New Insights into the Mechanism of Action of PirAB from Vibrio Parahaemolyticus
Abstract
:1. Introduction
2. Acute Hepatopancreatic Necrosis Disease in Penaeid Shrimp
2.1. Degree of Virulence
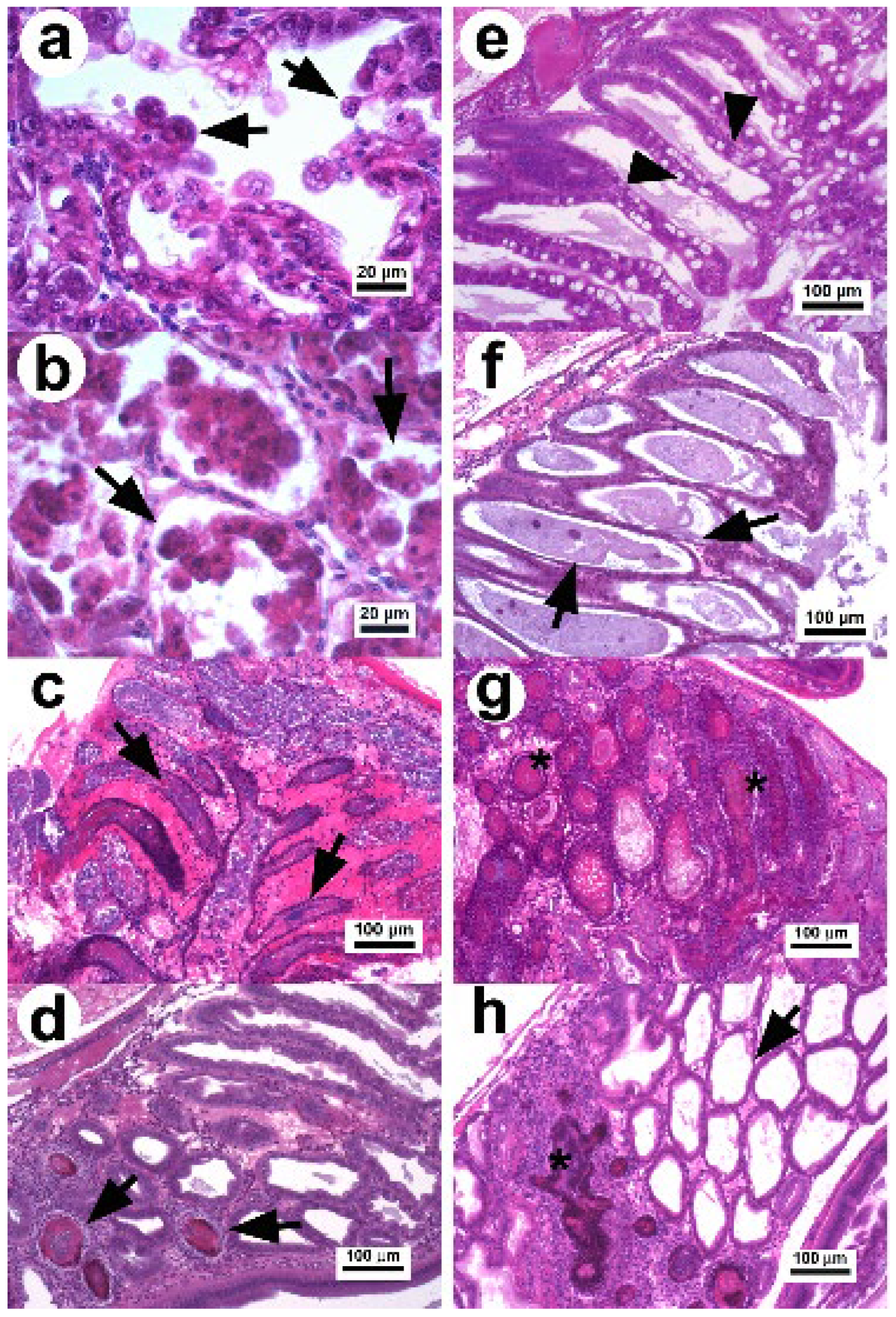
2.2. Histopathology of AHPND
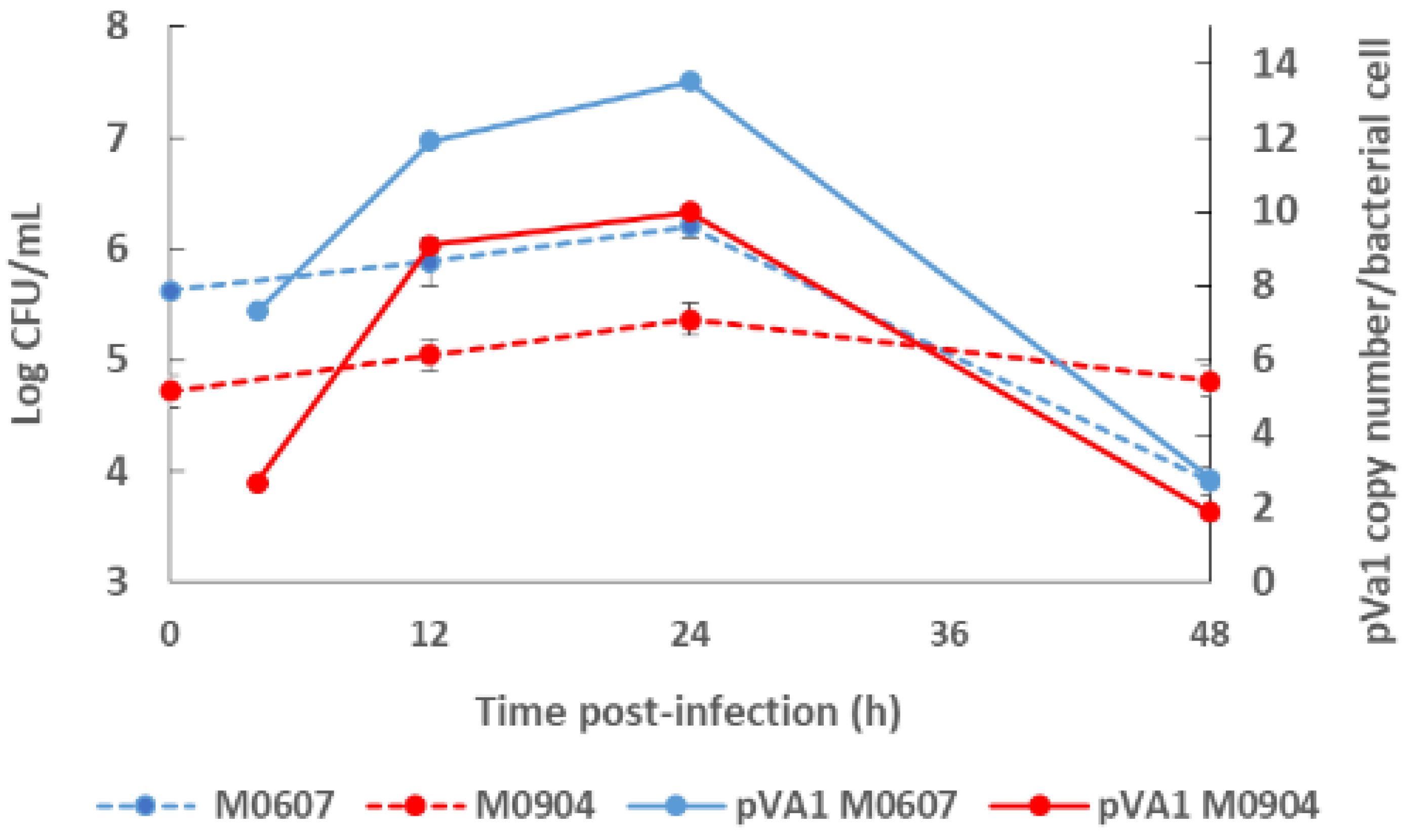
3. Virulence Plasmid pVa1
4. Changes in the Microbiota of Seawater
5. Factors That Could Induce or Inhibit Toxin Production
5.1. Quorum Sensing
5.2. Environmental Factors
5.3. Biofilm Formation
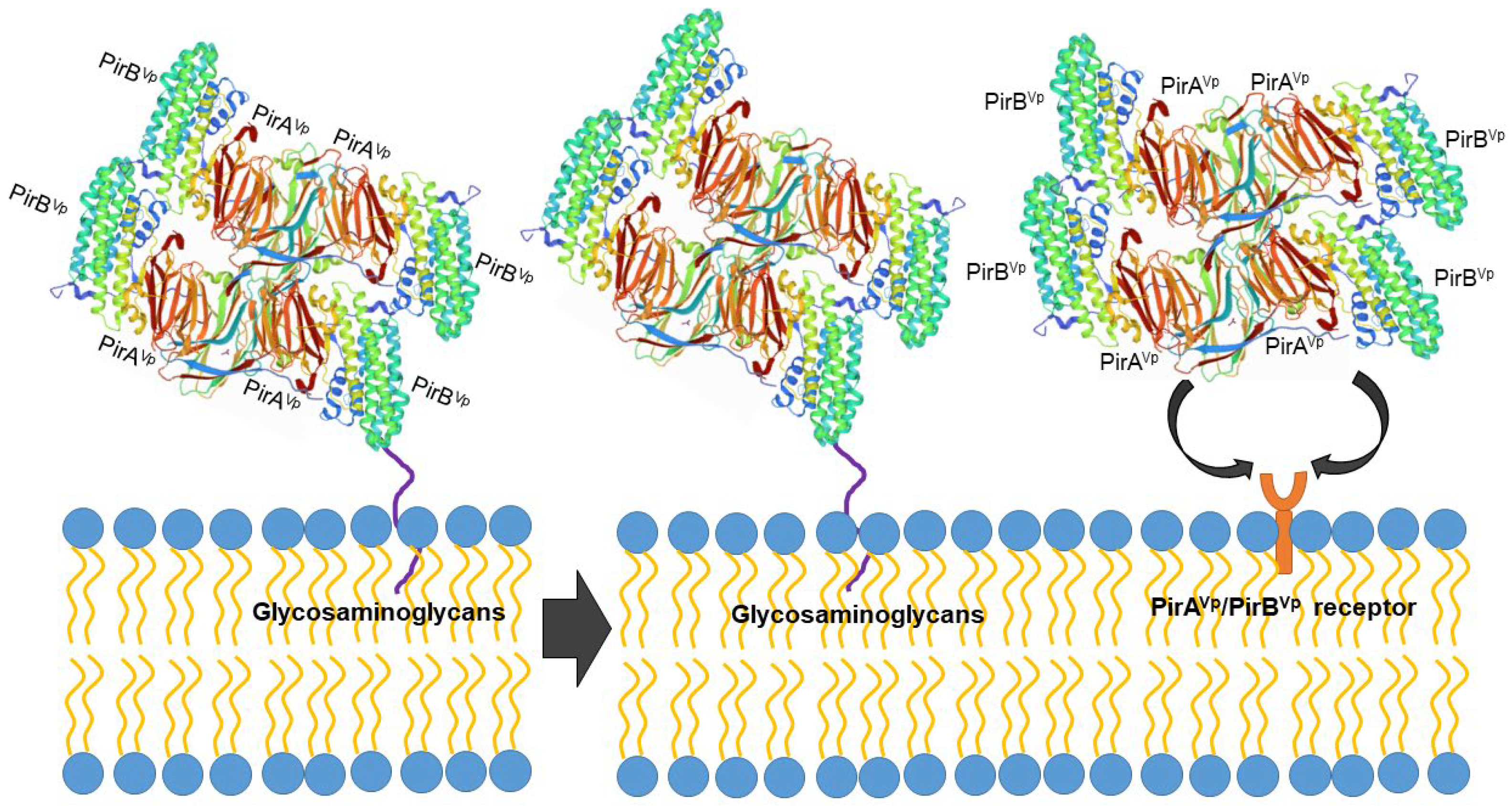
6. Search for Membrane Receptors of PirAVp and PirBVp
6.1. Biological Activities of the PirAVp and PirBVp Subunits
6.2. Expression of Mucin-like O-Glycosidic Structures in Shrimp
6.3. Receptor on Shrimp Hemocytes
7. Search of Potential Inhibitors of the PirABVp Toxin
8. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tran, L.H.; Nunan, L.; Redman, R.M.; Mohney, L.L.; Pantoja, C.R.; Fitzsimmons, K.; Lightner, D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting Penaeid shrimp. Dis. Aquat. Org. 2013, 105, 45–55. [Google Scholar]
- OIE. Manual of Diagnostic Tests for Aquatic Animals (2019); World Organisation for Animal Health: Paris, France, 2019. [Google Scholar]
- Joshi, J.; Srisala, J.; Truong, V.H.; Chen, I.T.; Nuangsaeng, B.; Suthienkul, O.; Lo, C.F.; Flegel, T.W.; Sritunyalucksana, K.; Thitamadee, S. Variation in Vibrio parahaemolyticus isolates from a single Thai shrimp farm experiencing an outbreak of acute hepatopancreatic necrosis disease (AHPND). Aquaculture 2014, 428, 297–302. [Google Scholar]
- Kondo, H.; Van, P.T.; Dang, L.T.; Hirono, I. Draft genome sequence of non-Vibrio parahaemolyticus acute hepatopancreatic necrosis disease strain KC13.17.5, isolated from diseased shrimp in Vietnam. Genome Announc. 2015, 3, e00978-15. [Google Scholar] [PubMed] [Green Version]
- Feng, B.; Liu, H.; Wang, M.; Sun, X.; Pan, Y. Diversity analysis of acute hepatopancreatic necrosis disease-positive Vibrio parahaemolyticus strains. Aquac. Fish. 2017, 2, 278–285. [Google Scholar]
- Nunan, L.; Lightner, D.; Pantoja, C.; Gomez-Jimenez, S. Detection of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Dis. Aquat. Org. 2014, 111, 81–86. [Google Scholar]
- Restrepo, L.; Bayot, B.; Betancourt, I.; Pinzón, A. Draft genome sequence of pathogenic bacteria Vibrio parahaemolyticus strain Ba94C2, associated with acute hepatopancreatic necrosis disease isolate from South America. Genom. Data 2016, 9, 143–144. [Google Scholar]
- Dhar, A.K.; Piamsomboon, P.; Caro, L.F.A.; Kanrar, S.; Adami, R., Jr.; Juan, Y.S. First report of acute hepatopancreatic necrosis disease (AHPND) occurring in the USA. Dis. Aquat. Org. 2019, 132, 241–247. [Google Scholar]
- Shinn, A.P.; Pratoomyot, J.; Griffiths, D.; Trong, T.Q.; Vu, N.T.; Jiravanichpaisal, J.; Briggs, M. Asian shrimp production and the economic costs of disease. Asian Fish. Sci. 2018, 31S, 29–58. [Google Scholar]
- Lee, C.T.; Chen, I.T.; Yang, Y.T.; Ko, T.P.; Huang, Y.T.; Huang, J.Y.; Huang, M.F.; Lin, S.J.; Chen, C.Y.; Lin, S.S.; et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 10798–10803. [Google Scholar]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano-Olvera, R.; Betancourt-Lozano, M.; Morales-Covarrubias, M.S. Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease (AHPND) of cultured shrimp (Litopenaeus vannamei) in northwestern Mexico. Appl. Environ. Microbiol. 2015, 81, 1689–1699. [Google Scholar]
- Liu, L.; Xiao, J.; Xia, X.; Pan, Y.; Yan, S.; Wang, Y. Draft genome sequence of Vibrio owensii strain SH-14, which causes shrimp acute hepatopancreatic necrosis disease. Genome Announc. 2015, 3, e01395-15. [Google Scholar] [PubMed] [Green Version]
- Liu, L.; Xiao, J.; Zhang, M.; Zhu, W.; Xia, X.; Dai, W.; Pan, Y.; Yan, S.; Wang, Y. A Vibrio owensii strain as the causative agent of AHPND in cultured shrimp, Litopenaeus vannamei. J. Invertebr. Pathol. 2018, 153, 156–164. [Google Scholar] [PubMed]
- Ahn, Y.S.; Piamsomboond, P.; Tang, K.F.J.; Han, J.E.; Kim, J.H. Complete genome sequence of AHPND-causing Vibrio campbellii LA16-V1 isolated from Penaeus vannamei cultured in a Latin American country. Genome Announc. 2017, 5, e01011-17. [Google Scholar] [PubMed] [Green Version]
