1. Introduction
Disseminated intravascular coagulation (DIC), a severe clinical condition caused by an underlying disease, involves a markedly continuous and widespread activation of coagulation in the circulating blood and the formation of numerous microvascular thrombi [
1,
2]. DIC is also caused by some snakebites [
3].
Yamakagashi (
Rhabdophis tigrinus), belonging to the family Colubridae, is a rear-fanged venomous snake. Its fangs are short with no groove. This venomous snake is widespread in East Asia. In Japan, it is commonly found in the paddy fields, and it feeds primarily on frogs [
4]. A severe case of its bite is rare because the venom can be introduced into the skin of the person only when it attacks with the rearmost fangs. However, several cases of bites requiring treatment are reported every year [
5]. Regarding the chemical characterization of
R. tigrinus venom, the higher molecular mass fraction of the venom contains a prothrombin activator. Both the prothrombin time (PT) and activated partial thromboplastin time of human plasma were found to be shortened by the addition of this snake venom. Thrombin formation was determined using SDS-PAGE and chromogenic substrates. The venom fractions also exhibited specific proteinase activity on human fibrinogen (FIB), but the substrates for matrix metalloproteinase, such as collagen and laminin, were not hydrolyzed [
6]. In an in vivo experiment conducted using mice, not only was local hemorrhage observed, but also systemic subcutaneous, pulmonary, and subendocardial hemorrhages as well. Microthrombi in the alveolar capillaries and glomerulus were also observed [
7]. In cases of bites, systemic hemorrhage, DIC, and acute renal failure have been observed [
8,
9], and two fatal cases of acute pulmonary edema and cerebral hemorrhage have also been reported [
10,
11].
Currently, an unapproved drug,
R. tigrinus antivenom, is used as a therapeutic agent for
R. tigrinus envenomation, but there are restrictions on its use.
R. tigrinus antivenom is a horse plasma-derived preparation immunized with
R. tigrinus venom, and when administered to humans, there is a risk of causing adverse reactions such as serum sickness and anaphylaxis due to animal protein. There are also several approved drugs already on the market for the treatment of DIC [
12,
13,
14]. Among those drugs there is no record of DIC treatment with venomous snakes, but recombinant thrombomodulin alpha (rTM) is often used as a drug that is always available in clinical practice [
15]. None of the approved DIC treatments listed here have been used to treat snake-venom-derived DIC. Therefore, in the future, we plan to investigate the therapeutic effect of snake venom on DIC. Thrombomodulin is a glycoprotein present on vascular endothelial cells, and it was revealed in 1982 by Dr. Esmon et al. from the University of Oklahoma, USA, as a physiological anticoagulation factor responsible for regulating blood coagulation in the body [
16]. The gene encoding thrombomodulin was isolated, and it was clarified that its active site exists in the extracellular domain. Moreover, only the extracellular domain in animal cells was successfully produced by genetic engineering. A clinical trial of this soluble human thrombomodulin was conducted, and a manufacturing and marketing approval application was submitted for the treatment of DIC in 2006, following which the drug was approved in January 2008 [
17,
18]. Studies have reported that rTM exerts a therapeutic effect on DIC caused by infectious diseases, malignant tumors, trauma, and gynecological diseases [
19,
20,
21]. The mechanism underlying the action of rTM involves promoting the activation of protein C by thrombin, and the generated activated protein C decomposes the coagulation-promoting factors Va and VIIIa using protein S as a coagulation factor to generate thrombin [
22]. Through this process, rTM suppresses the blood coagulation reaction.
In this study, we confirmed the effect of rTM on R. tigrinus venom in an in vitro blood coagulation system as a putative therapeutic agent for the DIC state of R. tigrinus bites. We also investigated the therapeutic effect of rTM in an in vivo rat DIC model.
