Providing Biological Plausibility for Exposure–Health Relationships for the Mycotoxins Deoxynivalenol (DON) and Fumonisin B1 (FB1) in Humans Using the AOP Framework
Abstract
1. Introduction
2. Results
2.1. Exposure Estimates
2.1.1. Dietary Exposure Estimates in the General Population
2.1.2. Exposure Estimates in the Occupational Setting
2.2. Health Effects
2.2.1. Deoxynivalenol
2.2.2. Fumonisin B1
2.3. Adverse Outcome Pathways
2.3.1. Deoxynivalenol
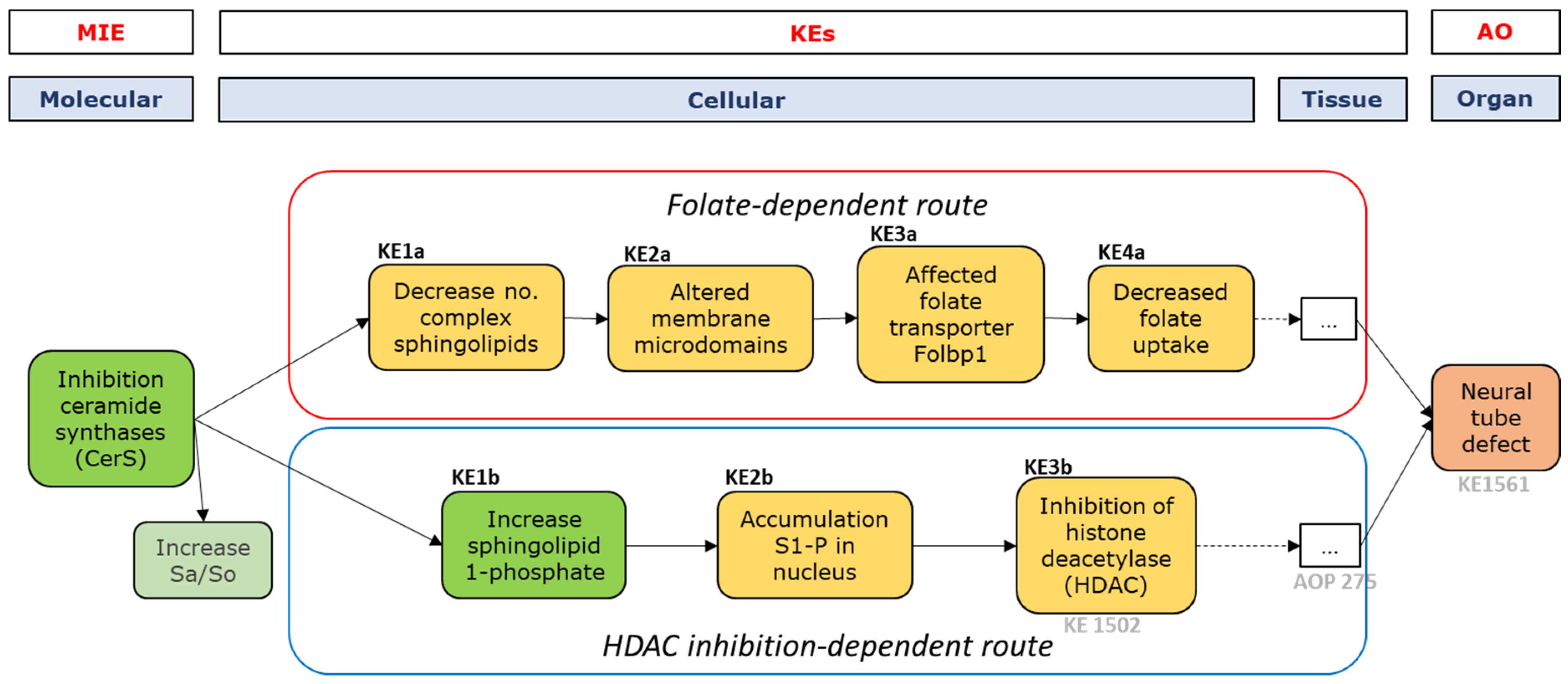
2.3.2. Fumonisin B1
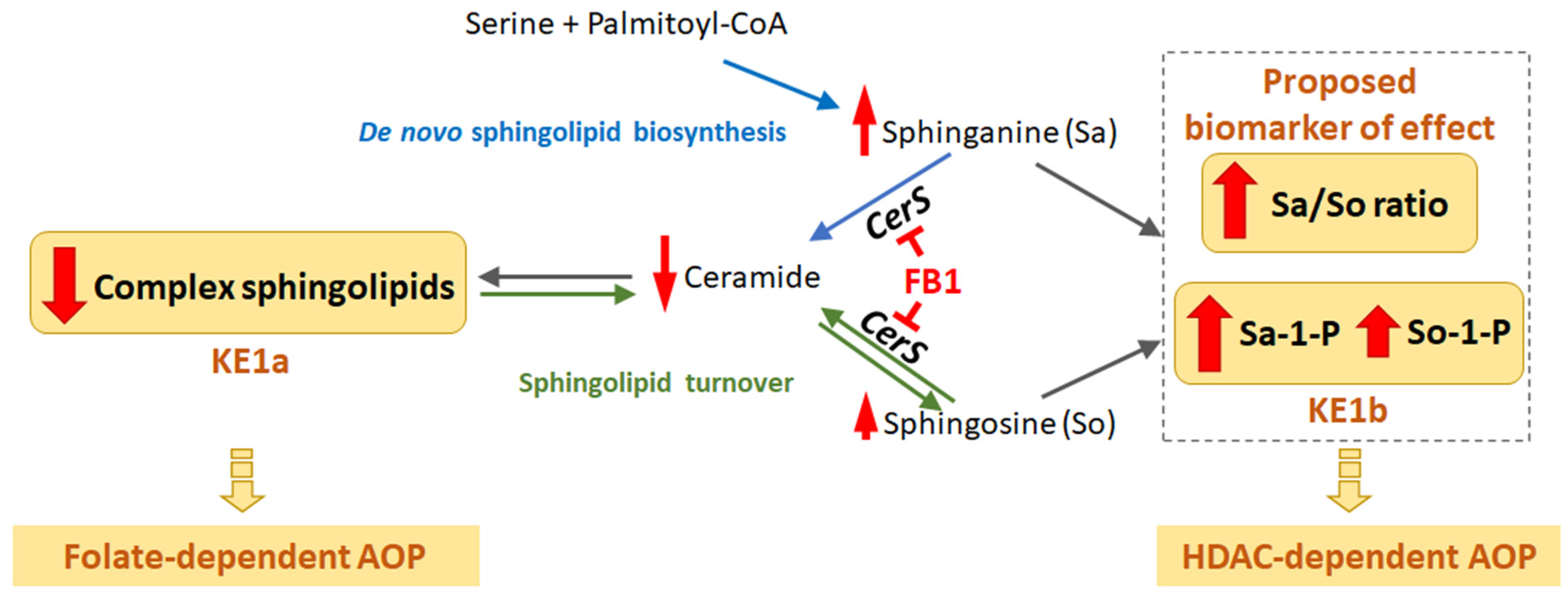
MIE and First Key Events—Sphingolipid Metabolism
Folate-Dependent Route, KE-1-4
HDAC Inhibition-Dependent Route, KE1-3
2.4. Establishing (Future) Exposure–Health Relationships
3. Discussion
3.1. Exposure–Health Relationships
3.1.1. Exposure Estimates
3.1.2. Biomarkers
3.2. Adverse Outcome Pathways
3.2.1. Applicability
3.2.2. Branching of the Adverse Outcome Pathway
3.3. Human Biomonitoring
3.4. Recommendations
4. Conclusions
5. Materials and Methods
5.1. Literature Searches
5.2. Appraisal of Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CAST. Mycotoxins: Risks in Plant, Animal and Human Systems; Council for Agricultural Science and Technology: Ames, IA, USA, 2003. [Google Scholar]
- Bernhoft, A.; Eriksen, G.S.; Sundheim, L.; Berntssen, M.; Brantsæter, A.L.; Brodal, G.; Tronsmo, A.M. Risk Assessment of Mycotoxins in Cereal Grain in Norway. Opinion of the Scientific Steering Committee of the Norwegian Scientific Committee for Food Safety; VKM Report; VKM: Oslo, Norway, 2013; Volume 21, pp. 1–287. [Google Scholar]
- EFSA. Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J. 2014, 12, 3916. [Google Scholar]
- EFSA; CONTAM; Knutsen, H.K.; Alexander, J.; Barregard, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar] [PubMed]
- Viegas, S.; Assunção, R.; Martins, C.; Nunes, C.; Osteresch, B.; Twarużek, M.; Kosicki, R.; Grajewski, J.; Ribeiro, E.; Viegas, C. Occupational Exposure to Mycotoxins in Swine Production: Environmental and Biological Monitoring Approaches. Toxins 2019, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Assunção, R.; Nunes, C.; Osteresch, B.; Twarużek, M.; Kosicki, R.; Grajewski, J.; Martins, C.; Alvito, P.; Almeida, A.; et al. Exposure Assessment to Mycotoxins in a Portuguese Fresh Bread Dough Company by Using a Multi-Biomarker Approach. Toxins 2018, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- De Boevre, M.; Di Mavungu, J.D.; Maene, P.; Audenaert, K.; Deforce, D.; Haesaert, G.; Eeckhout, M.; Callebaut, A.; Berthiller, F.; Van Peteghem, C.; et al. Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, J.; Rodríguez-Carrasco, Y.; Graziani, G.; Gaspari, A.; Ferrer, E.; Mañes, J.; Ritieni, A. Mycotoxin Occurrence and Risk Assessment in Gluten-Free Pasta through UHPLC-Q-Exactive Orbitrap MS. Toxins 2021, 13, 305. [Google Scholar] [CrossRef]
- López, P.; De Rijk, T.; Sprong, R.; Mengelers, M.; Castenmiller, J.; Alewijn, M. A mycotoxin-dedicated total diet study in the Netherlands in 2013: Part II—Occurrence. World Mycotoxin J. 2016, 9, 89–108. [Google Scholar] [CrossRef]
- Pleadin, J.; Frece, J.; Lešić, T.; Zadravec, M.; Vahčić, N.; Staver, M.M.; Markov, K. Deoxynivalenol and zearalenone in unprocessed cereals and soybean from different cultivation regions in Croatia. Food Addit. Contam. Part B Surveill. 2017, 10, 268–274. [Google Scholar] [CrossRef]
- Torović, L. Fusarium toxins in corn food products: A survey of the Serbian retail market. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1596–1609. [Google Scholar] [CrossRef]
- Warth, B.; Parich, A.; Atehnkeng, J.; Bandyopadhyay, R.; Schuhmacher, R.; Sulyok, M.; Krska, R. Quantitation of Mycotoxins in Food and Feed from Burkina Faso and Mozambique Using a Modern LC-MS/MS Multitoxin Method. J. Agric. Food Chem. 2012, 60, 9352–9363. [Google Scholar] [CrossRef]