- Dong, X.; Wang, H.; Xie, G.; Zou, P.; Guo, C.; Liang, Y.; Huang, J. An isolate of Vibrio campbellii carrying the pirVP gene causes acute hepatopancreatic necrosis disease. Emerg. Microbes Infect. 2017, 6, 1–3. [Google Scholar]
- Dong, X.; Wang, H.; Zou, P.; Chen, J.; Liu, Z.; Wang, X.; Huang, J. Complete genome sequence of Vibrio campbellii strain 20130629003S01 isolated from shrimp with acute hepatopancreatic necrosis disease. Gut Pathog. 2017, 9, 31–35. [Google Scholar]
- Restrepo, L.; Bayot, B.; Arciniegas, S.; Bajaña, L.; Betancourt, I.; Panchana, F.; Reyes Muñoz, A. PirVP genes causing AHPND identified in a new Vibrio species (Vibrio punensis) within the commensal Orientalis clade. Sci. Rep. 2018, 8, 13080. [Google Scholar]
- Xiao, J.; Liu, L.; Ke, Y.; Li, X.; Liu, Y.; Pan, Y.; Yan, S.; Wang, Y. Shrimp AHPND-causing plasmids encoding the PirAB toxins as mediated by pirAB-Tn903 are prevalent in various Vibrio species. Sci. Rep. 2017, 7, 42177. [Google Scholar]
- Dong, X.; Chen, J.; Song, J.; Wang, H.; Wang, W.; Ren, Y.; Guo, C.; Wang, X.; Tang, K.F.J.; Huang, J. Evidence of the horizontal transfer of pVA1-type plasmid from AHPND-causing V. campbellii to non-AHPND V. owensii. Aquaculture 2019, 503, 396–402. [Google Scholar]
- Dong, X.; Song, J.; Chen, J.; Bi, D.; Wang, W.; Ren, Y.; Wang, H.; Wang, G.; Tang, K.F.J.; Wang, X.; et al. Conjugative transfer of the pVA1-type plasmid carrying the pirABvp genes results in the formation of new AHPND-causing Vibrio. Front. Cell. Infect. Microbiol. 2019, 9, 195–205. [Google Scholar]
- FAO. Report of the FAO/MARD Technical Workshop on Early Mortality Syndrome (EMS) or Acute Hepatopancreatic Necrosis Syndrome (AHPNS) of Cultured Shrimp (under TCP/VIE/3304), Hanoi, Vietnam, 25 to 27 June 2013; Report No. 1053; FAO Fisheries and Aquaculture: Rome, Italy, 2013. [Google Scholar]
- Powers, Q.; Caro, L.F.A.; Fitzsimmons, K.M.; McLain, J.E.; Dhar, A.K. Crayfish (Cherax quadricarinatus) susceptibility to acute hepatopancreatic necrosis disease (AHPND). J. Invertebr. Pathol. 2021, 186, 107554. [Google Scholar]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano-Olvera, R.; Bolan-Mejía, C.; Aguilar-Rendon, K.G.; Enciso-Ibarra, J. Pathological, genomic and phenotypical characterization of Vibrio parahaemolyticus, causative agent of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Asian Fish. Sci. 2018, 31, 102–111. [Google Scholar]
- Lightner, D.V. A Handbook of Shrimp Pathology and Diagnostic Procedures for Diseases of Cultured Penaeid Shrimp; World Aquaculture Society: Baton Rouge, LA, USA, 1996. [Google Scholar]
- González-Gómez, J.P.; Soto-Rodriguez, S.; López-Cuevas, O.; Castro-del Campo, N.; Chaidez, C.; Gomez-Gil, B. Phylogenomic Analysis Supports Two Possible Origins for Latin American Strains of Vibrio parahaemolyticus Associated with Acute Hepatopancreatic Necrosis Disease (AHPND). Curr. Microbiol. 2020, 77, 3851–3860. [Google Scholar] [PubMed]
- Salyer, A.; Whitt, D. Bacterial Pathogenesis, a Molecular Approach, 2nd ed.; ASS Press: Washington, DC, USA, 2002. [Google Scholar]
- Donnenberg, M.S. Pathogenic strategies of enteric bacteria. Nature 2000, 406, 768–774. [Google Scholar] [PubMed]
- Casadevall, A.; Pirofski, L. Host-pathogen interactions: The attributes of virulence. J. Infect. Dis. 2001, 184, 337–344. [Google Scholar] [PubMed]
- Wurtzel, O.; Sesto, N.; Mellin, J.R.; Karunker, I.; Edelheit, S.; Bécavin, C.; Archambaud, C.; Cossart, P.; Sorek, R. Comparative transcriptomics of pathogenic and non-pathogenic Listeria species. Mol. Syst. Biol. 2012, 8, 583. [Google Scholar] [PubMed]
- Han, J.E.; Tang, K.F.J.; Tran, L.H.; Lightner, D.V. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis. Aquat. Org. 2015, 113, 33–40. [Google Scholar]
- Aguilar-Rendón, K.G.; Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano-Olvera, R.; Yáñez-Rivera, B. Water microbiome dynamics of Pacific white shrimp Penaeus vannamei infected with Vibrio parahaemolyticus strains responsible for acute hepatopancreatic necrosis disease. Aquaculture 2022, 551, 737871. [Google Scholar]