3. Discussion
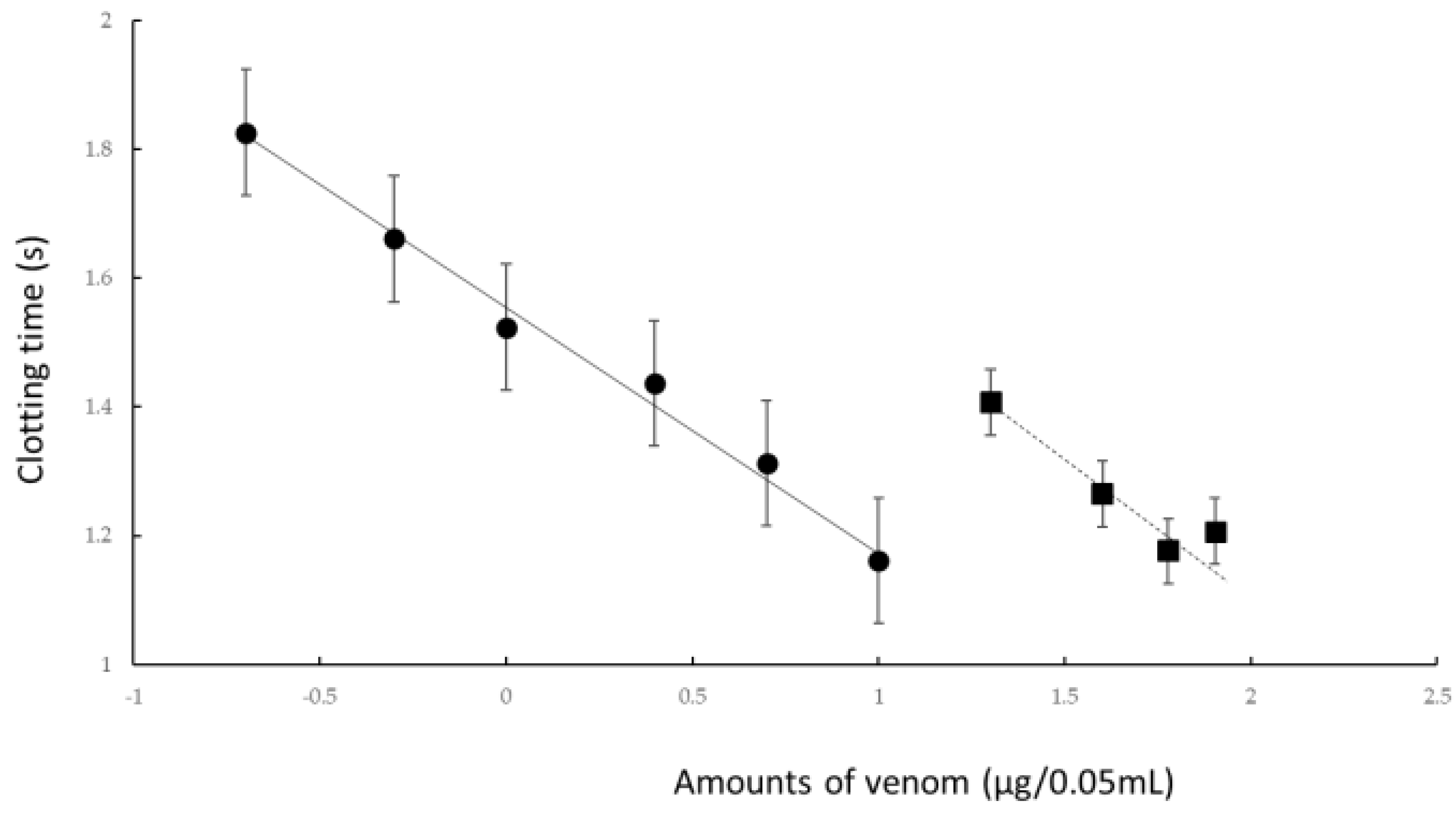
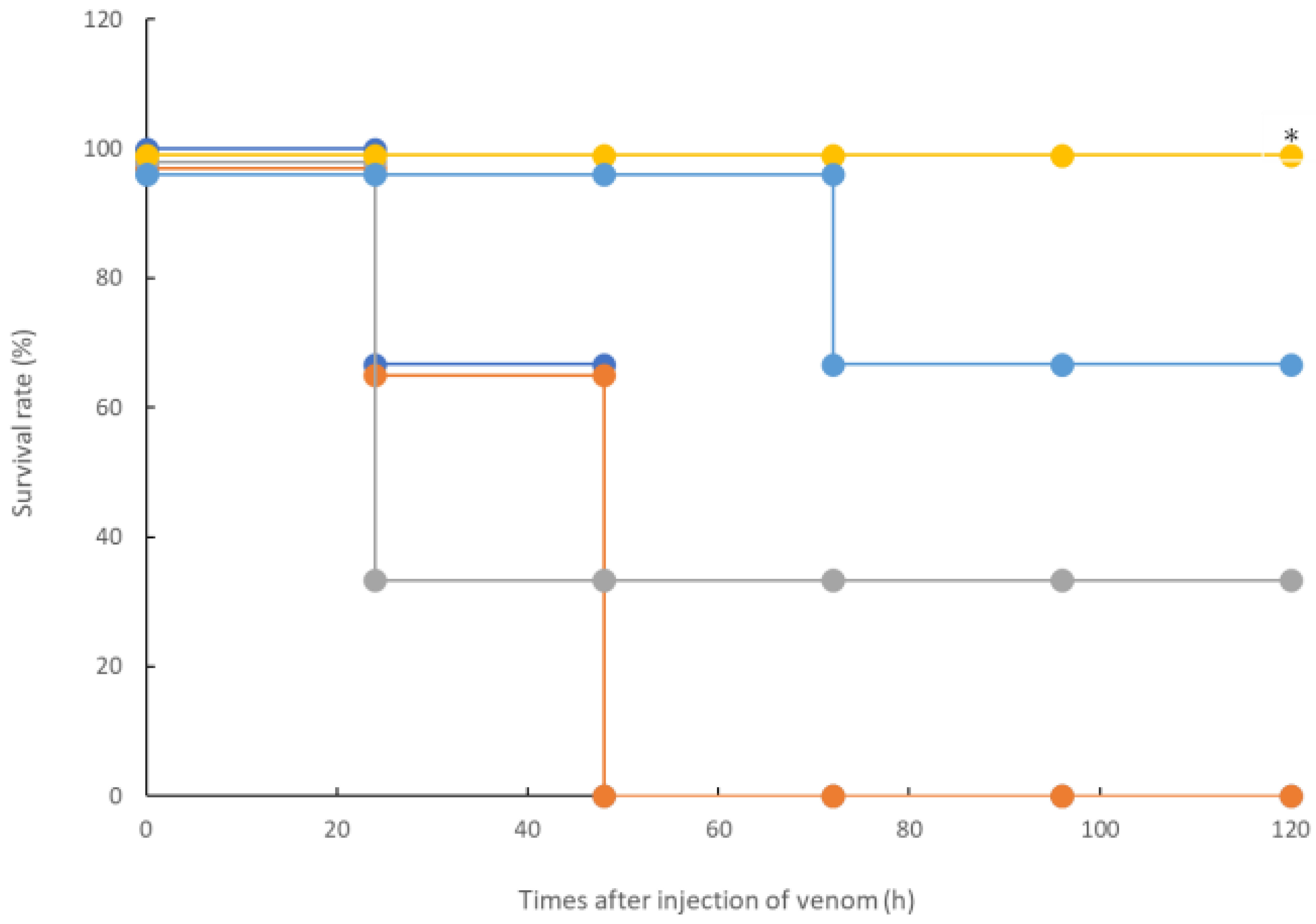
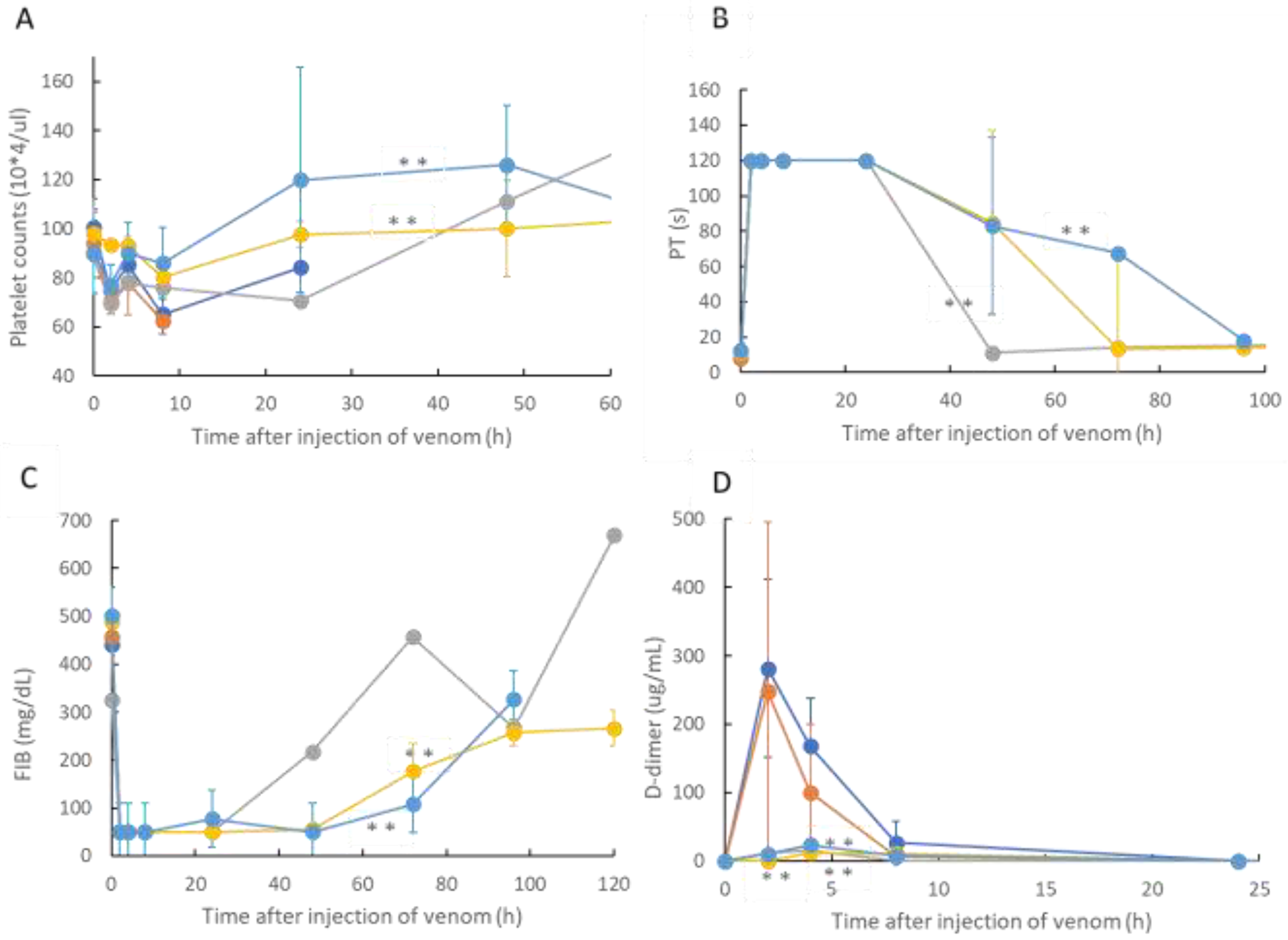
In this study, we confirmed the action of rTM on R. tigrinus venom using two methods, namely, in vitro and in vivo. First, in the in vitro blood coagulation system using standard human plasma, the administration of rTM canceled the blood coagulation activity of 2.1 mg of R. tigrinus venom per 1 mg. Second, the in vivo method saved 33.3%, 100%, and 66.7% of the rats after the administration of 1 mg/kg of rTM 0.5, 0.33, and 0.17 h after venom administration in the rat DIC model with R. tigrinus venom, respectively. The rat survivors recovered from thrombocytopenia, prolongation of PT, and decreased FIB concentrations, which represent the changes in blood coagulation markers that occur in the rat DIC model, with a slight transient increase in D-dimer levels.