- Streit, E.; Schwab, C.; Sulyok, M.; Naehrer, K.; Krska, R.; Schatzmayr, G. Multi-Mycotoxin Screening Reveals the Occurrence of 139 Different Secondary Metabolites in Feed and Feed Ingredients. Toxins 2013, 5, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Maggiore, A.; Afonso, A.; Barrucci, F.; De Sanctis, G. Climate change as a driver of emerging risks for food and feed safety, plant, animal health and nutritional quality. EFSA Support. Publ. 2020, 17, E1881. [Google Scholar] [CrossRef]
- HBM4EU. Deliverable 4.9 Scoping Documents for 2021 for the First and Second Second Round HBM4EU Priority Substances; HBM4EU: Brussels, Belgium, 2019. [Google Scholar]
- Luo, X.Y.; Li, Y.W.; Wen, S.F.; Hu, X. Food poisoning caused by scabby wheat and the detection of Fusarium mycotoxins. J. Hyg. Res. 1987, 16, 33–37. [Google Scholar]
- JECFA. Evaluation of Certain Contaminants in Food—Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 959; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- JECFA. Safety Evaluation of Certain Contaminants in Food—Prepared by the Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives—Deoxynivalenol Addendum; WHO Food Additives Series 63; WHO: Geneva, Switzerland, 2011; pp. 317–485. [Google Scholar]
- EFSA; CONTAM; Knutsen, H.K.; Barregard, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Appropriateness to set a group health-based guidance value for fumonisins and their modified forms. EFSA J. 2018, 16, e05172. [Google Scholar]
- Bondy, G.; Mehta, R.; Caldwell, D.; Coady, L.; Armstrong, C.; Savard, M.; Miller, J.D.; Chomyshyn, E.; Bronson, R.; Zitomer, N.; et al. Effects of long term exposure to the mycotoxin fumonisin B1 in p53 heterozygous and p53 homozygous transgenic mice. Food Chem. Toxicol. 2012, 50, 3604–3613. [Google Scholar] [CrossRef]
- Iverson, F.; Armstrong, C.; Nera, E.; Truelove, J.; Fernie, S.; Scott, P.; Stapley, R.; Hayward, S.; Gunner, S. Chronic feeding study of deoxynivalenol in B6C3F1 male and female mice. Teratog Carcinog Mutagen 1995, 15, 283–306. [Google Scholar] [CrossRef]
- EFSA. Cadmium in food Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2009, 7, 980. [Google Scholar]
- EFSA; CONTAM; Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; et al. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18, e06223. [Google Scholar]
- EFSA; CONTAM; Knutsen, H.K.; Alexander, J.; Barregard, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; et al. Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA J. 2018, 16, e05333. [Google Scholar]
- Leist, M.; Ghallab, A.; Graepel, R.; Marchan, R.; Hassan, R.; Bennekou, S.H.; Limonciel, A.; Vinken, M.; Schildknecht, S.; Waldmann, T.; et al. Adverse outcome pathways: Opportunities, limitations and open questions. Arch. Toxicol. 2017, 91, 3477–3505. [Google Scholar] [CrossRef]
- Vidal, A.; Claeys, L.; Mengelers, M.; Vanhoorne, V.; Vervaet, C.; Huybrechts, B.; De Saeger, S.; De Boevre, M. Humans significantly metabolize and excrete the mycotoxin deoxynivalenol and its modified form deoxynivalenol-3-glucoside within 24 hours. Sci. Rep. 2018, 8, 5255. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.W.; Currie, V.; Richardson, A.J.; Duncan, G.; Holtrop, G.; Farquharson, F.; Louis, P.; Pinton, P.; Oswald, I.P. Porcine Small and Large Intestinal Microbiota Rapidly Hydrolyze the Masked Mycotoxin Deoxynivalenol-3-Glucoside and Release Deoxynivalenol in Spiked Batch Cultures In Vitro. Appl. Environ. Microbiol. 2018, 84, e02106-17. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Bhatnagar, D.; Bui-Klimke, T.; Carbone, I.; Hellmich, R.; Munkvold, G.; Paul, P.; Payne, G.; Takle, E. Climate change impacts on mycotoxin risks in US maize. World Mycotoxin J. 2011, 4, 79–93. [Google Scholar] [CrossRef]
