Mycotoxins in Maize Silage from China in 2019
Abstract
:1. Introduction
2. Results
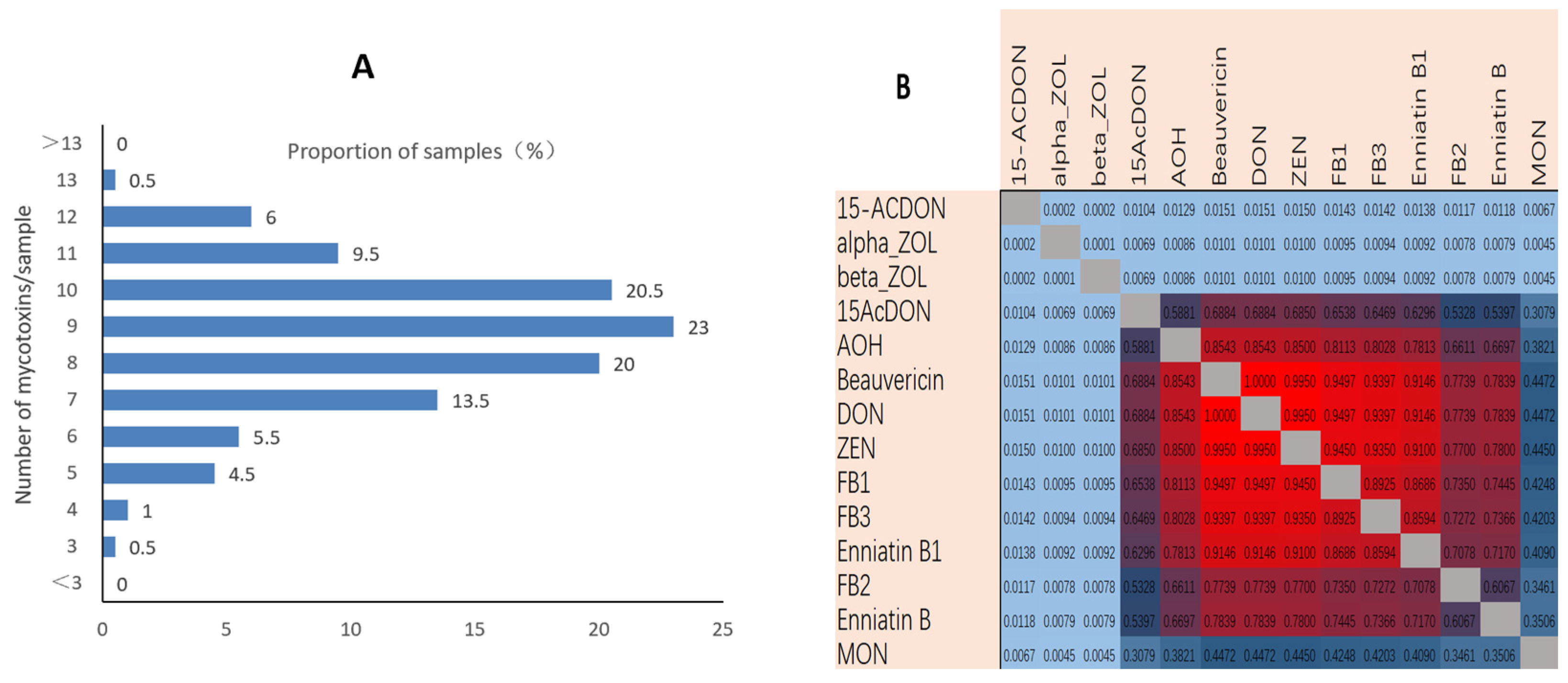
2.1. Mycotoxins in Maize Silage
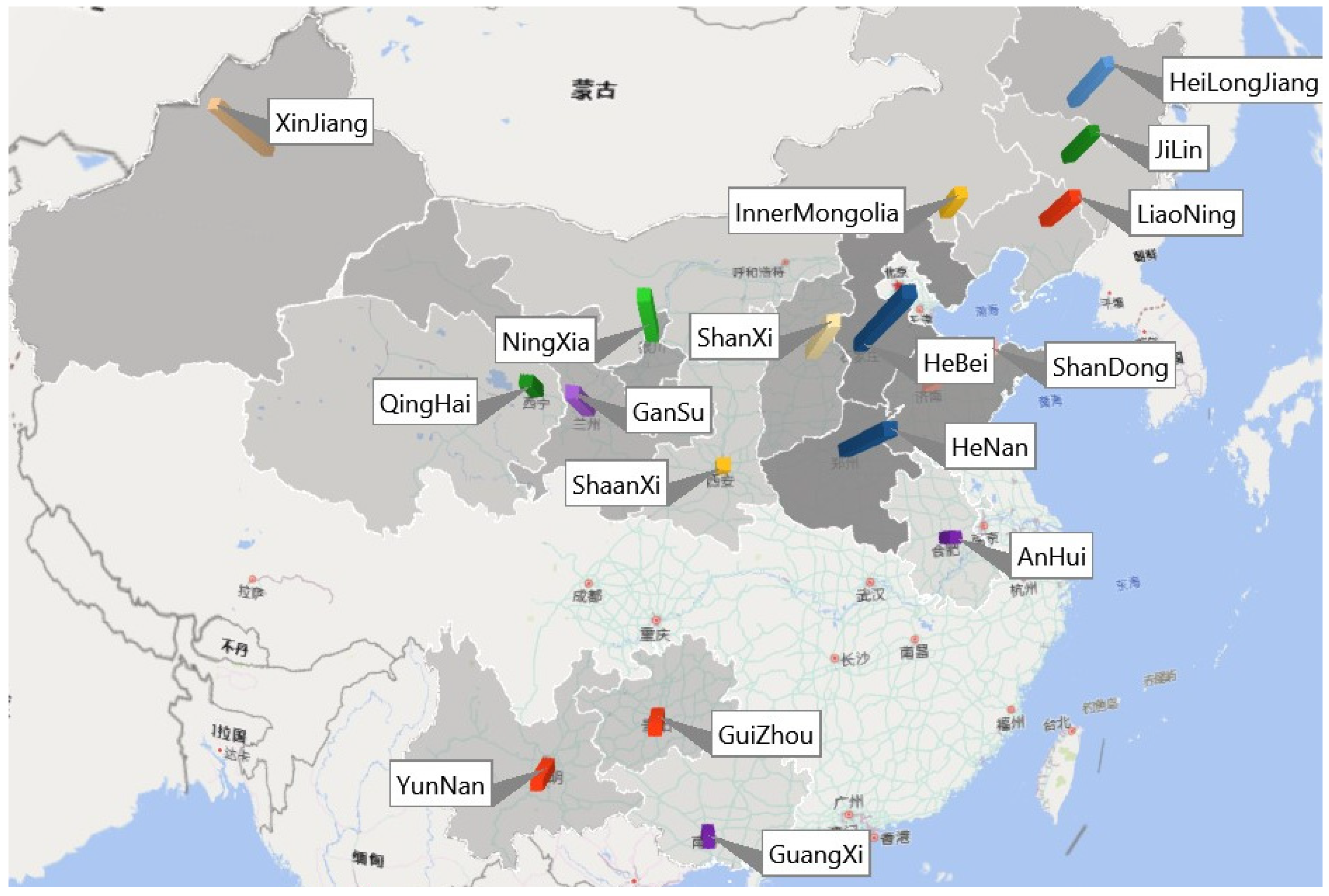
2.2. Sources of the Samples
2.3. Regulated Mycotoxins in Maize Silage
2.4. Trichothecenes in Maize Silage
2.5. Masked Mycotoxins and Metabolites in Maize Silage
2.6. Emerging Mycotoxins and Other Mycotoxins
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sample Collection
5.2. Silage Sampling
5.2.1. Sample Extraction and Preparation
5.2.2. Method Validation
5.3. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khodaei, D.; Javanmardi, F.; Khaneghah, A.M. The global overview of the occurrence of mycotoxins in cereals: A three-year survey. Curr. Opin. Food Sci. 2021, 39, 36–42. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D. Mycotoxin contamination in silages. West. Dairy News 2006, 6, 10. [Google Scholar]
- Kemboi, D.C.; Ochieng, P.E.; Antonissen, G.; Croubels, S.; Gathumbi, J.K. Multi-Mycotoxin Occurrence in Diary Cattle and Poultry Feeds and Feed Ingredients from Machakos Town, Kenya. Toxins 2020, 12, 762. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Multi-mycotoxins screening of feed and fee raw materials from Africa. World Mycotoxin J. 2018, 11, 369–383. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, J.C. Opinion of the scientific panel on contaminants in the food chain on a request from the Commission related to aflatoxin B1 as undesirable substance in animal feed. EFSA J. 2004, 2, 39. [Google Scholar]
- Battilani, P.; Palumbo, R.; Giorni, P.; Dall’Asta, C.; Dellafiora, L.; Gkrillas, A.; Oswald, I.P. Mycotoxin mixtures in food and feed: Holistic, innovative, flexible risk assessment modeling approach: MYCHIF. EFSA Support. Publ. 2020, 17, 1757. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Hassan, Y.I.; Watts, C.; Zhou, T. Innovative technologies for the mitigation of mycotoxins in animal feed and ingredients-A review of recent patents. Anim. Feed Sci. Technol. 2016, 216, 19–29. [Google Scholar] [CrossRef]
- Rodríguez-Blanco, M.; Ramos, A.J.; Sanchis, V.; Marín, S. Mycotoxin’s occurrence and fungal populations in different types of silages for dairy cows in Spain. Fungal Biol. 2019, 8, 6. [Google Scholar]
- Panasiuk, L.; Jedziniak, P.; Pietruszka, K.; Piatkowska, M.; Bocian, L. Frequency and levels of regulated and emerging mycotoxins in silage in Poland. Mycotoxin Res. 2019, 35, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Yao, E.; Zheng, J.; Liu, X.; Zhang, Y. Occurrence of mycotoxins in silage and its effects on ruminant health. China Feed 2019, 7, 9–13. [Google Scholar]