- Sirikharin, R.; Taengchaiyaphum, S.; Sanguanrut, P.; Chi, T.D.; Mavichak, R.; Proespraiwong, P.; Nuangsaeng, B.; Thitamadee, S.; Flegel, T.W.; Sritunyalucksana, K. Characterization and PCR detection of binary, Pir-Like toxins from Vibrio parahaemolyticus isolates that cause acute hepatopancreatic necrosis disease (AHPND) in shrimp. PLoS ONE 2015, 10, e0126987. [Google Scholar]
- Tinwongger, S.; Nochiri, Y.; Thawonsuwan, J.; Nozaki, R.; Kondo, H.; Awasthi, S.P.; Hinenoya, A.; Yamasaki, S.; Hirono, I. Virulence of acute hepatopancreatic necrosis disease PirAB-like relies on secreted proteins not on gene copy number. J. Appl. Microbiol. 2016, 121, 1755–1765. [Google Scholar]
- Aguilar-Rendón, K.G.; Lozano-Olvera, R.; Yáñez-Rivera, B.; Soto-Rodriguez, S.A. Bacteriological and histopathological analysis of Penaeus vannamei experimentally infected with Vibrio parahaemolyticus-AHPND strains. Dis. Aquat. Org. 2020, 140, 167–177. [Google Scholar]
- Hong, X.P.; Xu, D.; Zhuo, Y.; Liu, H.Q.; Lu, L.Q. Identification and pathogenicity of Vibrio parahaemolyticus isolates and immune responses of Penaeus (Litopenaeus) vannamei (Boone). J. Fish Dis. 2016, 39, 1085–1097. [Google Scholar] [PubMed]
- Phiwsaiya, K.; Charoensapsri, W.; Taengphu, S.; Dong, H.T.; Sangsuriya, P.; Nguyen, G.T.T.; Pham, H.Q.; Amparyup, P.; Sritunyalucksana, K.; Taengchaiyaphum, S.; et al. A natural Vibrio parahaemolyticus ΔpirAVppirBVp+ mutant kills shrimp but produces neither PirVp toxins nor acute hepatopancreatic necrosis disease lesions. Appl. Environ. Microbiol. 2017, 83, e00680-17. [Google Scholar] [PubMed] [Green Version]
- Han, J.E.; Choi, S.K.; Han, S.H.; Lee, S.C.; Jeon, H.J.; Lee, C.; Lee, K.J. Genomic and histopathological characteristics of Vibrio parahaemolyticus isolated from an acute hepatopancreatic necrosis disease outbreak in Pacific white shrimp (Penaeus vannamei) cultured in Korea. Aquaculture 2020, 524, 735284. [Google Scholar]
- Han, J.E.; Tang, K.F.J.; Aranguren, L.F.; Piamsomboon, P. Characterization and pathogenicity of acute hepatopancreatic necrosis disease natural mutants, pirABvp(-) Vibrio parahaemolyticus, and pirABvp (+) Vibrio campbellii strains. Aquaculture 2017, 470, 84–90. [Google Scholar]
- Vicente, A.; Taengphu, S.; Hung, A.L.; Mora, C.M.; Dong, H.T.; Senapin, S. Detection of Vibrio campbellii and V. parahaemolyticus carrying full-length pirABVp but only V. campbellii produces PirVp toxins. Aquaculture 2020, 519, 734708. [Google Scholar]
- Lightner, D.V.; Redman, R.M.; Pantoja, C.R.; Noble, B.L.; Tran, L.H. Early mortality syndrome affects shrimp in Asia. Glob. Aquac. Advocate 2012, 201, 40. [Google Scholar]
- Caro, L.F.A.; Mai, H.N.; Noble, B.; Dhar, A.K. Acute hepatopancreatic necrosis disease (VPAHPND), a chronic disease in shrimp (Penaeus vannamei) population raised in Latin America. J. Invertebr. Pathol. 2020, 174, 107424. [Google Scholar]
- Han, J.E.; Tang, K.F.J.; Lightner, D.V. Genotyping of virulence plasmid from Vibrio parahaemolyticus isolates causing acute hepatopancreatic necrosis disease in shrimp. Dis. Aquat. Org. 2015, 115, 245–251. [Google Scholar]
- Cardona, E.; Gueguen, Y.; Magré, K.; Lorgeoux, B.; Piquemal, D.; Pierrat, F.; Noguier, F.; Saulnier, D. Bacterial community characterization of water and intestine of the shrimp Litopenaeus stylirostris in a biofloc system. BMC Microbiol. 2016, 16, 157. [Google Scholar]
- Md Zoqratt, M.; Eng, W.W.H.; Thai, B.T.; Austin, C.M.; Gan, H.M. Microbiome analysis of Pacific white shrimp gut and rearing water from Malaysia and Vietnam: Implications for aquaculture research and management. PeerJ 2018, 6, e5826. [Google Scholar]
- Rungrassamee, W.; Klanchui, A.; Chaiyapechara, S.; Maibunkaew, S.; Tangphatsornruang, S.; Jiravanichpaisal, P.; Karoonuthaisiri, N. Bacterial population in intestines of the black tiger shrimp (Penaeus monodon) under different growth stages. PLoS ONE 2013, 8, 60802. [Google Scholar]
