Management of Double Sensitization to Vespids in Europe
Abstract
:1. Introduction
2. Venoms from Different Species Share Similar Allergens: Cross-Reactivity
2.1. Phospholipase A1 (Ves v 1; Pol d 1)
2.2. Hyaluronidase (Ves v 2; Pol d 2)
2.3. Dipeptidyl Peptidases IV (Ves v 3; Pol d 3)
2.4. Antigen 5 (Ves v 5; Pol d 5)
2.5. Vitellogenin (Ves v 6)
2.6. Serine Protease (Pol d 4)
2.7. Other Components
3. Tools to Identify the Culprit Insect: Helping with the Diagnosis
3.1. Visual Identification of the Insect
3.2. Skin Test
3.3. Serum Specific IgE Determinations against Whole Venom and Its Components (sIgE)
3.4. Serum Specific IgE Inhibition Assays (sIgE-INH)
3.5. Basophil Activation Test (BAT)
3.6. Serum Specific IgG4 (sIgG4) as a Marker of Exposure
3.7. Sting Challenge Test (SCT)
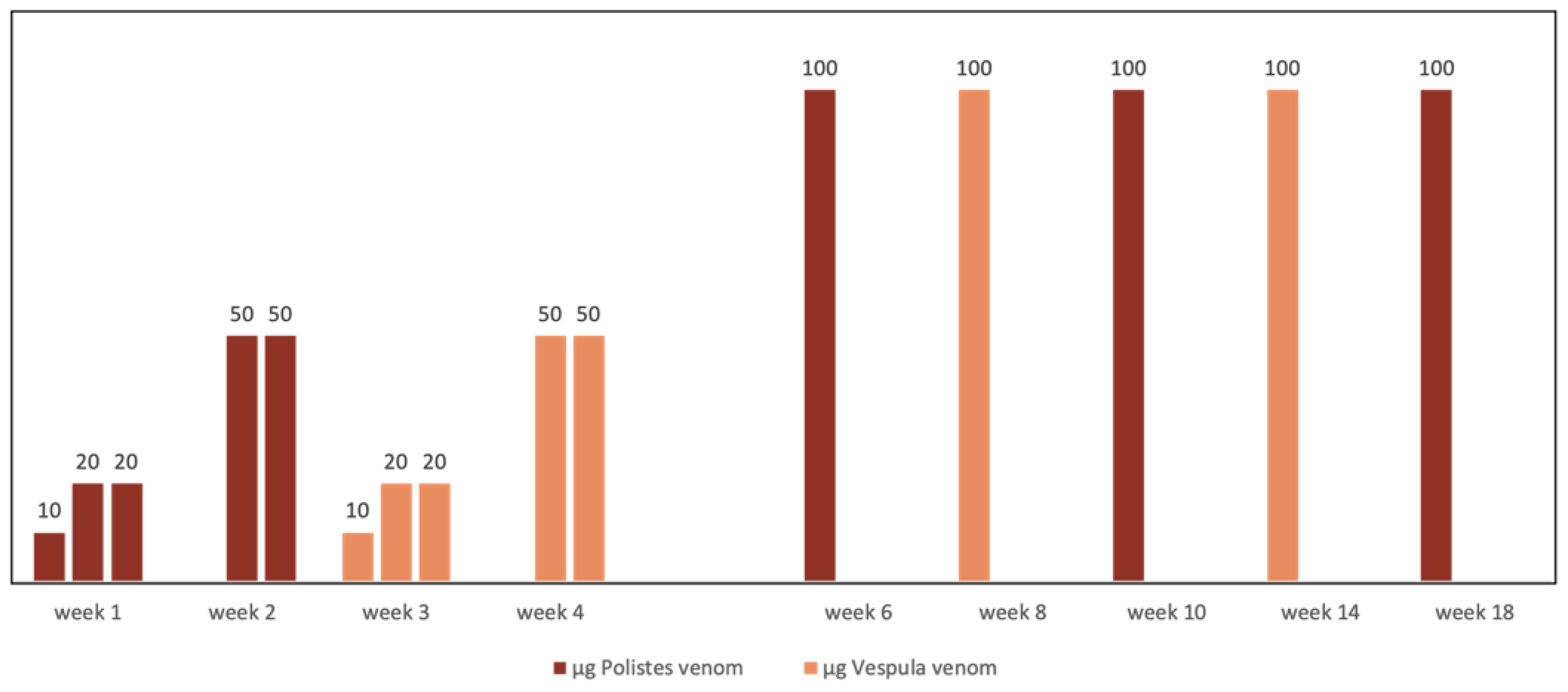
4. Management of Vespid Venom Immunotherapy (vvit) in Cases of Double Sensitization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schiener, M.; Graessel, A.; Ollert, M.; Schmidt-Weber, C.B.; Blank, S. Allergen-Specific Immunotherapy of Hymenoptera Venom Allergy—Also a Matter of Diagnosis. Hum. Vaccin. Immunother. 2017, 13, 2467–2481. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, S.J.P.-V. Orden Hymenoptera. Rev. IDE@—SEA 2015, 59, 1–36. [Google Scholar]
- De Villiers, M.; Kriticos, D.J.; Veldtman, R. Including Irrigation in Niche Modelling of the Invasive Wasp Vespula Germanica (Fabricius) Improves Model Fit to Predict Potential for Further Spread. PLoS ONE 2017, 12, e0181397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arca, M.; Mougel, F.; Guillemaud, T.; Dupas, S.; Rome, Q.; Perrard, A.; Muller, F.; Fossoud, A.; Capdevielle-Dulac, C.; Torres-Leguizamon, M.; et al. Reconstructing the Invasion and the Demographic History of the Yellow-Legged Hornet, Vespa Velutina, in Europe. Biol. Invasions 2015, 17, 2357–2371. [Google Scholar] [CrossRef]
- Vidal, C. The Asian Wasp Vespa Velutina Nigrithorax: Entomological and Allergological Characteristics. Clin. Exp. Allergy 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fichas—Portal SEAIC. Available online: https://www.seaic.org/alergia-abejas-y-avispas/vespidos-sociales-en-europa/fichas (accessed on 12 December 2021).
