The Role of Dietary Fiber and Gut Microbiome Modulation in Progression of Chronic Kidney Disease
Abstract
:1. Introduction
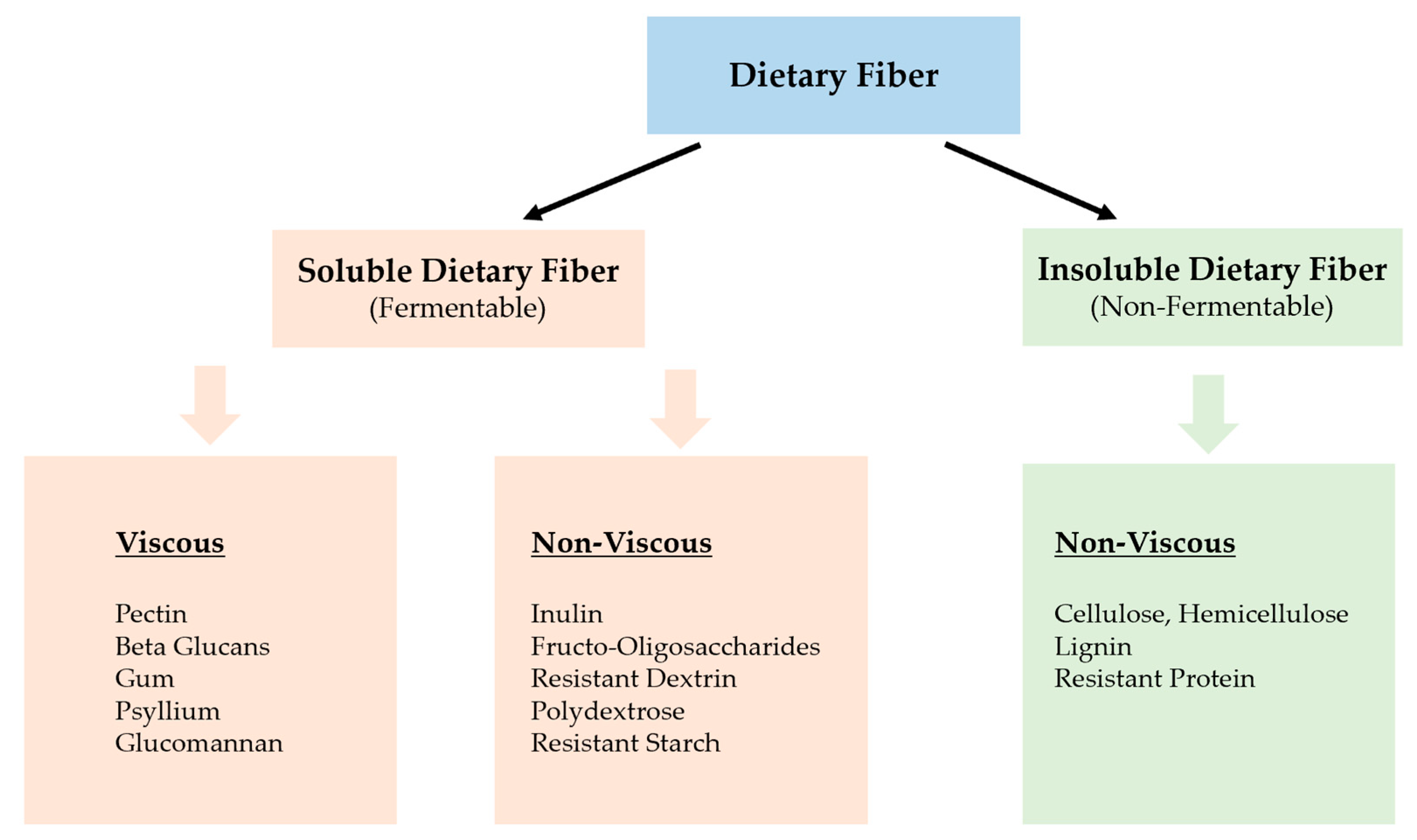
2. What Is Dietary Fiber
3. Health Benefits Effects of Dietary Fiber
4. The Composition of Healthy Gut Microbiota
5. Effect of Dietary Fiber Intake on Gut Microbiome
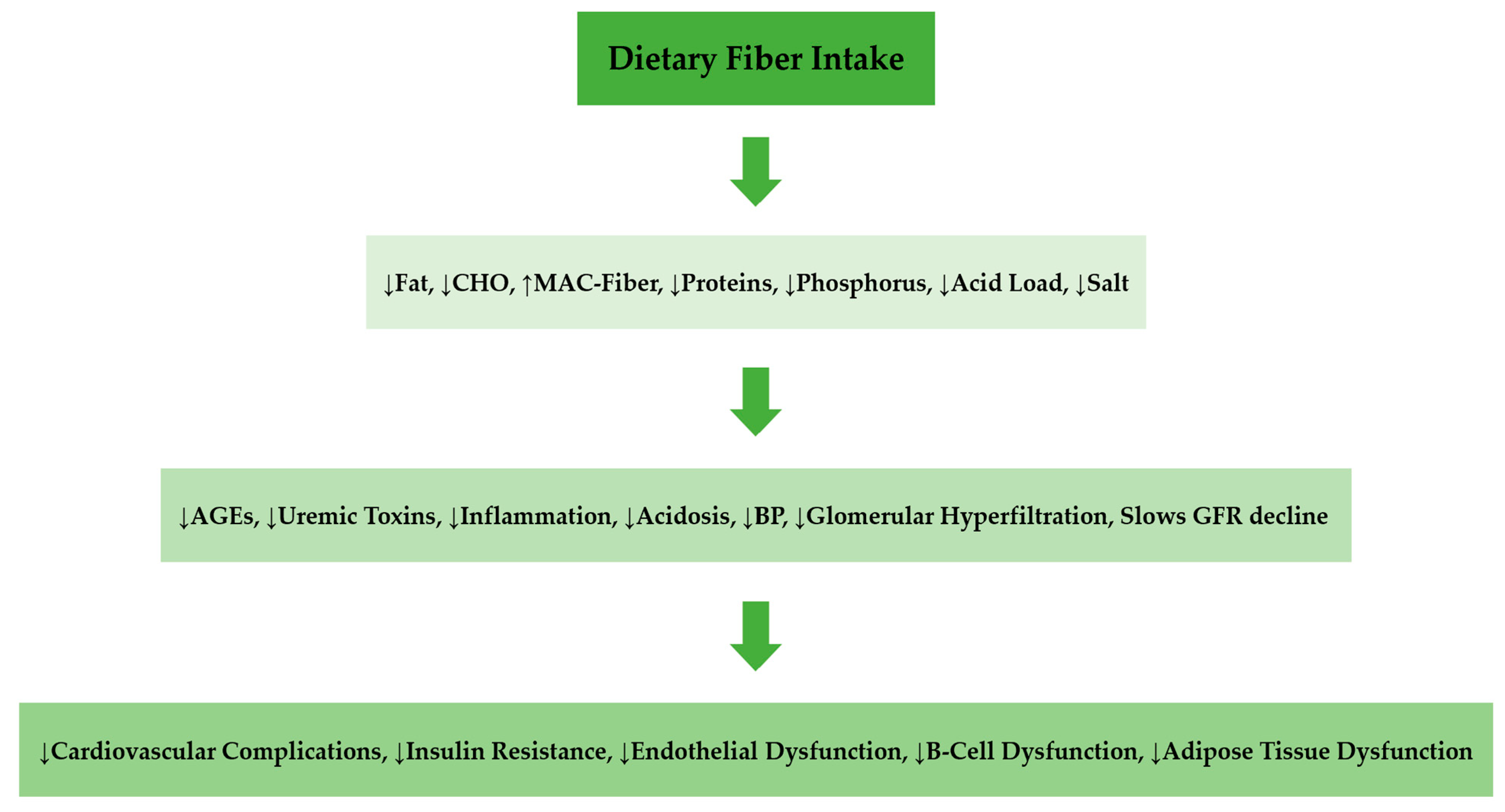
6. Role of Dietary Fiber in CKD Progression
7. Dietary Fiber in Renal Diet
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mandaliya, D.; Patel, S.; Seshadri, S. Fiber in our diet and its role in health and disease. In Functional Food and Human Health; Springer: Singapore, 2018; pp. 247–255. Available online: https://link.springer.com/chapter/10.1007/978-981-13-1123-9_12 (accessed on 15 November 2021).