- Halstensen, A.S.; Nordby, K.-C.; Eduard, W.; Klemsdal, S.S. Real-time PCR detection of toxigenic Fusarium in airborne and settled grain dust and associations with trichothecene mycotoxins. J. Environ. Monit. 2006, 8, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Straumfors, A.; Uhlig, S.; Eriksen, G.S.; Heldal, K.; Eduard, W.; Krska, R.; Sulyok, M. Mycotoxins and other fungal metabolites in grain dust from Norwegian grain elevators and compound feed mills. World Mycotoxin J. 2015, 8, 361–373. [Google Scholar] [CrossRef]
- Tangni, E.K.; Pussemier, L. Ergosterol and mycotoxins in grain dusts from fourteen Belgian cereal storages: A preliminary screening survey. J. Sci. Food Agric. 2007, 87, 1263–1270. [Google Scholar] [CrossRef]
- Viegas, C.; Fleming, G.T.A.; Kadir, A.; Almeida, B.; Caetano, L.A.; Gomes, A.Q.; Twarużek, M.; Kosicki, R.; Viegas, S.; Coggins, A.M.; et al. Occupational Exposures to Organic Dust in Irish Bakeries and a Pizzeria Restaurant. Microorganisms 2020, 8, 118. [Google Scholar] [CrossRef]
- Niculita-Hirzel, H.; Hantier, G.; Storti, F.; Plateel, G.; Roger, T. Frequent Occupational Exposure to Fusarium Mycotoxins of Workers in the Swiss Grain Industry. Toxins 2016, 8, 370. [Google Scholar] [CrossRef]
- Ndaw, S.; Remy, A.; Jargot, D.; Antoine, G.; Denis, F.; Robert, A. Mycotoxins Exposure of French Grain Elevator Workers: Biomonitoring and Airborne Measurements. Toxins 2021, 13, 382. [Google Scholar] [CrossRef]
- Ndaw, S.; Jargot, D.; Antoine, G.; Denis, F.; Melin, S.; Robert, A. Investigating Multi-Mycotoxin Exposure in Occupational Settings: A Biomonitoring and Airborne Measurement Approach. Toxins 2021, 13, 54. [Google Scholar] [CrossRef]
- Föllmann, W.; Ali, N.; Blaszkewicz, M.; Degen, G.H. Biomonitoring of Mycotoxins in Urine: Pilot Study in Mill Workers. J. Toxicol. Environ. Health Part A 2016, 79, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- LaKind, J.S.; Sobus, J.R.; Goodman, M.; Barr, D.B.; Furst, P.; Albertini, R.J.; Arbuckle, T.E.; Schoeters, G.; Tan, Y.M.; Teeguarden, J.; et al. A proposal for assessing study quality: Biomonitoring, Environmental Epidemiology, and Short-lived Chemicals (BEES-C) instrument. Environ. Int. 2014, 73, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Torres, O.; Showker, J.L.; Zitomer, N.C.; Matute, J.; Voss, K.A.; Waes, J.G.-V.; Maddox, J.R.; Gregory, S.G.; Ashley-Koch, A.E. The kinetics of urinary fumonisin B1 excretion in humans consuming maize-based diets. Mol. Nutr. Food Res. 2012, 56, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- De Santis, B.; Raggi, M.E.; Moretti, G.; Facchiano, F.; Mezzelani, A.; Villa, L.; Bonfanti, A.; Campioni, A.; Rossi, S.; Camposeo, S.; et al. Study on the Association among Mycotoxins and other Variables in Children with Autism. Toxins 2017, 9, 203. [Google Scholar] [CrossRef]
- Persson, E.C.; Sewram, V.; Evans, A.A.; London, W.T.; Volkwyn, Y.; Shen, Y.-J.; Van Zyl, J.A.; Chen, G.; Lin, W.; Shephard, G.S.; et al. Fumonisin B1 and risk of hepatocellular carcinoma in two Chinese cohorts. Food Chem. Toxicol. 2012, 50, 679–683. [Google Scholar] [CrossRef]
- Howard, P.C.; Eppley, R.M.; Stack, M.E.; Warbritton, A.; Voss, K.A.; Lorentzen, R.J.; Kovach, R.M.; Bucci, T.J. Fumonisin b1 carcinogenicity in a two-year feeding study using F344 rats and B6C3F1 mice. Environ. Health Perspect. 2001, 109 (Suppl. 2), 277–282. [Google Scholar] [CrossRef]
- Gelderblom, W.; Marasas, W.; Lebepe-Mazur, S.; Swanevelder, S.; Abel, S. Cancer initiating properties of fumonisin B1 in a short-term rat liver carcinogenesis assay. Toxicology 2008, 250, 89–95. [Google Scholar] [CrossRef]
- EPHPP, Project EPHP. Quality Assessment Tool for Quantitative Studies; Effective Public Health Practice Project: Amilton, ON, Canada, 1998. [Google Scholar]
