil, Y.; Maillaud, C.; Mengant, S.; Peres, H. Fisherman dying of his own fishing. Toxicol. Anal. Clin. 2015, 27, S30–S31. [Google Scholar]
- Brun, R.; Schmitt, C.; Simon, N.; de Haro, L. New tetrodotoxism poisoning overseas: Reunion collective Lagocephalus sceleratus poisoning. Toxicol. Anal. Clin. 2014, 26, 215–216. [Google Scholar]
- Cook, J. The Journals of Captain James Cook on his Voyages of Discovery; The Hakluyt Society at the University Press: London, UK, 1955. [Google Scholar]
- Endean, R.; Griffith, J.K.; Robins, J.J.; Monks, S.A. Multiple toxins in a specimen of the narrow-barred Spanish mackerel, Scomberomorus commersoni. Toxicon 1993, 31, 195–204. [Google Scholar] [CrossRef]
- Noguchi, T.; Arakawa, O. Tetrodotoxin-distribution and accumulation in aquatic organisms, and cases of human poisoning. Mar. Drugs 2008, 28, 220–242. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, T.; Arakawa, O.; Takatani, T. TTX accumulation in pufferfish. Comp. Biochem. Physiol. Part D Genom. Proteom. 2006, 1, 145–152. [Google Scholar]
- Azman, A.M.N.; Samsur, M.; Othman, M. Distribution of tetrodotoxin among tissues of puffer fish from Sabah and Sarawak waters. Sains Malays. 2014, 43, 1003–1011. [Google Scholar]
- Sims, J.K.; Ostman, D.C. Pufferfish poisoning: Emergency diagnosis and management of mild human tetrodotoxication. Ann. Emerg. Med. 1986, 15, 1094–1098. [Google Scholar] [CrossRef]
- Ikeda, K.; Emoto, Y.; Tatsuno, R.; Wang, J.J.; Ngy, L.; Taniyama, S.; Takatani, T.; Arakawa, O. Maturation-associated changes in toxicity of the pufferfish Takifugu poecilonotus. Toxicon 2010, 55, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Toxicovigilance Imputability Method. Version 7.6. Available online: https://tv.antipoison.fr/v7.6/Notice_methode_imputabilite_v7.6.pdf (accessed on 1 February 2022).
- Persson, H.E.; Sjöberg, G.K.; Haines, J.A.; de Garbino, J.P. Poisoning severity score. Grading of acute poisoning. J. Toxicol. Clin. Toxicol. 1998, 36, 205–213. [Google Scholar] [CrossRef]
| Signs and Symptoms | Number of Cases (n = 44) and Percentage (%) | |
|---|---|---|
| Total neurologic signs | 36 (81.8%) | |
| • Paresthesia | 33 (75.0%) | |
| • Sensitivity disorder | 15 (34.1%) | |
| • Vertigo | 11 (25.0%) | |
| • Ataxia | 10 (22.7%) | |
| • Dysarthria | 8 (18.2%) | |
| • Headache | 7 (15.9%) | |
| • Tetraparesis | 7 (15.9%) | |
| • Reflex disorder | 6 (13.6%) | |
| • Mydriasis | 5 (11.4%) | |
| Total gastrointestinal signs | 24 (54.5%) | |
| • Nausea | 16 (36.4%) | |
| • Vomiting | 10 (22.7%) | |
| • Abdominal pain | 7 (15.9%) | |
| Total general signs | 23 (52.3%) | |
| • Muscle weakness | 16 (36.4%) | |
| • Arthralgias, myalgias | 6 (13.6%) | |
| • Shivering | 6 (13.6%) | |
| • Others | 9 (20.4%) | |
| Total cardiologic signs | 8 (18.2%) | |
| • Bradycardia | 6 (13.6%) | |
| Total respiratory signs | 7 (15.9%) | |
| Total cutaneous signs | 7 (15.9%) | |
| • Pruritus | 5 (11.4%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouel, P.; Gatti, C.M.i.; de Haro, L.; Liautaud, A.; Langrand, J.; Boucaud-Maitre, D. Tetrodotoxin Poisoning in Mainland France and French Overseas Territories: A Review of Published and Unpublished Cases. Toxins 2022, 14, 351. https://doi.org/10.3390/toxins14050351
Gouel P, Gatti CMi, de Haro L, Liautaud A, Langrand J, Boucaud-Maitre D. Tetrodotoxin Poisoning in Mainland France and French Overseas Territories: A Review of Published and Unpublished Cases. Toxins. 2022; 14(5):351. https://doi.org/10.3390/toxins14050351
Chicago/Turabian StyleGouel, Pierrick, Clémence Mahana iti Gatti, Luc de Haro, Alice Liautaud, Jérôme Langrand, and Denis Boucaud-Maitre. 2022. "Tetrodotoxin Poisoning in Mainland France and French Overseas Territories: A Review of Published and Unpublished Cases" Toxins 14, no. 5: 351. https://doi.org/10.3390/toxins14050351
APA StyleGouel, P., Gatti, C. M. i., de Haro, L., Liautaud, A., Langrand, J., & Boucaud-Maitre, D. (2022). Tetrodotoxin Poisoning in Mainland France and French Overseas Territories: A Review of Published and Unpublished Cases. Toxins, 14(5), 351. https://doi.org/10.3390/toxins14050351