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. Available online: https://academic.oup.com/nutritionreviews/article (accessed on 15 November 2021). [CrossRef] [PubMed]
- Khoury, T.; Tzukert, K.; Abel, R.; Abu Rmeileh, A.; Levi, R.; Ilan, Y. The gut-kidney axis in chronic renal failure: A new potential target for therapy. Hemodial. Int. 2017, 21, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Tucker, K.L. Health benefits of cereal fiber: A review of clinical trials. Nutr. Res. Rev. 2011, 24, 118–131. [Google Scholar] [CrossRef] [Green Version]
- Su, G.; Qin, X.; Yang, C.; Sabatino, A.; Kelly, J.T.; Avesani, C.M.; Carrero, J.J. Fiber intake and health in people with chronic kidney disease. Clin. Kidney J. 2021, 2021, 169. Available online: https://academic.oup.com/ckj/advance-article/doi/10.1093/ckj/sfab169 (accessed on 9 February 2022). [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. Available online: https://www.sciencedirect.com (accessed on 15 November 2021). [CrossRef] [PubMed] [Green Version]
- Deehan, E.C.; Duar, R.M.; Armet, A.M.; Perez-Muñoz, M.E.; Jin, M.; Walter, J. Modulation of the gastrointestinal microbiome with nondigestible fermentable carbohydrates to improve human health. Microbiol. Spectr. 2017, 5, 5. Available online: https://journals.asm.org/doi/full/10.1128 (accessed on 15 November 2021). [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fiber Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. Available online: https://www.mdpi.com/2072-6643/13/5/1655 (accessed on 9 February 2022). [CrossRef]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Shewry, P.R. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. Available online: https://www.tandfonline.com/doi/full/10.1080/10408398.2014.939263 (accessed on 15 November 2021). [CrossRef] [Green Version]
- Ma, Y.; Griffith, J.A.; Chasan-Taber, L.; Olendzki, B.C.; Jackson, E.; Stanek, E.J., III; Ockene, I.S. Association between dietary fiber and serum C-reactive protein. Am. J. Clin. Nutr. 2006, 83, 760–766. Available online: https://academic.oup.com/ajcn/article/83/4/760/4649102 (accessed on 15 November 2021). [CrossRef]
- Arranz, S.; Remon, A.M.; Raventos, R.M.; Estruch, R.L. Effects of dietary fiber intake on cardiovascular risk factors. In Recent Advances in Cardiovascular Risk Factors; Intech Open Science/Open Minds: London, UK, 2021; Volume 978, pp. 459–488. [Google Scholar]
- Othman, R.A.; Moghadasian, M.H. Beyond cholesterol-lowering effects of plant sterols: Clinical and experimental evidence of anti-inflammatory properties. Nutr. Rev. 2011, 69, 371–382. Available online: https://academic.oup.com/nutritionreviews/article/69/7/371/1937270 (accessed on 20 November 2021). [CrossRef]
- Tuan, J.; Chen, Y.X. Dietary and lifestyle factors associated with colorectal cancer risk and interactions with microbiota: Fiber, red or processed meat and alcoholic drinks. Gastrointest. Tumors 2016, 3, 17–24. Available online: https://www.karger.com/Article/Abstract/442831 (accessed on 20 November 2021). [CrossRef] [PubMed]
- Rajput, P.; Prajapati, B.; kumar Jena, P.; Seshadri, S. The role of gut microbiota produced Short Chain Fatty Acids (SCFAs) in adiposity and inflammation in obesity and type 2 Diabetes. In Proceedings of the 6th World Congress of Biotechnology, Newq Delhi, India, 5–7 October 2015; Available online: https://www.researchgate.net/profile/Parth- (accessed on 20 November 2021).