- Shimshon, J.A.; Cuneah, O.; Sulyok, M.; Krska, R.; Galon, N.; Sharir, B.; Shlosberg, A. Mycotoxins in corn and wheat silage in Israel. Food Addit. Contam. 2013, 30, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.; Heutte, N.; Sage, L.; Pottier, D.; Bouchart, V.; Lebailly, P.; Garon, D. Toxigenic fungi and mycotoxins in mature corn silage. Food Chem. Toxicol. 2007, 45, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Mcelhinne, C.; Danaher, M.; Elliott, C.T.; O’Kiely, P. Mycotoxins in farm silages—A 2-year Irish national survey. Grass Forage Sci. 2016, 71, 339–352. [Google Scholar] [CrossRef]
- Li, S.; Lü, S.; Li, F.; Tang, S.; Xu, Q. Detection and Analysis on Mycotoxins in Whole Corn silage in North Shandong. China Herbiv. Sci. 2018, 38, 27–29. [Google Scholar]
- GB 2761-2017; National Food Safety Standard-Maximum Levels of Mycotoxins in Foods. Standardization Administration of the People’s Republic of China, Standards Press of China: Beijing, China, 2017.
- GB 13078-2017; Hygienical Standard for Feeds. Standardization Administration of the People’s Republic of China, Standards Press of China: Beijing, China, 2017.