- Blanca, M.; Garcia, F.; Miranda, A.; Carmona, M.J.; Garcia, J.; Fernandez, J.; Terrados, S.; Vega, J.M.; Juarez, C. Determination of IgE Antibodies to Polistes Dominulus, Vespula Germanica and Vespa Crabro in Sera of Patients Allergic to Vespids. Allergy 1991, 46, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Severino, M.G.; Campi, P.; MacChia, D.; Manfredi, M.; Turillazzi, S.; Spadolini, I.; Bilò, M.B.; Bonifazi, F. European Polistes Venom Allergy. Allergy 2006, 61, 860–863. [Google Scholar] [CrossRef]
- Caruso, B.; Bonadonna, P.; Severino, M.G.; Manfredi, M.; Dama, A.; Schiappoli, M.; Rizzotti, P.; Senna, G.; Passalacqua, G. Evaluation of the IgE Cross-Reactions among Vespid Venoms. A Possible Approach for the Choice of Immunotherapy. Allergy 2007, 62, 561–564. [Google Scholar] [CrossRef]
- Straumann, F.; Bucher, C.; Wüthrich, B. Double Sensitization to Honeybee and Wasp Venom: Immunotherapy with One or with Both Venoms? Int. Arch. Allergy Immunol. 2000, 123, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, R.I.; Vega, A.; Marqués, L.; Miranda, A.; Fernández, J.; Soriano, V.; Cruz, S.; Domínguez-Noche, C.; Sánchez-Morillas, L.; Armisen-Gil, M.; et al. Component-Resolved Diagnosis of Vespid Venom-Allergic Individuals: Phospholipases and Antigen 5s Are Necessary to Identify Vespula or Polistes Sensitization. Allergy 2012, 67, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pimiento, A.J.; Alonso-González, L.; Prieto-Lastra, L.; Rodríguez-Cabreros, M.I.; Iglesias-Cadarso, A.; Rodríguez-Mosquera, M. Anaphylaxis to Hymenoptera Sting: Study of 113 Patients. Med. Clin. 2005, 125, 417–420. [Google Scholar] [CrossRef]
- Alfaya Arias, T.; Soriano Gómis, V.; Soto Mera, T.; Vega Castro, A.; Vega Gutiérrez, J.M.; Alonso Llamazares, A.; Antolín Amérigo, D.; Carballada Gonzalez, F.J.; Dominguez Noche, C.; Gutierrez Fernandez, D.; et al. Key Issues in Hymenoptera Venom Allergy: An Update. J. Investig. Allergol. Clin. Immunol. 2017, 27, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galindo-Bonilla, P.A.; Galán-Nieto, A.; Alfaya-Arias, T.; García-Rodríguez, C.; de la Roca-Pinzón, F.; Feo-Brito, F. Component-Resolved Diagnosis in Vespid Venom-Allergic Individuals. Allergol. Immunopathol. 2015, 43, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Quercia, O.; Cova, V.; Martini, M.; Cortellini, G.; Murzilli, F.; Bignardi, D.; Cilia, M.; Scarpa, A.; Bilò, M.B. CAP-Inhibition, Molecular Diagnostics, and Total IgE in the Evaluation of Polistes and Vespula Double Sensitization. Int. Arch. Allergy Immunol. 2018, 177, 365–369. [Google Scholar] [CrossRef]