- Mattace Raso, G.; Simeoli, R.; Russo, R.; Iacono, A.; Santoro, A.; Paciello, O.; Meli, R. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS ONE 2013, 8, e68626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. Available online: https://www.sciencedirect.com/science/article/pii/S009286741301550X (accessed on 20 November 2021). [CrossRef] [Green Version]
- Anderson, J.W.; Pasupuleti, V.; Anderson, J. Dietary fiber and associated phytochemicals in prevention and reversal of diabetes. In Nutraceuticals Glycemic Health Type 2 Diabetes; John Wiley & Sons: Hoboken, NJ, USA, 2008; Chapter 7; pp. 97–125. [Google Scholar]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Bell, J.D. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. Available online: https://www.nature.com/articles/ncomms4611 (accessed on 20 November 2021). [CrossRef] [PubMed] [Green Version]
- O’Keefe, S.J.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Zoetendal, E.G. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015, 6, 6342. Available online: https://www.nature.com/articles (accessed on 20 November 2021). [CrossRef] [PubMed] [Green Version]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fiber in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. Available online: https://www.nature.com/articles/s41575-020-00375-4 (accessed on 9 February 2022). [CrossRef]
- Patterson, M.A.; Maiya, M.; Stewart, M.L. Resistant starch content in foods commonly consumed in the United States: A narrative review. J. Acad. Nutr. Diet. 2020, 120, 230–244. Available online: https://www.sciencedirect.com/science/article/abs/pii/S2212267219315540 (accessed on 20 November 2021). [CrossRef]
- Kizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Cuppari, L. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- De Boer, I.H.; Caramori, M.L.; Chan, J.C.; Heerspink, H.J.; Hurst, C.; Khunti, K.; Rossing, P. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128001004000039 (accessed on 30 November 2021).
- Yang, H.L.; Feng, P.; Xu, Y.; Hou, Y.Y.; Ojo, O.; Wang, X.H. The Role of Dietary Fiber Supplementation in Regulating Uremic Toxins in Patients with Chronic Kidney Disease: A Meta-Analysis of Randomized Controlled Trials. J. Ren. Nutr. 2021, 31, 438–447. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1051227620302910 (accessed on 9 February 2022). [CrossRef] [PubMed]
- Kim, S.M.; Han Song, I. The clinical impact of gut microbiota in chronic kidney disease. Korean J. Intern. Med. 2020, 35, 1305. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7652652/ (accessed on 30 November 2021). [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. Available online: https://www.nature.com/articles/nri.2016.42 (accessed on 30 November 2021). [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. Available online: https://www.science.org/doi/abs/10.1126/science.1110591 (accessed on 30 November 2021). [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. Available online: https://doi.org/10.3390/microorganisms7010014 (accessed on 30 November 2021). [CrossRef] [Green Version]
- Kanbay, M.; Onal, E.M.; Afsar, B.; Dagel, T.; Yerlikaya, A.; Covic, A.; Vaziri, N.D. The crosstalk of gut microbiota and chronic kidney disease: Role of inflammation, proteinuria, hypertension, and diabetes mellitus. Int. Urol. Nephrol. 2018, 50, 1453–1466. Available online: https://link.springer.com/article/10.1007/s11255-018-1873-2 (accessed on 30 November 2021). [CrossRef] [Green Version]
- Dominguez-Bello, M.G.; Blaser, M.J.; Ley, R.E.; Knight, R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology 2011, 140, 1713–1719. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0016508511001600 (accessed on 30 November 2021). [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. Available online: https://www.nature.com/articles/nrg3182 (accessed on 6 January 2022). [CrossRef] [Green Version]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Flint, H.J. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. Available online: https://www.nature.com/articles/ismej2010118 (accessed on 6 January 2022). [CrossRef]