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of Aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Yin, S.; Qu, Y. Quality Analysis of Whole Plant Corn Silage in Different Regions in 2018. J. Heilongjiang Bayi Agric. Univ. 2019, 31, 55–59. [Google Scholar]
- Kalac, P.; Woolford, M.K. A review of some aspects of possible associations between the feedings of silage and animal health. Br. Vet. J. 1982, 138, 305. [Google Scholar] [CrossRef]
- Richard, E.; Heutte, N.; Bouchart, V.; Garon, D. Evaluation of fungal contamination and mycotoxin production in maize silage. Anim. Feed Sci. Technol. 2009, 148, 309–320. [Google Scholar] [CrossRef]
- Reisinger, N.; Schürer-Waldheim, S.; Mayer, E.; Debevere, S.; Antonissen, G.; Sulyok, M.; Nagl, V. Mycotoxin Occurrence in Maize Silage-A Neglected Risk for Bovine Gut Health. Toxins 2019, 11, 577. [Google Scholar] [CrossRef] [Green Version]
- Escriva, L.; Font, G.; Manyes, L. In vivo toxicity studies of fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef]
- Yan, P.; Liu, Z.; Liu, S.; Yao, L.; Liu, Y.; Wu, Y.; Gong, Z. Natural Occurrence of Deoxynivalenol and Its Acetylated Derivatives in Chinese Maize and Wheat Collected in 2017. Toxins 2020, 12, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yang, L.; Wang, L.; Chang, F.; Yang, L. Survey of 11 mycotoxins in wheat flour in Hebei province, China. J. Food Addit. Contam. Part B 2015, 8, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Jestoi, M. Emerging fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Some Naturally Occurring Substances; Monographs, International Agency on for Research Cancer: Lyon, France, 1976; Volume 10, pp. 245–251. [Google Scholar]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef] [PubMed]
- Schoevers, E.J.; Santos, R.R.; Fink-Gremmels, J.; Roelen, B. Toxicity of beauvericin on porcine oocyte maturation and preimplantation embryo development. Reprod. Toxicol. 2016, 65, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Albonico, M.; Schutz, L.F.; Caloni, F.; Cortinovis, C.; Spicer, L.J. In vitro effects of the Fusarium mycotoxins fumonisin B1 and beauvericin on bovine granulosa cell proliferation and steroid production. Toxicon 2017, 128, 38–45. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]


| Mycotoxin | Positive Samples (n) | Positive Samples (%) | Median Concentration (μg/kg) | 75th Percentile (μg/kg) | 95th Percentile (μg/kg) | Maximum Concentration (μg/kg) |
|---|---|---|---|---|---|---|
| Regulated mycotoxins (except ergot alkaloids) | ||||||
| Aflatoxin B1 (AFB1) | 0 | 0 | - | - | - | - |
| Aflatoxin B2 (AFB2) | 0 | 0 | - | - | - | - |
| Aflatoxin G1 (AFG1) | 0 | 0 | - | - | - | - |
| Aflatoxin G2 (AFG2) | 0 | 0 | - | - | - | - |
| Deoxynivalenol (DON) | 199 | 99.5 | 315 | 611 | 1884 | 3587 |
| Fumonisin B1 (FB1) | 144 | 72.0 | 61.6 | 121 | 261 | 558 |