- Claeys, L.; Romano, C.; De Ruyck, K.; Wilson, H.; Fervers, B.; Korenjak, M.; Zavadil, J.; Gunter, M.J.; De Saeger, S.; De Boevre, M.; et al. Mycotoxin exposure and human cancer risk: A systematic review of epidemiological studies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1449–1464. [Google Scholar] [CrossRef]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill, A.H., Jr.; Rothman, K.J.; Hendricks, K.A. Exposure to Fumonisins and the Occurrence of Neural Tube Defects along the Texas–Mexico Border. Environ. Health Perspect. 2006, 114, 237–241. [Google Scholar] [CrossRef]
- Gelineau-van Waes, J.; Voss, K.A.; Stevens, V.L.; Speer, M.C.; Riley, R.T. Chapter 5 Maternal Fumonisin Exposure as a Risk Factor for Neural Tube Defects. Adv. Food Nutr. Res. 2019, 56, 145–181. [Google Scholar]
- A Venter, P.; Christianson, A.L.; Hutamo, C.M.; Makhura, M.P.; Gericke, G.S. Congenital anomalies in rural black South African neonates—A silent epidemic? S. Afr. Med. J. 1995, 85, 15–20. [Google Scholar] [PubMed]
- Moore, C.A.; Li, S.; Li, Z.; Hong, S.X.; Gu, H.Q.; Berry, R.J.; Mulinare, J.; Erickson, J.D. Elevated rates of severe neural tube defects in a high-prevalence area in northern China. Am. J. Med. Genet. 1997, 73, 113–118. [Google Scholar] [CrossRef]
- Lian, Z.H.; Yang, H.Y.; Li, Z. Neural tube defects in Beijing-Tianjin area of China. Urban-rural distribution and some other epidemiological characteristics. J. Epidemiol. Community Health 1987, 41, 259–262. [Google Scholar] [CrossRef]
- Ncayiyana, D.J. Neural tube defects among rural blacks in a Transkei district—A preliminary report and analysis. S. Afr. Med. J. 1986, 69, 618–620. [Google Scholar]
- Flynn, T.J.; Stack, M.E.; Troy, A.L.; Chirtel, S.J. Assessment of the embryotoxic potential of total hydrolysis product of Fumonisin B1 using cultured organegenesis-staged rat embyros. Food Chem. Toxicol. 1997, 35, 1135–1141. [Google Scholar] [CrossRef]
- Gelineau-van Waes, J.; Rainey, M.A.; Maddox, J.R.; Voss, K.A.; Sachs, A.J.; Gardner, N.M.; Wilberding, J.D.; Riley, R.T. Increased sphingoid base-1-phosphates and failure of neural tube closure after exposure to fumonisin or FTY720. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Gelineau-van Waes, J.; Starr, L.; Maddox, J.; Aleman, F.; Voss, K.A.; Wilberding, J.; Riley, R.T. Maternal fumonisin exposure and risk for neural tube defects: Mechanisms in an in vivo mouse model. Birth Defects Res. Part A Clin. Mol. Teratol. 2005, 73, 487–497. [Google Scholar] [CrossRef]
- Voss, K.A.; Riley, R.T.; Gelineau-van Waes, J.G.-V. Fumonisin B1 induced neural tube defects were not increased in LM/Bc mice fed folate-deficient diet. Mol. Nutr. Food Res. 2014, 58, 1190–1198. [Google Scholar] [CrossRef]
- Voss, K.; Riley, R.; Gelineau-van Waes, J.G.-V. Fetotoxicity and neural tube defects in CD1 mice exposed to the mycotoxin fumonisin. BJSM Mycotoxins 2006, 2006, 67–72. [Google Scholar] [CrossRef]
- Voss, K.A.; Riley, R.T.; Snook, M.E.; Waes, J.G.-V. Reproductive and Sphingolipid Metabolic Effects of Fumonisin B1 and its Alkaline Hydrolysis Product in LM/Bc Mice: Hydrolyzed Fumonisin B1 Did Not Cause Neural Tube Defects. Toxicol. Sci. 2009, 112, 459–467. [Google Scholar] [CrossRef]
- Liao, Y.J.; Yang, J.R.; Chen, S.E.; Wu, S.J.; Huang, S.Y.; Lin, J.J.; Chen, L.R.; Tang, P.C. Inhibition of fumonisin B1 cytotoxicity by nanosilicate platelets during mouse embryo development. PLoS ONE 2014, 9, e112290. [Google Scholar] [CrossRef]
- Sadler, T.; Merrill, A.H.; Stevens, V.L.; Sullards, M.C.; Wang, E.; Wang, P. Prevention of fumonisin B1-induced neural tube defects by folic acid. Teratology 2002, 66, 169–176. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Ksieniewicz-Woźniak, E.; Szymczyk, K.; Ędrzejczak, R.J. Modified Fusarium Mycotoxins in Cereals and Their Products—Metabolism, Occurrence, and Toxicity: An Updated Review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef]