- Martínez, I.; Kim, J.; Duffy, P.R.; Schlegel, V.L.; Walter, J. Resistant starch types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE 2010, 5, e15046. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0015046 (accessed on 6 January 2022). [CrossRef] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. Available online: https://www.sciencedirect.com/science/article/pii/S009286741630592X (accessed on 6 January 2022). [CrossRef] [PubMed] [Green Version]
- Tomaro-Duchesneau, C.; Saha, S.; Malhotra, M.; Coussa-Charley, M.; Kahouli, I.; Jones, M.L.; Prakash, S. Probiotic ferulic acid esterase active Lactobacillus fermentum NCIMB 5221 APA microcapsules for oral delivery: Preparation and in vitro characterization. Pharmaceuticals 2012, 5, 236–248. Available online: https://www.mdpi.com/1424-8247/5/2/236 (accessed on 6 January 2022). [CrossRef] [PubMed]
- Baye, K.; Guyot, J.P.; Mouquet-Rivier, C. The unresolved role of dietary fibers on mineral absorption. Crit. Rev. Food Sci. Nutr. 2017, 57, 949–957. Available online: https://www.tandfonline.com/doi/abs/10.1080/10408398.2014.953030 (accessed on 6 January 2022). [CrossRef] [PubMed]
- Kim, K.M.; Oh, H.J.; Choi, H.Y.; Lee, H.; Ryu, D.R. Impact of chronic kidney disease on mortality: A nationwide cohort study. Kidney Res. Clin. Pract. 2019, 38, 382. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6727899 (accessed on 11 January 2022). [CrossRef] [Green Version]
- Mafra, D.; Borges, N.; Alvarenga, L.; Esgalhado, M.; Cardozo, L.; Lindholm, B.; Stenvinkel, P. Dietary components that may influence the disturbed gut microbiota in chronic kidney disease. Nutrients 2019, 11, 496. Available online: https://www.mdpi.com/2072-6643/11/3/496 (accessed on 11 January 2022). [CrossRef] [Green Version]
- Tayebi-Khosroshahi, H.; Habibzadeh, A.; Niknafs, B.; Ghotaslou, R.; Sefidan, F.Y.; Ghojazadeh, M.; Parkhide, S. The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial. J. Ren. Inj. Prev. 2016, 5, 162. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5040005 (accessed on 11 January 2022). [CrossRef] [Green Version]
- Poesen, R.; Evenepoel, P.; de Loor, H.; Delcour, J.A.; Courtin, C.M.; Kuypers, D.; Meijers, B. The influence of prebiotic arabinoxylan oligosaccharides on microbiota derived uremic retention solutes in patients with chronic kidney disease: A randomized controlled trial. PLoS ONE 2016, 11, e0153893. [Google Scholar] [CrossRef]
- Meijers, B.K.; De Preter, V.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. p-Cresyl sulfate serum concentrations in hemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol. Dial. Transplant. 2010, 25, 219–224. Available online: https://academic.oup.com/ndt/article/25/1/219/1909853 (accessed on 11 January 2022). [CrossRef] [Green Version]
- pChiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.A.; Darling, P.B. Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2015, 69, 761–768. Available online: https://www.nature.com/articles/ejcn2014237 (accessed on 11 January 2022). [CrossRef] [Green Version]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. Available online: https://www.mdpi.com/2072-6643/9/9/1021 (accessed on 11 January 2022). [CrossRef]
- D’Alessandro, C.; Piccoli, G.B.; Calella, P.; Brunori, G.; Pasticci, F.; Egidi, M.F.; Cupisti, A. “Dietaly”: Practical issues for the nutritional management of CKD patients in Italy. BMC Nephrol. 2016, 17, 102. Available online: https://bmcnephrol.biomedcentral.com/articles/10.1186/s12882-016-0296-5 (accessed on 11 January 2022). [CrossRef] [PubMed]