| Fumonisin B2 (FB2) | 120 | 60.0 | 31.4 | 54.6 | 108 | 198 |
| Fumonisin B3 (FB3) | 65 | 32.5 | 18.8 | 30.3 | 49.7 | 79.5 |
| Ochratoxin A (OTA) | 0 | 0 | - | - | - | - |
| Zearalenone (ZEN) | 159 | 79.5 | 38.7 | 82.7 | 201 | 832 |
| Patulin (PAT) | 1 | 0.5 | 257 | 257 | 257 | 257 |
| ergot alkaloids | ||||||
| Agroclavine | 0 | 0 | - | - | - | - |
| Ergine | 0 | 0 | - | - | - | - |
| Ergocornine | 0 | 0 | - | - | - | - |
| Ergocorninine | 0 | 0 | - | - | - | - |
| Ergocristine | 0 | 0 | - | - | - | - |
| Ergocristinine | 0 | 0 | - | - | - | - |
| Ergocryptine | 1 | 0.5 | 15.3 | 15.3 | 15.3 | 15.3 |
| Ergocryptinine | 0 | 0 | - | - | - | - |
| Ergometrine | 0 | 0 | - | - | - | - |
| Ergometrinine | 0 | 0 | - | - | - | - |
| Ergosine | 0 | 0 | - | - | - | - |
| Ergotamine | 0 | 0 | - | - | - | - |
| Dihydrolysergol | 0 | 0 | - | - | - | - |
| Elymoclavine | 0 | 0 | - | - | - | - |
| Type-A trichothecenes | ||||||
| Diacetoxyscirpenol (DAS) | 0 | 0 | - | - | - | - |
| HT-2 Toxin (HT-2) | 8 | 4.0 | 31.6 | 49.4 | 64.2 | 68.5 |
| Neosolaniol (NEO) | 0 | 0 | - | - | - | - |
| T-2 Toxin (T-2) | 3 | 1.5 | 6.3 | 6.3 | 6.3 | 6.3 |
| Type-B trichothecenes | ||||||
| 15-Acetoxyscirpenol (15-ACDAS) | 3 | 1.5 | 68.5 | 74.3 | 79.0 | 80.2 |
| 15-Acetyldeoxynivalenol (15-ACDON) | 137 | 68.5 | 61.3 | 89.9 | 247 | 411 |
| 3-Acetyldeoxynivalenol (3-ACDON) | 0 | 0 | - | - | - | - |
| Nivalenol (NIV) | 10 | 5.0 | 362 | 996 | 1253 | 1302 |
| Fusarenon X (FUX) | 0 | 0 | - | - | - | - |
| Modified mycotoxins | ||||||
| Deoxynivalenol-3-Glucoside (D3G) | 0 | 0 | - | - | - | - |
| α-Zearalenol (α-ZEL) | 2 | 1.0 | 9.8 | 11.1 | 12.2 | 12.5 |
| β-Zearalenol (β-ZEL) | 2 | 1.0 | 15.7 | 20.1 | 23.6 | 24.5 |
| Emerging mycotoxins | ||||||
| Alternariol (AOH) | 170 | 85.0 | 17.0 | 29.8 | 87.4 | 225 |
| Beauvericin (BEA) | 199 | 99.5 | 22.3 | 37.3 | 130 | 315 |
| Enniatin A (ENNA) | 161 | 80.5 | 20.6 | 30.1 | 49.1 | 307 |
| Enniatin A1 (ENNA1) | 11 | 5.5 | 1.56 | 2.22 | 4.34 | 5.08 |
| Enniatin B (ENNB) | 70 | 35.0 | 2.09 | 8.54 | 37.1 | 84.1 |
| Enniatin B1 (ENNB1) | 145 | 72.5 | 1.98 | 3.15 | 14.7 | 56.0 |
| Moniliformin (MON) | 89 | 44.5 | 5.21 | 11.7 | 55.0 | 116 |
| Mycophenolic acid (MPA) | 3 | 1.5 | 48.3 | 95.6 | 133 | 143 |
| Sterigmatocystin (STG) | 1 | 0.5 | 146 | 146 | 146 | 146 |
| Penicillin Acid (PEA) | 0 | 0 | - | - | - | - |
| Roquefortine C (ROC) | 3 | 1.5 | 10.3 | 14.2 | 17.3 | 18.0 |
| T-2 Triol | 0 | 0 | - | - | - | - |
| T-2-Tetraol | 0 | 0 | - | - | - | - |
| Gliotoxin | 0 | 0 | - | - | - | - |
| Ochratoxin B (OTB) | 0 | 0 | - | - | - | - |
| Zearalanol (ZAN) | 4 | 2.0 | 7.43 | 8.28 | 9.32 | 9.58 |
| Regions | BEA(μg/kg) | ||
|---|---|---|---|
| Average | Median | Maximum | |
| Central China (CT area) | 25.4 | 14.0 | 152.0 |
| Northeast (NE area) | 24.2 | 16.2 | 128.0 |
| Southwest (SW area) | 18.5 | 11.6 | 91.1 |