- Zhang, M.; Sun, Y.; Chen, K.; Yu, N.; Zhou, Z.; Chen, L.; Du, Z.; Li, E. Characterization of the intestinal microbiota in Pacific white shrimp, Litopenaeus vannamei, fed diets with different lipid sources. Aquaculture 2014, 434, 449–455. [Google Scholar]
- Tzeng, T.D.; Pao, Y.Y.; Chen, P.; Weng, F.; Jean, W.D.; Wang, D. Effects of host phylogeny and habitats on gut microbiomes of oriental river prawn (Macrobrachium nipponense). PLoS ONE 2015, 10, e0132860. [Google Scholar]
- Zhang, H.; Sun, Z.; Liu, B.; Xuan, Y.; Jiang, M.; Pan, Y.; Zhang, Y. Dynamic changes of microbial communities in Litopenaeus vannamei cultures and the effects of environmental factors. Aquaculture 2016, 455, 97–108. [Google Scholar]
- Holt, C.C.; Bass, D.; Stentiford, G.D.; van der Giezen, M. Understanding the role of the shrimp gut microbiome in health and disease. J. Invertebr. Pathol. 2021, 186, 107387. [Google Scholar] [PubMed]
- Chen, W.Y.; Ng, T.H.; Wu, J.H.; Chen, J.W.; Wang, H.C. Microbiome dynamics in a shrimp grow-out pond with possible outbreak of acute hepatopancreatic necrosis disease. Sci. Rep. 2017, 7, 9395. [Google Scholar]
- Yao, Z.; Yang, K.; Huang, L.; Huang, X.; Qiuqian, L.; Wang, K.; Zhang, D. Disease outbreak accompanies the dispersive structure of shrimp gut bacterial community with a simple core microbiota. AMB Express 2018, 8, 120. [Google Scholar]
- Yang, Q.; Dong, X.; Xie, G.; Fu, S.; Zou, P.; Sun, J.; Wang, Y.; Huang, J. Comparative genomic analysis unravels the transmission pattern and intra-species divergence of acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus strains. Mol. Genet. Genom. 2019, 294, 1007–1022. [Google Scholar]
- Kohl, K.D.; Dearing, M.D. The woodrat gut microbiota as an experimental system for understanding microbial metabolism of dietary toxins. Front. Microbiol. 2016, 7, 1165. [Google Scholar]
- Xiong, J.; Zhu, J.; Dai, W.; Dong, C.; Qiu, Q.; Li, C. Integrating gut microbiota immaturity and disease discriminatory taxa to diagnose the initiation and severity of shrimp disease. Environ. Microbiol. 2017, 19, 1490–1501. [Google Scholar]
- Yu, Y.; Yang, H.; Li, J.; Zhang, P.; Wu, B.; Zhu, B.; Zhang, Y.; Fang, W. Putative type VI secretion systems of Vibrio parahaemolyticus contribute to adhesion to cultured cell monolayers. Arch. Microbiol. 2012, 194, 827–835. [Google Scholar] [PubMed]
- Lien, Y.W.; Lai, E.M. Type VI secretion effectors: Methodologies and biology. Front. Cell. Infect. Microbiol. 2017, 7, 254. [Google Scholar] [PubMed] [Green Version]
- Pinoargote, G.; Flores, G.; Cooper, K.; Ravishankar, S. Effects on survival and bacterial community composition of the aquaculture water and gastrointestinal tract of shrimp (Litopenaeus vannamei) exposed to probiotic treatments after an induced infection of acute hepatopancreatic necrosis disease. Aquaculture 2018, 49, 3270–3288. [Google Scholar]
- Diéguez, A.L.; Balboa, S.; Magnesen, T.; Romalde, J.L. Neptuniibacter pectenicola sp. nov. and Neptuniibacter marinus sp. nov., two novel species isolated from a Great scallop (Pecten maximus) hatchery in Norway and amended description of the genus Neptuniibacter. Syst. Appl. Microbiol. 2017, 40, 80–85. [Google Scholar]
- Salomon, D.; Gonzalez, H.; Updegraff, B.L.; Orth, K. Vibrio parahaemolyticus Type VI secretion system 1 is activated in marine conditions to target bacteria, and is differentially regulated from system 2. PLoS ONE 2013, 8, e61086. [Google Scholar]
- Victorio-De Los Santos, M.; Vibanco-Pérez, N.; Soto-Rodriguez, S.; Pereyra, A.; Zenteno, E.; Cano-Sánchez, P. The B Subunit of PirAB(vp) Toxin Secreted from Vibrio parahaemolyticus Causing AHPND Is an Amino Sugar Specific Lectin. Pathogens 2020, 9, 182. [Google Scholar]
- Fu, S.; Wang, L.; Tian, H.; Wei, D.; Liu, Y. Pathogenicity and genomic characterization of Vibrio parahaemolyticus strain PB1937 causing shrimp acute hepatopancreatic necrosis disease in China. Ann. Microbiol. 2018, 68, 175–184. [Google Scholar]