- Joshi, S.; McMacken, M.; Kalantar-Zadeh, K. Plant-based diets for kidney disease: A guide for clinicians. Am. J. Kidney Dis. 2021, 77, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. Available online: https://jasn.asnjournals.org/content/25/4/657.short (accessed on 11 January 2022). [CrossRef] [PubMed] [Green Version]
- Lau, W.L.; Vaziri, N.D. The leaky gut and altered microbiome in chronic kidney disease. J. Ren. Nutr. 2017, 27, 458–461. Available online: https://www.sciencedirect.com/science/article (accessed on 11 January 2022). [CrossRef] [PubMed] [Green Version]
- Hobby, G.P.; Karaduta, O.; Dusio, G.F.; Singh, M.; Zybailov, B.L.; Arthur, J.M. Chronic kidney disease and the gut microbiome. Am. J. Physiol. Ren. Physiol. 2019, 316, F1211–F1217. Available online: https://journals.physiology.org/doi/full (accessed on 11 January 2022). [CrossRef]
- Koppe, L.; Fouque, D.; Soulage, C.O. The role of gut microbiota and diet on uremic retention solutes production in the context of chronic kidney disease. Toxins 2018, 10, 155. Available online: https://www.mdpi.com/2072-6651/10/4/155/htm (accessed on 11 January 2022). [CrossRef] [Green Version]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-supplemented vegetarian very low–protein diet and CKD progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. Available online: https://jasn.asnjournals.org/content/27/7/2164 (accessed on 11 January 2022). [CrossRef] [Green Version]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. Available online: https://cjasn.asnjournals.org/content/9/9/1603 (accessed on 11 January 2022). [CrossRef] [Green Version]
- Xu, X.; Li, Z.; Chen, Y.; Liu, X.; Dong, J. Dietary fibre and mortality risk in patients on peritoneal dialysis. Br. J. Nutr. 2019, 122, 996–1005. [Google Scholar] [CrossRef] [Green Version]
- Adair, K.E.; Bowden, R.G. Ameliorating chronic kidney disease using a whole food plant-based diet. Nutrients 2020, 12, 1007. [Google Scholar] [CrossRef] [Green Version]
- King, D.E.; Mainous III, A.G.; Lambourne, C.A. Trends in dietary fiber intake in the United States, 1999–2008. J. Acad. Nutr. Diet. 2012, 112, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Camerotto, C.; Cupisti, A.; D’Alessandro, C.; Muzio, F.; Gallieni, M. Dietary fiber and gut microbiota in renal diets. Nutrients 2019, 11, 2149. Available online: https://www.mdpi.com/2072-6643/11/9/2149 (accessed on 11 January 2022). [CrossRef] [PubMed] [Green Version]
- Zimmer, C. Fiber Is Good for You. Now Scientists May Know Why. The New York Times, 2018. Available online: https://www.nytimes.com/2018/01/01/science/food-fiber-microbiome-inflammation.html(accessed on 11 January 2022).
- Zhao, J.; Ning, X.; Liu, B.; Dong, R.; Bai, M.; Sun, S. Specific alterations in gut microbiota in patients with chronic kidney disease: An updated systematic review. Ren. Fail. 2021, 43, 102–112. Available online: https://www.tandfonline.com/doi/epub/10.1080/0886022X.2020.1864404 (accessed on 24 February 2022). [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranganathan, N.; Anteyi, E. The Role of Dietary Fiber and Gut Microbiome Modulation in Progression of Chronic Kidney Disease. Toxins 2022, 14, 183. https://doi.org/10.3390/toxins14030183
Ranganathan N, Anteyi E. The Role of Dietary Fiber and Gut Microbiome Modulation in Progression of Chronic Kidney Disease. Toxins. 2022; 14(3):183. https://doi.org/10.3390/toxins14030183
Chicago/Turabian StyleRanganathan, Natarajan, and Emmanuel Anteyi. 2022. "The Role of Dietary Fiber and Gut Microbiome Modulation in Progression of Chronic Kidney Disease" Toxins 14, no. 3: 183. https://doi.org/10.3390/toxins14030183
APA StyleRanganathan, N., & Anteyi, E. (2022). The Role of Dietary Fiber and Gut Microbiome Modulation in Progression of Chronic Kidney Disease. Toxins, 14(3), 183. https://doi.org/10.3390/toxins14030183