- Chen, L.; Peng, Z.; Nüssler, A.K.; Liu, L.; Yang, W. Current and prospective sights in mechanism of deoxynivalenol-induced emesis for future scientific study and clinical treatment. J. Appl. Toxicol. 2017, 37, 784–791. [Google Scholar] [CrossRef]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Terciolo, C.; Maresca, M.; Pinton, P.; Oswald, I.P. Review article: Role of satiety hormones in anorexia induction by Trichothecene mycotoxins. Food Chem. Toxicol. 2018, 121, 701–714. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Nepovimova, E.; Wang, Y.; Yang, H.; Li, L.; Zhang, X.; Kuca, K. Antioxidant agents against trichothecenes: New hints for oxidative stress treatment. Oncotarget 2017, 8, 110708–110726. [Google Scholar] [CrossRef]
- Chlebicz, A.; Śliżewska, K. In Vitro Detoxification of Aflatoxin B1, Deoxynivalenol, Fumonisins, T-2 Toxin and Zearalenone by Probiotic Bacteria from Genus Lactobacillus and Saccharomyces cerevisiae Yeast. Probiotics Antimicrob. Proteins 2020, 12, 289–301. [Google Scholar] [CrossRef]
- Heusinkveld, H.J.; Staal, Y.C.M.; Baker, N.C.; Daston, G.; Knudsen, T.B.; Piersma, A. An ontology for developmental processes and toxicities of neural tube closure. Reprod. Toxicol. 2021, 99, 160–167. [Google Scholar] [CrossRef]
- Copp, A.J.; Greene, N. Genetics and development of neural tube defects. J. Pathol. 2009, 220, 217–230. [Google Scholar] [CrossRef]
- Greene, N.D.; Copp, A.J. Neural tube defects. Annu. Rev. Neurosci. 2014, 37, 221–242. [Google Scholar] [CrossRef]
- Blom, H.J.; Shaw, G.M.; den Heijer, M.D.; Finnell, R.H. Neural tube defects and folate: Case far from closed. Nat. Rev. Neurosci. 2006, 7, 724–731. [Google Scholar] [CrossRef]
- Murko, C.; Lagger, S.; Steiner, M.; Seiser, C.; Schoefer, C.; Pusch, O. Histone deacetylase inhibitor Trichostatin A induces neural tube defects and promotes neural crest specification in the chicken neural tube. Differentiation 2013, 85, 55–66. [Google Scholar] [CrossRef]
- Voss, K.A.; Riley, R.T.; Moore, N.D.; Burns, T.D. Alkaline cooking (nixtamalisation) and the reduction in the in vivo toxicity of fumonisin-contaminated corn in a rat feeding bioassay. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2013, 30, 1415–1421. [Google Scholar] [CrossRef]
- Sassa, T.; Hirayama, T.; Kihara, A. Enzyme Activities of the Ceramide Synthases CERS2–6 Are Regulated by Phosphorylation in the C-terminal Region. J. Biol. Chem. 2016, 291, 7477–7487. [Google Scholar] [CrossRef]
- Riley, R.T.; Enongene, E.; Voss, K.A.; Norred, W.P.; Meredith, F.I.; Sharma, R.P.; Spitsbergen, J.; Williams, D.E.; Carlson, D.B.; Merrill, A.H., Jr. Sphingolipid perturbations as mechanisms for fumonisin carcinogenesis. Environ. Health Perspect. 2001, 109 (Suppl. 2), 301–308. [Google Scholar]
- Merrill, A.H., Jr.; Sullards, M.C.; Wang, E.; Voss, K.A.; Riley, R.T. Sphingolipid Metabolism: Roles in Signal Transduction and Disruption by Fumonisins. Environ. Health Perspect. 2001, 109, 283. [Google Scholar] [CrossRef]
- Turner, N.; Lim, X.Y.; Toop, H.D.; Osborne, B.; Brandon, A.E.; Taylor, E.N.; Fiveash, C.E.; Govindaraju, H.; Teo, J.D.; McEwen, H.P.; et al. A selective inhibitor of ceramide synthase 1 reveals a novel role in fat metabolism. Nat. Commun. 2018, 9, 3165. [Google Scholar] [CrossRef]
- Wang, E.; Norred, W.P.; Bacon, C.W.; Riley, R.T.; Merrill, A.H., Jr. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 1991, 266, 14486–14490. [Google Scholar] [CrossRef]
- Riley, R.T.; Merrill, A.H., Jr. Ceramide synthase inhibition by fumonisins: A perfect storm of perturbed sphingolipid metabolism, signaling, and disease. J. Lipid Res. 2019, 60, 1183–1189. [Google Scholar] [CrossRef]
- Wangia, R.N.; Githanga, D.P.; Xue, K.S.; Tang, L.; Anzala, O.A.; Wang, J.-S. Validation of urinary sphingolipid metabolites as biomarker of effect for fumonisins exposure in Kenyan children. Biomarkers 2019, 24, 379–388. [Google Scholar] [CrossRef]