- Li, P.; Kinch, L.N.; Ray, A.; Dalia, A.B.; Cong, Q.; Nunan, L.M.; Camilli, A.; Grishin, N.V.; Salomon, D.; Orth, K. Acute hepatopancreatic necrosis disease-causing Vibrio parahaemolyticus strains maintain an antibacterial type VI secretion system with versatile effector repertoires. Appl. Environ. Microbiol. 2017, 83, e00737-17. [Google Scholar]
- Johnson, D.I. Bacterial virulence factors. In Bacterial Pathogens and Their Virulence Factors; Johnson, D.I., Ed.; Springer International Publishing: New York, NY, USA, 2018; pp. 1–38. [Google Scholar]
- Russell, A.B.; Singh, P.; Brittnacher, M.; Bui, N.K.; Hood, R.D.; Carl, M.A.; Agnello, D.M.; Schwarz, S.; Goodlett, D.R.; Vollmer, W.; et al. A widespread bacterial type VI secretion effector superfamily identified using a heuristic approach. Cell Host Microbe 2012, 11, 538–549. [Google Scholar]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [PubMed]
- Federle, M.J.; Bassler, B.L. Interspecies communication in bacteria. J. Clin. Investig. 2003, 112, 1291–1299. [Google Scholar] [PubMed] [Green Version]
- Wellington, S.; Greenberg, E.P. Quorum sensing signal selectivity and the potential for interspecies cross talk. mBio 2019, 10, e00146-19. [Google Scholar] [PubMed] [Green Version]
- Makino, K.; Oshima, K.; Kurokawa, K.; Yokoyama, K.; Uda, T.; Tagomori, K.; Iijima, Y.; Najima, M.; Nakano, M.; Yamashita, A.; et al. Genome sequence of Vibrio parahaemolyticus: A pathogenic mechanism distinct from that of V. cholerae. Lancet 2003, 361, 743–749. [Google Scholar] [PubMed]
- Jaques, S.; McCarter, L.L. Three new regulators of swarming in Vibrio parahaemolyticus. J. Bacteriol. 2006, 188, 2625–2635. [Google Scholar]
- Eickhoff, M.J.; Fei, C.; Huang, X.; Bassler, B.L. LuxT controls specific quorum-sensing-regulated behaviors in Vibrionaceae spp. via repression of qrr1, encoding a small regulatory RNA. PLoS Genet. 2021, 17, e1009336. [Google Scholar]
- Zhang, Y.; Qiu, Y.; Tan, Y.; Guo, Z.; Yang, R.; Zhou, D. Transcriptional regulation of opaR, qrr2-4 and aphA by the master quorum-sensing regulator OpaR in Vibrio parahaemolyticus. PLoS ONE 2012, 7, e34622. [Google Scholar]
- Pumkaew, M.; Taengchaiyaphum, S.; Powtongsook, S.; Pungrasmi, W.; Sritunyalucksana, K. Production of acute hepatopancreatic necrosis disease toxin is affected by addition of cell-free supernatant prepared from Al-2-producing Vibrio harveyi mutant. J. World Aquac. Soc. 2019, 50, 878–886. [Google Scholar]
- Federle, M.J. Autoinducer-2-based chemical communication in bacteria: Complexities of interspecies signaling. Contrib. Microbiol. 2009, 16, 18–32. [Google Scholar]
- Takemura, A.F.; Chien, D.M.; Polz, M.F. Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front. Microbiol. 2014, 5, 38. [Google Scholar]
- Williams, S.L.; Jensen, R.V.; Kuhn, D.D.; Stevens, A.M. Analyzing the metabolic capabilities of a Vibrio parahaemolyticus strain that causes Early Mortality Syndrome in shrimp. Aquaculture 2017, 476, 44–48. [Google Scholar]
- Fu, S.; Wei, D.; Yang, Q.; Xie, G.; Pang, B.; Wang, Y.; Lan, R.; Wang, Q.; Dong, X.; Zhang, X.; et al. Horizontal plasmid transfer promotes the dissemination of Asian acute hepatopancreatic necrosis disease and provides a novel mechanism for genetic exchange and environmental adaptation. mSystems 2020, 5, e00799. [Google Scholar] [PubMed] [Green Version]
- Pragthong, P.; Chirapongsatonkul, N. Temperature-dependent expression of virulence genes in Vibrio parahaemolyticus AHPND strain (VpAHPND). Int. J. Agric. Technol. 2020, 16, 1185–1198. [Google Scholar]
- Schofield, P.J.; Noble, B.L.; Caro, L.F.A.; Mai, H.N.; Padilla, T.J.; Millabas, J.; Dhar, A.K. Pathogenicity of Acute Hepatopancreatic Necrosis Disease (AHPND) on the freshwater prawn, Macrobrachium rosenbergii, and Pacific White Shrimp, Penaeus vannamei, at various salinities. Aquac. Res. 2021, 52, 1480–1489. [Google Scholar]
- López-Cervantes, G.; Álvarez-Ruiz, P.; Luna-Suárez, S.; Luna-González, A.; Esparza-Leal, H.M.; Castro-Martínez, C.; Gámez-Jiménez, C.; Soto-Alcalá, J. Temperature and salinity modulate virulence and PirA gene expression of Vibrio parahaemolyticus, the causative agent of AHPND. Aquac. Int. 2021, 29, 743–756. [Google Scholar]