As the in vitro blood coagulation system consisted only of human standard plasma, calcium chloride, and
R. tigrinus venom, rTM may act directly on
R. tigrinus venom to suppress its blood coagulation effect. However, coagulation occurs even when calcium chloride is added to human standard plasma without the addition of
R. tigrinus venom, which was performed as a control experiment, and was heated at 37 °C. When rTM was added to this reaction, a concentration-dependent prolongation effect of the added rTM was observed on the coagulation time. This clearly showed that rTM does not act directly on
R. tigrinus venom. Moreover, as no experiments have been conducted with the addition of rTM inhibitors, the mechanism by which the activity of
R. tigrinus venom is canceled has not yet been elucidated in the in vitro experiments that were conducted in this study. Comparing the cancelling effects of
R tigrinus antivenom on the same measurement system, 1 mg of rTM is equivalent to 60% of one vial of
R. tigrinus antivenom [
24].
Next, the effect of rTM on the rat DIC model treated with
R. tigrinus venom showed that it could partially or completely save the rat with the administration of 1 mg/kg of rTM within 0.5 h of the venom administration (
Figure 2). This observation reflects the above-described in vitro experimental results (
Figure 1). However, even when the same amount of rTM was administered 2 h after the administration of venom, no life-saving effect was observed (
Figure 2 and
Figure 3). Considering the relationship between the administration time and life-saving effect, the administered venom is carried systemically by the bloodstream, and the rat is saved by the administration of rTM within 0.5 h after the administration of venom before exerting its effect. Nevertheless, according to the in vitro experimental results, it is unlikely that the administered rTM will have a direct effect on the toxic neutralization of
R. tigrinus venom. Compared with the effect of
R. tigrinus antivenom in the in vivo experimental system, the life-saving effect on rats was similar. In addition, a comparison of the changes in blood coagulation markers in life-saving rats that recovered from thrombocytopenia, prolongation of PT, and decreased FIB concentrations was observed [
23]. The difference in the effects of rTM and
R. tigrinus antivenom in the in vivo experimental system appeared in the amount of D-dimer as a blood coagulation marker. Antivenom had little effect on the appearance of D-dimer, but rTM significantly suppressed the appearance of D-dimer [
23]. D-dimer is normally undetectable or only detectable at a very low level unless the animal body is forming and breaking down significant blood clots. A positive or elevated D-dimer test result may indicate that the animal has a blood clotting condition, but it doesn’t guarantee that they have one [
24]. In the rat DIC model, D-dimer increased, and coagulation occurred systemically, but the fact that D-dimer was hardly detected within 0.5 h of administration of rTM (
Figure 3D) indicates that coagulation was suppressed. It was speculated that the difference between the two was due to the difference in the mechanism of action.
rTM is a glycoprotein present on vascular endothelial cells and acts as a physiological anticoagulation factor responsible for regulating blood coagulation in the body [
16,
17,
18]. rTM has been used to treat DIC caused by infectious diseases, cancer, and sepsis [
19,
20,
21]. Regarding the possibility of treating DIC caused by snake venom, it was found that the administration of
R. tigrinus venom to the rat DIC model could save the lives of rats. A horse-specific antibody against the venom has been used for bites caused by
R. tigrinus. However, owing to the prolonged transport time of this antibody, it is difficult to administer it to patients with rapid bites. Therefore, as rTM is always available in all medical institutions as an approved drug for DIC treatment, it is easy to administer it to patients bitten by
R. tigrinus. Our experimental results showed that the toxic activity could not be canceled unless rTM was administered to the bite patient in a short time after being bitten by
R. tigrinus. Therefore, rTM can be considered a potential therapeutic agent for bites caused by
R. tigrinus.The mechanism underlying the action of rTM involves promoting the activation of protein C by thrombin, and the generated activated protein C uses protein S as a coagulation factor to decompose the coagulation promoters Va and VIIIa to produce thrombin [
22]. Through this process, rTM suppresses the blood coagulation reaction. The mechanism underlying the life-saving effect of rTM in the rat DIC model treated with
R. tigrinus venom was revealed in the in vitro and in vivo experiments using inhibitors for each reaction of the anticoagulant as described earlier.
From the results of the in vitro and in vivo experiments conducted in this study, rTM did not directly neutralize R. tigrinus, unlike antivenom, and the survival of the rats was within 0.5 h after administration of the venom. Therefore, considering future clinical use, it is necessary to consider: (1) dealing with the toxicity of R. tigrinus venom other than DIC, such as hemolytic activity, for patients with R. tigrinus bites; and (2) it has the characteristic that it is required to be administered as soon as possible. In addition, bleeding and damage to the liver and bile duct system have been reported as side effects of high doses of rTM, so this point must also be taken into consideration.