- Riley, R.T.; Torres, O.; Matute, J.; Gregory, S.G.; Ashley-Koch, A.E.; Showker, J.L.; Mitchell, T.R.; Voss, K.A.; Maddox, J.R.; Waes, J.B.G.-V. Evidence for fumonisin inhibition of ceramide synthase in humans consuming maize-based foods and living in high exposure communities in Guatemala. Mol. Nutr. Food Res. 2015, 59, 2209–2224. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dudás, I.; Vereczkey, A.; Bánhidy, F. Folate Deficiency and Folic Acid Supplementation: The Prevention of Neural-Tube Defects and Congenital Heart Defects. Nutrients 2013, 5, 4760–4775. [Google Scholar] [CrossRef]
- Sato, K. Why is folate effective in preventing neural tube closure defects? Med. Hypotheses 2019, 134, 109429. [Google Scholar] [CrossRef]
- Stevens, V.L.; Tang, L. Fumonisin B1-induced sphingolipid depletion inhibits vitamin uptake via the glycosylphosphatidylinositol-anchored folate receptor. J. Biol. Chem. 1997, 272, 18020–18025. [Google Scholar] [CrossRef]
- Chatterjee, S.; Smith, E.R.; Hanada, K.; Stevens, V.L.; Mayor, S. GPI anchoring leads to sphingolipid-dependent retention of endocytosed proteins in the recycling endosomal compartment. EMBO J. 2001, 20, 1583–1592. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Riley, R.T.; Hendricks, K.A.; Stevens, V.L.; Sadler, T.W.; Gelineau-van Waes, J.; Missmer, S.A.; Cabrera, J.; Torres, O.; Gelderblom, W.C.A.; et al. Fumonisins Disrupt Sphingolipid Metabolism, Folate Transport, and Neural Tube Development in Embryo Culture and In Vivo: A Potential Risk Factor for Human Neural Tube Defects among Populations Consuming Fumonisin-Contaminated Maize. J. Nutr. 2004, 134, 711–716. [Google Scholar] [CrossRef]
- Mullen, T.D.; Hannun, Y.A.; Obeid, L.M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 2012, 441, 789–802. [Google Scholar] [CrossRef]
- Futerman, A.H.; Hannun, Y.A. The complex life of simple sphingolipids. EMBO Rep. 2004, 5, 777–782. [Google Scholar] [CrossRef]
- Naslavsky, N.; Shmeeda, H.; Friedlander, G.; Yanai, A.; Futerman, A.H.; Barenholz, Y.; Taraboulos, A. Sphingolipid Depletion Increases Formation of the Scrapie Prion Protein in Neuroblastoma Cells Infected with Prions. J. Biol. Chem. 1999, 274, 20763–20771. [Google Scholar] [CrossRef]
- Yoo, H.-S.; Norred, W.P.; Showker, J.; Riley, R.T. Elevated Sphingoid Bases and Complex Sphingolipid Depletion as Contributing Factors in Fumonisin-Induced Cytotoxicity. Toxicol. Appl. Pharmacol. 1996, 138, 211–218. [Google Scholar] [CrossRef]
- Mitsuda, T.; Furukawa, K.; Fukumoto, S.; Miyazaki, H.; Urano, T.; Furukawa, K. Overexpression of Ganglioside GM1 Results in the Dispersion of Platelet-derived Growth Factor Receptor from Glycolipid-enriched Microdomains and in the Suppression of Cell Growth Signals. J. Biol. Chem. 2002, 277, 11239–11246. [Google Scholar] [CrossRef]
- Puff, N.; Watanabe, C.; Seigneuret, M.; Angelova, M.I.; Staneva, G. Lo/Ld phase coexistence modulation induced by GM1. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2105–2114. [Google Scholar] [CrossRef][Green Version]
- Mayor, S.; Sabharanjak, S.; Maxfield, F.R. Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 1998, 17, 4626–4638. [Google Scholar] [CrossRef]
- Refaei, M.; Leventis, R.; Silvius, J.R. Assessment of the Roles of Ordered Lipid Microdomains in Post-Endocytic Trafficking of Glycosyl-Phosphatidylinositol-Anchored Proteins in Mammalian Fibroblasts. Traffic 2011, 12, 1012–1024. [Google Scholar] [CrossRef]
- Sharom, F.J.; Lehto, M.T. Glycosylphosphatidylinositol-anchored proteins: Structure, function, and cleavage by phosphatidylinositol-specific phospholipase C. Biochem. Cell Biol. 2002, 80, 535–549. [Google Scholar] [CrossRef]
- Menegola, E.; Di Renzo, F.; Broccia, M.L.; Prudenziati, M.; Minucci, S.; Massa, V.; Giavini, E. Inhibition of histone deacetylase activity on specific embryonic tissues as a new mechanism for teratogenicity. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2005, 74, 392–398. [Google Scholar] [CrossRef]
- AOPwiki. Histone Deacetylase Inhibition Leads to Neural Tube Defects 2021. Available online: https://aopwiki.org/aops/275. (accessed on 1 January 2022).