| Northwest (NW area) | 28.2 | 19.7 | 225.0 |
| Regions | ENNA | ENNA1 | ENNB | ENNB1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | Median | Maximum | Average | Median | Maximum | Average | Median | Maximum | Average | Median | Maximum | |
| CT area | 18.9 | 15.0 | 55.4 | 1.23 | 1.23 | 1.23 | 2.01 | 1.55 | 3.44 | 1.68 | 1.36 | 6.00 |
| NE area | 24.2 | 23.1 | 55.0 | 2.63 | 1.50 | 5.08 | 2.74 | 1.52 | 17.3 | 3.34 | 2.19 | 27.8 |
| SW area | 27.0 | 18.4 | 91.1 | ND | ND | ND | 15.5 | 2.86 | 84.1 | 4.23 | 2.04 | 31.5 |
| NW area | 29.5 | 22.3 | 307 | 2.03 | 1.56 | 3.60 | 9.54 | 3.03 | 64.8 | 5.99 | 2.50 | 56.0 |
| Regions | MON | ||
|---|---|---|---|
| Average | Median | Maximum | |
| CT area | 12.8 | 5.6 | 98.7 |
| NE area | 10.5 | 5.2 | 35.9 |
| SW area | 21.0 | 10.9 | 99.1 |
| NW area | 10.2 | 3.8 | 116.0 |
| Regions | STG | MPA | ||||
|---|---|---|---|---|---|---|
| Average | Median | Maximum | Average | Median | Maximum | |
| CT area | ND * | ND * | ND * | 95.6 | 95.6 | 143 |
| NE area | ND * | ND * | ND * | 41.5 | 41.5 | 41.5 |
| SW area | 146.0 | 146.0 | 146.0 | ND * | ND * | ND * |
| NW area | ND * | ND * | ND * | ND * | ND * | ND * |
| Regions | AOH | ZAN | ||||
|---|---|---|---|---|---|---|
| Average | Median | Maximum | Average | Median | Maximum | |
| CT area | 25.4 | 14.0 | 152.0 | ND * | ND * | ND * |
| NE area | 24.2 | 16.2 | 128.0 | ND * | ND * | ND * |
| SW area | 18.5 | 11.6 | 91.1 | ND * | ND * | ND * |
| NW area | 28.2 | 19.7 | 225.0 | 7.3 | 7.4 | 9.6 |
| Column | Phenomenex Gemini C18 (3.0 × 100 mm; 3 µm) with suitable pre-column (optional) |
| Gradient | 0 min–20% B 2 min–20% B 8 min–65% B 10 min–80% B 11 min–95% B 13 min–95% B 13.1 min–20% B 17 min–20% B |
| Temperature | 45 °C |
| Flow rate | 0.5 mL/min |
| Injection volume | Sample: 10 µL 13C-iStd-Mix: 2 µL |
| Injection program | Wash needle in Flushport for 15 s Draw 2 µL from Vial 1 Wash needle in Flushport for 15 s Draw def. amount of sample Wash needle in Flushport for 15 s Inject |
| Running Time | 17 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Zhao, L.; Chen, Y.; Gao, H.; Hua, Y.; Yuan, X.; Yang, H. Mycotoxins in Maize Silage from China in 2019. Toxins 2022, 14, 241. https://doi.org/10.3390/toxins14040241
Zhang D, Zhao L, Chen Y, Gao H, Hua Y, Yuan X, Yang H. Mycotoxins in Maize Silage from China in 2019. Toxins. 2022; 14(4):241. https://doi.org/10.3390/toxins14040241
Chicago/Turabian StyleZhang, Dawei, Liansheng Zhao, Yakun Chen, Heyang Gao, Yu Hua, Xianjun Yuan, and Hailin Yang. 2022. "Mycotoxins in Maize Silage from China in 2019" Toxins 14, no. 4: 241. https://doi.org/10.3390/toxins14040241
APA StyleZhang, D., Zhao, L., Chen, Y., Gao, H., Hua, Y., Yuan, X., & Yang, H. (2022). Mycotoxins in Maize Silage from China in 2019. Toxins, 14(4), 241. https://doi.org/10.3390/toxins14040241