- Soto-Rodriguez, S.; Lozano Olvera, R.; Palacios-Gonzalez, D.; Bolan-Mejía, M.; Aguilar-Rendon, K. Characterization and growth conditions of Vibrio parahaemolyticus strains with different virulence degrees that cause acute hepatopancreatic necrosis disease in Litopenaeus vannamei. J. World Aquac. Soc. 2019, 50, 1002–1015. [Google Scholar]
- Sultana, T.; Haque, M.; Salam, M.; Alam, M. Effect of aeration on growth and production of fish in intensive aquaculture system in earthen ponds. J. Bangladesh Agric. Univ. 2017, 15, 113–122. [Google Scholar]
- Kumar, V.; Roy, S.; Baruah, K.; Van Haver, D.; Impens, F.; Bossier, P. Environmental conditions steer phenotypic switching in acute hepatopancreatic necrosis disease-causing Vibrio parahaemolyticus, affecting PirAVP/PirBVP toxins production. Environ. Microbiol. 2020, 22, 4212–4230. [Google Scholar]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microb. 2019, 17, 247–260. [Google Scholar]
- Mizan, M.F.; Jahid, I.K.; Kim, M.; Lee, K.H.; Kim, T.J.; Ha, S.D. Variability in biofilm formation correlates with hydrophobicity and quorum sensing among Vibrio parahaemolyticus isolates from food contact surfaces and the distribution of the genes involved in biofilm formation. Biofouling 2016, 32, 497–509. [Google Scholar]
- Zhang, Y.; Hu, L.; Osei-Adjei, G.; Zhang, Y.; Yang, W.; Yin, Z.; Lu, R.; Sheng, X.; Yang, R.; Huang, X.; et al. Autoregulation of ToxR and Its Regulatory Actions on Major Virulence Gene Loci in Vibrio parahaemolyticus. Front. Cell. Infect. Microbiol. 2018, 8, 291. [Google Scholar] [PubMed]
- Yildiz, F.H.; Visick, K.L. Vibrio biofilms: So much the same yet so different. Trends Microbiol. 2009, 17, 109–118. [Google Scholar] [PubMed] [Green Version]
- Henke, J.M.; Bassler, B.L. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 2004, 186, 3794–3805. [Google Scholar] [PubMed] [Green Version]
- Zhu, J.; Miller, M.B.; Vance, R.E.; Dziejman, M.; Bassler, B.L.; Mekalanos, J.J. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2002, 99, 3129–3134. [Google Scholar]
- Sharon, N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 527–537. [Google Scholar]
- Hao, J.; Zhang, Y.; Fu, S.; Lu, Y.; Hua, X.; Liu, Y. Pathogenicity and protein analysis of photorhabdus insect-related (Pir) toxin PirAB revealed PirABvp is a host-specific toxin. Aquaculture 2019, 500, 290–299. [Google Scholar]
- Lin, S.J.; Hsu, K.C.; Wang, H.C. Structural insights into the cytotoxic mechanism of Vibrio parahaemolyticus PirAvp and PirBvp toxins. Mar. Drugs 2017, 15, 373. [Google Scholar]
- De Los Santos, M.V.; Sánchez-Salgado, J.L.; Pereyra, A.; Zenteno, E.; Vibanco-Pérez, N.; Ramos-Clamont Montfort, G.; Soto-Rodriguez, S.A. The Vibrio parahaemolyticus subunit toxin PirB(vp) recognizes glycoproteins on the epithelium of the Penaeus vannamei hepatopancreas. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 257, 110673. [Google Scholar]
- Lin, S.J.; Chen, Y.F.; Hsu, K.C.; Chen, Y.L.; Ko, T.P.; Lo, C.F.; Wang, H.C.; Wang, H.C. Structural insights to the heterotetrameric interaction between the Vibrio parahaemolyticus PirAvp and PirBvp toxins and activation of the cry-like pore-forming domain. Toxins 2019, 11, 233. [Google Scholar]
- Sengupta, A.; Sarkar, A.; Priya, P.; Ghosh Dastidar, S.; Das, S. New insight to structure-function relationship of GalNAc mediated primary interaction between insecticidal Cry1Ac toxin and HaALP receptor of Helicoverpa armigera. PLoS ONE 2013, 8, e78249. [Google Scholar]
- Kitami, M.; Kadotani, T.; Nakanishi, K.; Atsumi, S.; Higurashi, S.; Ishizaka, T.; Watanabe, A.; Sato, R. Bacillus thuringiensis cry toxins bund specifically to various proteins via domain III, which had a galactose-binding domain-like fold. Biosci. Biotechnol. Biochem. 2011, 75, 305–312. [Google Scholar] [PubMed]
- Fantus, I.G.; Goldberg, H.J.; Whiteside, C.I. The Hexosamine Biosynthesis Pathway, 1st ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 117–133. [Google Scholar]
- Xie, X.-L.; Huang, Q.-S.; Wang, Y.; Ke, C.-H.; Chen, Q.-X. Modification and Modificatory Kinetics of the Active Center of Prawn β-N-Acetyl-D-glucosaminidase. J. Biomol. Struct. Dyn. 2009, 26, 781–786. [Google Scholar] [PubMed]