- Gardner, N.M.; Riley, R.T.; Showker, J.L.; Voss, K.A.; Sachs, A.J.; Maddox, J.R.; Waes, J.B.G.-V. Elevated nuclear sphingoid base-1-phosphates and decreased histone deacetylase activity after fumonisin B1 treatment in mouse embryonic fibroblasts. Toxicol. Appl. Pharmacol. 2016, 298, 56–65. [Google Scholar] [CrossRef]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of Histone Acetylation in the Nucleus by Sphingosine-1-Phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef]
- Riccio, A. New Endogenous Regulators of Class I Histone Deacetylases. Sci. Signal. 2010, 3, pe1. [Google Scholar] [CrossRef] [PubMed]
- Blaho, V.A.; Hla, T. An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 2014, 55, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, N.; Okada, T.; Hayashi, S.; Fujita, T.; Jahangeer, S.; Nakamura, S.-I. Sphingosine Kinase 2 Is a Nuclear Protein and Inhibits DNA Synthesis. J. Biol. Chem. 2003, 278, 46832–46839. [Google Scholar] [CrossRef] [PubMed]
- Mizugishi, K.; Yamashita, T.; Olivera, A.; Miller, G.F.; Spiegel, S.; Proia, R.L. Essential Role for Sphingosine Kinases in Neural and Vascular Development. Mol. Cell. Biol. 2005, 25, 11113–11121. [Google Scholar] [CrossRef]
- Mengelers, M.; Zeilmaker, M.; Vidal, A.; De Boevre, M.; De Saeger, S.; Hoogenveen, R. Biomonitoring of Deoxynivalenol and Deoxynivalenol-3-glucoside in Human Volunteers: Renal Excretion Profiles. Toxins 2019, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- van den Brand, A.D.; Hoogenveen, R.; Mengelers, M.J.B.; Zeilmaker, M.; Eriksen, G.S.; Uhlig, S.; Brantsæter, A.L.; Dirven, H.A.; Husøy, T. Modelling the Renal Excretion of the Mycotoxin Deoxynivalenol in Humans in an Everyday Situation. Toxins 2021, 13, 675. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Thiel, P.G.; Sydenham, E.W.; Alberts, J.F. Biliary excretion of the mycotoxin fumonisin B1 in rats. Food Chem. Toxicol. 1994, 32, 489–491. [Google Scholar] [CrossRef]
- Prelusky, D.B.; Trenholm, H.L.; Savard, M.E. Pharmacokinetic fate of 14C-Labelled fumonisin B1 in Swine. Nat. Toxins 1994, 2, 73–80. [Google Scholar] [CrossRef]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis 2010, 31, 71–82. [Google Scholar] [CrossRef]
- Li, M.; Sun, M.; Hong, X.; Duan, J.; Du, D. Survey of Deoxynivalenol Contamination in Agricultural Products in the Chinese Market Using An ELISA Kit. Toxins 2018, 11, 6. [Google Scholar] [CrossRef]
- Tantaoui-Elaraki, A.; Riba, A.; Oueslati, S.; Zinedine, A. Toxigenic fungi and mycotoxin occurrence and prevention in food and feed in northern Africa—A review. World Mycotoxin J. 2018, 11, 385–400. [Google Scholar] [CrossRef]
- HBM4EU. ICI Report 2nd Round Substances—Mycotoxins/Round_01/2020—Deoxynivalenol Biomarkers in Urine; HBM4EU: Brussels, Belgium, 2020. [Google Scholar]
- Turner, P.C.; White, K.L.; Burley, V.J.; Hopton, R.P.; Rajendram, A.; Fisher, J.; Cade, J.E.; Wild, C.P. A comparison of deoxynivalenol intake and urinary deoxynivalenol in UK adults. Biomarkers 2010, 15, 553–562. [Google Scholar] [CrossRef]
- HBM4EU. D14.5—Selection Criteria and Inventory of Effect Biomarkers for the 2nd Set of Substances; HBM4EU: Brussels, Belgium, 2020. [Google Scholar]
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.; Latiff, A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2008. Food Chem. Toxicol. 2019, 129, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Van Der Westhuizen, L.; Sewram, V. Biomarkers of exposure to fumonisin mycotoxins: A review. Food Addit. Contam. 2007, 24, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.A.; Bacon, C.W.; Norred, W.P.; Chapin, R.E.; Chamberlain, W.J.; Plattner, R.D.; Meredith, F.I. Studies on the reproductive effects of Fusarium moniliforme culture material in rats and the biodistribution of [14C] fumonisin B1 in pregnant rats. Nat. Toxins 1996, 4, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Cortinovis, C.; Pizzo, F.; Spicer, L.; Caloni, F. Fusarium mycotoxins: Effects on reproductive function in domestic animals—A review. Theriogenology 2013, 80, 557–564. [Google Scholar] [CrossRef]
- Voss, K.A.; Riley, R.T.; Norred, W.P.; Bacon, C.W.; Meredith, F.I.; Howard, P.C.; Plattner, R.D.; Collins, T.F.; Hansen, D.K.; Porter, J.K. An overview of rodent toxicities: Liver and kidney effects of fumonisins and Fusarium moniliforme. Environ. Health Perspect. 2001, 109 (Suppl. 2), 259–266. [Google Scholar]
- Lumsangkul, C.; Chiang, H.-I.; Lo, N.-W.; Fan, Y.-K.; Ju, J.-C. Developmental Toxicity of Mycotoxin Fumonisin B1 in Animal Embryogenesis: An Overview. Toxins 2019, 11, 114. [Google Scholar] [CrossRef]
- Del Gaudio, I.; Sasset, L.; Lorenzo, A.D.; Wadsack, C. Sphingolipid Signature of Human Feto-Placental Vasculature in Preeclampsia. Int. J. Mol. Sci. 2020, 21, 1019. [Google Scholar] [CrossRef]
- Padmanabhan, R. Etiology, pathogenesis and prevention of neural tube defects. Congenit. Anom. 2006, 46, 55–67. [Google Scholar] [CrossRef]
- Babenko, N.A.; Kharchenko, V.S. Effects of inhibitors of key enzymes of sphingolipid metabolism on insulin-induced glucose uptake and glycogen synthesis in liver cells of old rats. Biochemistry 2015, 80, 104–112. [Google Scholar] [CrossRef]
- Scarlatti, F.; Bauvy, C.; Ventruti, A.; Sala, G.; Cluzeaud, F.; Vandewalle, A.; Ghidoni, R.; Codogno, P. Ceramide-mediated Macroautophagy Involves Inhibition of Protein Kinase B and Up-regulation of Beclin 1. J. Biol. Chem. 2004, 279, 18384–18391. [Google Scholar] [CrossRef]