Regarding the limitations of this study, although we demonstrated the results of in vitro and in vivo experiments, the number of experiments in each model was as small as three. Moreover, because only one concentration of rTM was used, the conclusions drawn from the experimental results are limited. In the future, the effect of rTM on R. tigrinus venom should be further clarified by conducting studies with consideration of the rTM concentration, frequency of administration, and administration time even for in vivo experiments.
5. Materials and Methods
5.1. Blood Coagulation Assay
The R. tigrinus venom solutions were prepared with different concentrations in Owren’s buffer (Helena Biosciences Europe; London, UK). For determining the coagulation time due to R. tigrinus venom, 0.05 mL of venom and 0.05 mL of CaCl2(25 mM) were mixed at 37 °C and 0.05 mL of standard human plasma (98406012: Sysmex Co., Kobe, Japan) was added to the semi-automatic blood coagulation measuring device (CA-101: Sysmex Co., Kobe, Japan). The human plasma used in this experiment was produced from healthy human blood that was negative for HIV, Hepatitis B, and C virus; was treated with citric acid; stabilized with HEPRS buffer (12 g/L); and then lyophilized. When dissolved in 1 mL of distilled water for injection, the components are blood coagulation factors II, V, VII, VIII, IX, X, XI, XII, and XIII, as well as protein C, antithrombin III, C1 inhibitor, and VWF. This is included in the concentration of 0.87 to 1.17 IU/mL. In addition, the fibrinogen concentration is 2.49 mg/mL. The rTM used in this experiment has an activity of 6700 U/mg. Next, to confirm the effect of rTM, 0.025 mL of venom and 0.025 mL of rTM were added, 0.05 mL of CaCl2 was mixed at 37 °C, and 0.05 mL of human plasma was added to this solution. The CA-101 device senses the addition of human plasma, heats it at 37 °C while stirring the contents with a rotor in the cuvette, and measures the time until the purification of the coagulated mass. The comparison between the coagulation activity of R. tigrinus venom and the suppression of its activity by rTM was performed using the parallel line assay method.
5.2. Animal Preparation
Male Sprague Dawley rats (JAPAN SLC, Inc., Shizuoka, Japan) aged 12 weeks were housed in separate cages in a temperature-controlled room under a 12 h/12 h light/dark cycle. They were fed on a standard laboratory diet and given water ad libitum. The weight of the rats used in the experiment ranged from 300 to 350 g. All surgical and experimental procedures were approved by the Animal Care and Use Committee and conformed to the Guidelines for Animal Experimentation (approval number: 118065, approval date: 13 December 2018).
Under general anesthesia using 4% isoflurane induction, a polyethylene catheter (PE-60) was inserted into the femoral artery of the rat for bleeding. Another catheter (PE-50) was inserted into the femoral vein for the administration of saline solution and drugs. All catheters were filled with heparinized saline (100 U/mL), placed under the skin, and then opened under the neck to prevent the catheter from coming off when the animal awakened. After placing these two catheters, the rats were subjected to the experiment after a 48-h post-treatment recovery period. Blood sampling and drug administration to the rats were performed after anesthesia induction with 4% isoflurane.
5.3. Snake Venom
R. tigrinus venom was extracted from the Duvernoy’s gland. Toxic glands were collected from 100 male and female
R. tigrinus measuring ≥80 cm in body length from throughout Japan. The glands were excised, cut into small pieces, and centrifuged with distilled water, after which the supernatant was lyophilized [
25]. The venom powder was dissolved in distilled water and centrifuged again to remove the mucous substance that was contained during venom extraction. The lyophilized venom was stored in a refrigerator.
The intravenous LD50 value of the venom was 5.3 μg/20 g mouse [
10].