- Song, Y.; Evenseth, L.M.; Iguchi, T.; Tollefsen, K.E. Release of chitobiase as an indicator of potential molting disruption in juvenile Daphnia magna exposed to the ecdysone receptor agonist 20-hydroxyecdysone. J. Toxicol. Environ. Health Part A 2017, 80, 954–962. [Google Scholar]
- Ettrich, R.; Kopecký, V., Jr.; Hofbauerová, K.; Baumruk, V.; Novák, P.; Pompach, P.; Man, P.; Plíhal, O.; Kutý, M.; Kulik, N.; et al. Structure of the dimeric N-glycosylated form of fungal beta-N-acetyl hexosaminidase revealed by computer modeling, vibrational spectroscopy, and biochemical studies. BMC Struct. Biol. 2007, 7, 32. [Google Scholar]
- Weitz, G.; Proia, R.L. Analysis of the glycosylation and phosphorylation of the alpha-subunit of the lysosomal enzyme, beta- hexosaminidase A, by site-directed mutagenesis. J. Biol. Chem. 1992, 267, 10039–10044. [Google Scholar]
- Zhu, F.; Li, D.; Chen, K. Structures and functions of invertebrate glycosylation. Open Biol. 2019, 9, 180232. [Google Scholar]
- Erlandson, M.A.; Toprak, U.; Hegedus, D.D. Role of the peritrophic matrix in insect-pathogen interactions. J. Insect Physiol. 2019, 117, 103894. [Google Scholar]
- Zhang, Z.; Wang, F.; Chen, C.; Zheng, Z.; Aweya, J.J.; Zhang, Y. Glycosylation of hemocyanin in Litopenaeus vannamei is an antibacterial response feature. Immunol. Lett. 2017, 192, 42–47. [Google Scholar]
- Zhang, Z.; Li, R.; Aweya, J.J.; Wang, F.; Zhong, M.; Zhang, Y. Identification and characterization of glycosylation sites on Litopenaeus vannamei hemocyanin. FEBS Lett. 2019, 593, 820–830. [Google Scholar]
- Du, X.-J.; Wang, J.-X.; Liu, N.; Zhao, X.-F.; Li, F.; Xiang, J. Identification and molecular characterization of a peritrophin-like protein from fleshy prawn (Fenneropenaeus chinensis). Mol. Immunol. 2006, 43, 1633–1644. [Google Scholar]
- Wang, L.; Li, F.; Xiang, J. A new shrimp peritrophin-like gene from Exopalaemon carinicauda involved in white spot syndrome virus (WSSV) infection. Fish Shellfish Immunol. 2013, 35, 840–846. [Google Scholar] [PubMed]
- Soonthornchai, W.; Rungrassamee, W.; Karoonuthaisiri, N.; Jarayabhand, P.; Klinbunga, S.; Söderhäll, K.; Jiravanichpaisal, P. Expression of immune-related genes in the digestive organ of shrimp, Penaeus monodon, after an oral infection by Vibrio harveyi. Dev. Comp. Immunol. 2010, 34, 19–28. [Google Scholar] [PubMed]
- Duan, Y.; Wang, Y.; Liu, Q.; Dong, H.; Li, H.; Xiong, D.; Zhang, J. Changes in the intestine microbial, digestion and immunity of Litopenaeus vannamei in response to dietary resistant starch. Sci. Rep. 2019, 9, 6464. [Google Scholar] [PubMed]
- Duan, Y.; Yun, W.; Ding, X.; Xiong, D.; Zhang, J. Response of intestine microbiota, digestion, and immunity in Pacific white shrimp Litopenaeus vannamei to dietary succinate. Aquaculture 2019, 517, 734762. [Google Scholar]
- Wang, Z.; Zhou, J.; Li, J.; Zou, J.; Fan, L. The immune defense response of Pacific white shrimp (Litopenaeus vannamei) to temperature fluctuation. Fish Shellfish Immunol. 2020, 103, 103–110. [Google Scholar]
- Kumar, V.; Nguyen, D.V.; Baruah, K.; Bossier, P. Probing the mechanism of VPAHPND extracellular proteins toxicity purified from Vibrio parahaemolyticus AHPND strain in germ-free Artemia test system. Aquaculture 2019, 504, 414–419. [Google Scholar]
- Zheng, Z.; Wang, F.; Aweya, J.J.; Li, R.; Yao, D.; Zhong, M.; Li, S.; Zhang, Y. Comparative transcriptomic analysis of shrimp hemocytes in response to acute hepatopancreas necrosis disease (AHPND) causing Vibrio parahaemolyticus infection. Fish Shellfish Immunol. 2018, 74, 10–18. [Google Scholar]
- Soberón, M.; Pardo, L.; Muñóz-Garay, C.; Sánchez, J.; Gómez, I.; Porta, H.; Bravo, A. Pore formation by Cry toxins. Adv. Exp. Med. Biol. 2010, 677, 127–142. [Google Scholar]
- Luangtrakul, W.; Boonchuen, P.; Jaree, P.; Kumar, R.; Wang, H.-C.; Somboonwiwat, K. Cytotoxicity of Vibrio parahaemolyticus AHPND toxin on shrimp hemocytes, a newly identified target tissue, involves binding of toxin to aminopeptidase N1 receptor. PLoS Pathog. 2021, 17, e1009463. [Google Scholar]