- Ross, M.M.; Piorczynski, T.B.; Harvey, J.; Burnham, T.S.; Francis, M.; Larsen, M.W.; Roe, K.; Hansen, J.M.; Stark, M.R. Ceramide: A novel inducer for neural tube defects. Dev. Dyn. 2019, 248, 979–996. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.-N.; Zhu, Z.; Itokazu, Y.; Wang, G.; Dinkins, M.B.; Zhong, L.; Lin, H.-P.; Elsherbini, A.; Leanhart, S.; Jiang, X.; et al. Novel function of ceramide for regulation of mitochondrial ATP release in astrocytes. J. Lipid Res. 2018, 59, 488–506. [Google Scholar] [CrossRef] [PubMed]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef] [PubMed]
- Mollinedo, F.; Gajate, C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: Implications in tumor progression and therapy: Thematic review series: Biology of lipid rafts. J. Lipid Res. 2020, 61, 611–635. [Google Scholar] [CrossRef] [PubMed]
- D’Aprile, C.; Prioni, S.; Mauri, L.; Prinetti, A.; Grassi, S. Lipid rafts as platforms for sphingosine 1-phosphate metabolism and signalling. Cell. Signal. 2021, 80, 109929. [Google Scholar] [CrossRef]
- Renwick, J.H.; Claringbold, W.D.B.; Earthy, M.E.; Few, J.D.; Carolines, A.; McLean, S. Neural-tube defects produced in Syrian hamsters by potato glycoalkaloids. Teratology 1984, 30, 371–381. [Google Scholar] [CrossRef]
- Ni, W.; Tian, T.; Zhang, L.; Li, Z.; Wang, L.; Ren, A. Maternal periconceptional consumption of sprouted potato and risks of neural tube defects and orofacial clefts. Nutr. J. 2018, 17, 112. [Google Scholar] [CrossRef]
- Mandimika, T.; Baykus, H.; Poortman, J.; Garza, C.; Kuiper, H.; Peijnenburg, A. Induction of the cholesterol biosynthesis pathway in differentiated Caco-2 cells by the potato glycoalkaloid alpha-chaconine. Food Chem. Toxicol. 2007, 45, 1918–1927. [Google Scholar] [CrossRef]
- Meena, M.; Samal, S. Alternaria host-specific (HSTs) toxins: An overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 2019, 6, 745–758. [Google Scholar] [CrossRef]
- Topi, D.; Tavčar-Kalcher, G.; Pavšič-Vrtač, K.; Babič, J.; Jakovac-Strajn, B. Alternaria mycotoxins in grains from Albania: Alternariol, alternariol monomethyl ether, tenuazonic acid and tentoxin. World Mycotoxin J. 2019, 12, 89–99. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Multi-mycotoxin screening of feed and feed raw materials from Africa. World Mycotoxin J. 2018, 11, 369–383. [Google Scholar] [CrossRef]
- HBM4EU. HBM4EU ICI Report Mycotoxins (DON) in Urine Round 3; HBM4EU: Brussels, Belgium, 2020. [Google Scholar]
- Zare Jeddi, M.; Boon, P.E.; Cubadda, F.; Hoogenboom, L.R.; Mol, H.; Verhagen, H.; Sijm, D.T. A vision on the ‘foodture’ role of dietary exposure sciences in the interplay between food safety and nutrition. Trends Food Sci. Technol. 2022, 120, 288–300. [Google Scholar] [CrossRef]
- Sewram, V.; Mshicileli, N.; Shephard, G.S.; Marasas, W.F.O. Fumonisin mycotoxins in human hair. Biomarkers 2003, 8, 110–118. [Google Scholar] [CrossRef]
- Sewram, V.; Nair, J.J.; Nieuwoudt, T.W.; Gelderblom, W.C.A.; Marasas, W.F.O.; Shephard, G.S. Assessing chronic exposure to fumonisin mycotoxins: The use of hair as a suitable noninvasive matrix. J. Anal. Toxicol. 2001, 25, 450–455. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Total DON 1 [4] TDI 3 1 µg/kg bw/day [4] | Total Fumonisins 2 [3] TDI 1 µg/kg bw/day [19] | |||
|---|---|---|---|---|
| Mean (LB-UB 4) | P95 5 (LB-UB) | Mean (LB-UB) | P95 (LB-UB) | |
| Infants and children | 0.2–2.0 | 0.7–3.7 | 0.04–1.8 | 0.2–4.1 |
| Adults | 0.3–0.7 | 0.5–1.4 | 0.05–0.6 | 0.09–1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Brand, A.D.; Bajard, L.; Steffensen, I.-L.; Brantsæter, A.L.; Dirven, H.A.A.M.; Louisse, J.; Peijnenburg, A.; Ndaw, S.; Mantovani, A.; De Santis, B.; et al. Providing Biological Plausibility for Exposure–Health Relationships for the Mycotoxins Deoxynivalenol (DON) and Fumonisin B1 (FB1) in Humans Using the AOP Framework. Toxins 2022, 14, 279. https://doi.org/10.3390/toxins14040279
van den Brand AD, Bajard L, Steffensen I-L, Brantsæter AL, Dirven HAAM, Louisse J, Peijnenburg A, Ndaw S, Mantovani A, De Santis B, et al. Providing Biological Plausibility for Exposure–Health Relationships for the Mycotoxins Deoxynivalenol (DON) and Fumonisin B1 (FB1) in Humans Using the AOP Framework. Toxins. 2022; 14(4):279. https://doi.org/10.3390/toxins14040279
Chicago/Turabian Stylevan den Brand, Annick D., Lola Bajard, Inger-Lise Steffensen, Anne Lise Brantsæter, Hubert A. A. M. Dirven, Jochem Louisse, Ad Peijnenburg, Sophie Ndaw, Alberto Mantovani, Barbara De Santis, and et al. 2022. "Providing Biological Plausibility for Exposure–Health Relationships for the Mycotoxins Deoxynivalenol (DON) and Fumonisin B1 (FB1) in Humans Using the AOP Framework" Toxins 14, no. 4: 279. https://doi.org/10.3390/toxins14040279
APA Stylevan den Brand, A. D., Bajard, L., Steffensen, I.-L., Brantsæter, A. L., Dirven, H. A. A. M., Louisse, J., Peijnenburg, A., Ndaw, S., Mantovani, A., De Santis, B., & Mengelers, M. J. B. (2022). Providing Biological Plausibility for Exposure–Health Relationships for the Mycotoxins Deoxynivalenol (DON) and Fumonisin B1 (FB1) in Humans Using the AOP Framework. Toxins, 14(4), 279. https://doi.org/10.3390/toxins14040279