5.4. Experimental Protocols
The rats were initially randomly divided into five groups to evaluate lethality as follows: (a) 300 μg of R. tigrinus venom was intramuscularly administered; 1 mg/kg of rTM was administered (b) 2, (c) 0.5, (d) 0.33, and (e) 0.17 h after R. tigrinus venom (300 μg) administration (each time: n = 3, respectively). Blood samples were collected at 0, 2, 4, 8, 24, 48, 72, 96, and 120 h after the start of the experiment (1500 μL of blood was collected at each time point). An equal volume of saline was administered through the femoral vein after bleeding. Blood samples were anticoagulated with sodium citrate and centrifuged immediately at 3000× g for 10 min, and the plasma supernatant was separated.
5.5. Test Principle in the Measurement of Platelet Count
Platelet counts were evaluated using an automated system on ADVIA 2120i (Siemens Diagnostic Solutions, Milan, Italy). ADVIA counts platelets by flow cytometry based on the principle of light scattering. The platelets are identified by their size (<30 FL, low-angle light scatter) and refractive index (n = 1.35–1.40 or high-angle light scatter). After blood was collected from the rats, EDTA was added to whole blood to prevent coagulation. The cells were stored at 4 °C until the end of the experiment, and after the listed experiments were completed, the platelet count was measured with ADVIA 2120i.
5.6. Test Principle in the Measurement of PT
An individual plasma sample was placed in a measuring test tube of CA-50 AutoAnalyzer (Sysmex Co., Kobe, Japan). The coagulation reaction detection method irradiates red light (660 nm) onto a mixture of blood plasma and reagent, detects the change in turbidity (when fibrin clots are formed) as the change in scattered light, and measures the coagulation time (s). It detects within the maximum detection time and measures the result. The typical maximum detection time is 120 s for PT. The coefficient of variation (CV) of the PT measurement is ≤2%. The data for CV are variation coefficients for the coagulation times of the change in activity (%) obtained from ten analyses of Dade Behring Ci-Trol Level 1 (control plasma) with a PT reagent.
5.7. Test Principle in the Measurement of FIB Concentration
An individual plasma sample diluted one-tenth with specific reagents was placed in a measuring test tube of CA-50 AutoAnalyzer (Sysmex Co., Kobe, Japan). The coagulation reaction detection method irradiates red light (660 nm) onto a mixture of blood plasma and reagent, detects the change in turbidity (when fibrin clots are formed) as the change in scattered light, and measures the coagulation time (s). The range of analysis for the FIB concentration can be from 50 to 450 mg/dL. The CV of FIB measurement is ≤4%. The data for CV are variation coefficients of the coagulation times of the change in activity (%) taken from ten analyses of Dade Behring Ci-Trol Level 1 (control plasma) with Dade Behring Fibrinogen Determination Reagents.
5.8. Test Principle in the Measurement of D-Dimer Levels
The individual plasma levels of D-dimer were measured using a latex photometric immunoassay (LPIA)-NV7 with an LPIA ACE D-Dimer II Kit (LSI Medience, Tokyo, Japan), as reported previously [
26]. Briefly, 4 μL of plasma was dispensed with 144 μL of R-1 solution into the reaction cuvette. After a 2-min stabilizing period at 37 °C, 48 μL of R-2 suspension was dispensed, which contained latex particles coated with the anti-D-dimer antibody JIF-23. The increase in turbidity was evaluated during the 7-min reaction period at 37 °C. The analysis range for D-dimer levels was 0.5–48 μg/mL, and samples beyond this range were remeasured with dilution. The within-run CV was <10%. The cross-reactivity to rat D-dimer was confirmed in the preliminary test using rat plasma samples treated with calcium ions and different concentrations of tissue plasminogen activator.
5.9. Statistical Analysis
The uniformity of data distribution in each group was assessed using Bart-lett’s test. As a result, when comparing data groups with confirmed homogeneity, we used a bidirectional analysis method to compare the groups. We also used the Mann–Whitney U test to compare data groups with negative homogeneity. In the in vitro blood coagulation system comparison, the parallel line assay method was used. The survival study used Kaplan–Meier analysis to analyze the data. In addition, the binary placement method was used to compare the blood coagulation markers. Statistical analyses were conducted using the JMP version 11 software (SAS Institute, Cary, NC, USA). A two-sided probability value of <0.05 was considered statistically significant in all analyses.