- Estrada, N.; Velázquez, E.; Rodríguez-Jaramillo, C.; Ascencio, F. Carbohydrate moieties and cytoenzymatic characterization of hemocytes in white leg shrimp Litopenaeus vannamei. Int. J. Cell Biol. 2016, 2016, 9032181. [Google Scholar]
- Kuyucak, S.; Norton, R.S. Computational approaches for designing potent and selective analogs of peptide toxins as novel therapeutics. Future Med. Chem. 2014, 6, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, J.H.; Wong, W.L.; Wong, F.C.; Chai, T.T. Targeting PirAvp and PirBvp toxins of Vibrio parahaemolyticus with oilseed peptides: An in silico approach. Antibiotics 2021, 10, 1211. [Google Scholar] [PubMed]
- Shao, J.; Zhao, W.; Han, S.; Chen, Y.; Wang, B.; Wang, L. Partial replacement of fishmeal by fermented soybean meal in diets for juvenile white shrimp (Litopenaeus vannamei). Aquac. Nutr. 2019, 25, 145–153. [Google Scholar]
| Strain | Origin | Shrimp Size (g) | Density (CFU/mL) | Histo. | First Dead-100% Mortality (h) | Reference |
|---|---|---|---|---|---|---|
| Vp 13-028A/3 | Vietnam | 0.5–2.0 | 2 × 106 | Yes | <24–48 | [1] |
| Vp 3HP | Thailand | ~2.0 | 1 × 106 | Yes | ND-24 | [3] |
| Vp S02 | China | ~2.0 | 1 × 106 | Yes | ND-24 | [3] |
| Vp 13-306D/4 | Mexico | ~2.0 | ND | Yes | >24–72 | [6] |
| Vp 13-511A/1 | Mexico | ~3.0 | 2 × 106 | Yes | ND–24 | [6] |
| Vp M0607 | Mexico | 0.5–1.0 | 7.8 × 106 | Yes | 15–48 * | [11] |
| Vp M0802 | Mexico | 0.5–1.0 | 3.3 × 106 | Yes | 7–25 | [11] |
| Vp M0904 | Mexico | 0.5–1.0 | 2.2 × 106 | Yes | 4–17 | [11] |
| Vp 2S01 | China | ~ 1.0 | 1 × 106 | Yes | 3–18 | [16] |
| Vp-BA94C2 | Latin America | 2.5 ± 0.5 | 2 × 106 | Yes | 6–70 | [17] |
| Vp D6 | Thailand | 3 5 | 1 × 106 | ND | 144–216 | [33] |
| Vp D6 | Thailand | 0.82 | 5 × 105 | ND | 24–96 | [33] |
| Vp GD10 | China | ~2.0 | ~×106 | Yes | <24–72 | [35] |
| Vp 5HP | Thailand | 1.8 ± 0.2 | ~×106 | Yes | >24–96 * | [36] |
| Vp XN89 | Vietnam | 1.8 ± 0.2 | ~×106 | Yes | >24–96 * | [36] |
| Vp 15-250/20 | Latin America | 1–1.5 | 2 × 106 | Yes | <12–168 * | [37] |
| Vp 19-021-D1 | Korea | 1–1.5 | 2 × 106 | Yes | <12–168 * | [37] |
| Vp 19-022-A1 | Korea | 1–1.5 | 2 × 106 | Yes | <12–168 * | [37] |
| Vp C3 | Thailand | 2.0 | 2 × 105 | Yes | ND-72 | [38] |
| Vpu-BA55 | Latin America | 2.5 ± 0.5 | 2 × 106 | Yes | 8–70 * | [17] |
| Vc 20130629003S01 | China | ~1.0 | 2 ×106 | Yes | 12–36 | [16] |
| Vc 16-904/1 | Latin America | 2.0 | 2 × 105 | Yes | ND-72 | [38] |
| Vc 20130629003S01 | China | ~1.0 | 1 × 106 | Yes | 3–24 | [15] |
| Vc 34 | Peru | 1.2 | ~×106 | Yes | <24–120 | [39] |
| Vc 36 | Peru | 1.2 | ~×106 | Not | <24–120 | [39] |
| Vc 43 | Peru | 1.2 | ~×106 | Not | <24–120 | [39] |
| Vo SH-14 | China | 0.5–2.0 | ~×106 | Yes | 12–96 | [13] |
| Vo SH-14 | China | 0.5–2.0 | ~×106 | ND | <20–40 * | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Rodriguez, S.A.; Lozano-Olvera, R.; Ramos-Clamont Montfort, G.; Zenteno, E.; Sánchez-Salgado, J.L.; Vibanco-Pérez, N.; Aguilar Rendón, K.G. New Insights into the Mechanism of Action of PirAB from Vibrio Parahaemolyticus. Toxins 2022, 14, 243. https://doi.org/10.3390/toxins14040243
Soto-Rodriguez SA, Lozano-Olvera R, Ramos-Clamont Montfort G, Zenteno E, Sánchez-Salgado JL, Vibanco-Pérez N, Aguilar Rendón KG. New Insights into the Mechanism of Action of PirAB from Vibrio Parahaemolyticus. Toxins. 2022; 14(4):243. https://doi.org/10.3390/toxins14040243
Chicago/Turabian StyleSoto-Rodriguez, Sonia A., Rodolfo Lozano-Olvera, Gabriela Ramos-Clamont Montfort, Edgar Zenteno, José Luis Sánchez-Salgado, Norberto Vibanco-Pérez, and Karla G. Aguilar Rendón. 2022. "New Insights into the Mechanism of Action of PirAB from Vibrio Parahaemolyticus" Toxins 14, no. 4: 243. https://doi.org/10.3390/toxins14040243
APA StyleSoto-Rodriguez, S. A., Lozano-Olvera, R., Ramos-Clamont Montfort, G., Zenteno, E., Sánchez-Salgado, J. L., Vibanco-Pérez, N., & Aguilar Rendón, K. G. (2022). New Insights into the Mechanism of Action of PirAB from Vibrio Parahaemolyticus. Toxins, 14(4), 243. https://doi.org/10.3390/toxins14040243