- Schiener, M.; Eberlein, B.; Moreno-Aguilar, C.; Pietsch, G.; Serrano, P.; McIntyre, M.; Schwarze, L.; Russkamp, D.; Biedermann, T.; Spillner, E.; et al. Application of Recombinant Antigen 5 Allergens from Seven Allergy-Relevant Hymenoptera Species in Diagnostics. Allergy 2017, 72, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Kosnik, M.; Korosec, P.; Silar, M.; Music, E.; Erzen, R. Wasp Venom Is Appropriate for Immunotherapy of Patients with Allergic Reaction to the European Hornet Sting. Croat Med. J. 2002, 43, 25–27. [Google Scholar] [PubMed]
- Rodríguez-Vázquez, V.; Armisén, M.; Gómez-Rial, J.; Lamas-Vázquez, B.; Vidal, C. Immunotherapy with Vespula Venom for Vespa Velutina Nigrithorax Anaphylaxis: Preliminary Clinical and Immunological Results. Clin. Exp. Allergy 2022, 52, 345–347. [Google Scholar] [CrossRef] [PubMed]
- WHO/IUIS Allergen Nomenclature Home Page. Available online: http://allergen.org (accessed on 12 December 2021).
- Allergome. Available online: https://www.allergome.org/index.php (accessed on 12 December 2021).
- Chugo, S.; Lizaso, M.T.; Alvarez, M.J.; Arroabaren, E.; Lizarza, S.; Tabar, A.I. Vespa Velutina Nigritorax: A New Causative Agent in Anaphylaxis. J. Investig. Allergol. Clin. Immunol. 2015, 25, 231–232. [Google Scholar]
- Vidal, C.; Armisén, M.; Monsalve, R.; González-Vidal, T.; Lojo, S.; López-Freire, S.; Méndez, P.; Rodríguez, V.; Romero, L.; Galán, A.; et al. Anaphylaxis to Vespa Velutina Nigrithorax: Pattern of Sensitization for an Emerging Problem in Western Countries. J. Investig. Allergol. Clin. Immunol. 2021, 31, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27 (Suppl. S2), 1–250. [Google Scholar] [CrossRef] [PubMed]
- BLAST: Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 12 December 2021).
- Blank, S.; Neu, C.; Hasche, D.; Bantleon, F.I.; Jakob, T.; Spillner, E. Polistes Species Venom is Devoid of Carbohydrate-Based Cross-Reactivity and Allows Interference-Free Diagnostics. J. Allergy Clin. Immunol. 2013, 131, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Bilò, M.B.; Martini, M.; Bonadonna, P.; Cinti, B.; Da Re, M.; Gabrielli, O.; Olivieri, F.; Salgarolo, V.; Zanoni, G.V.D. Prevalence of Pol d 1 Sensitization in Polistes Dominula Allergy and Its Diagnostic Role in Vespid Double-Positivity. J. Allergy Clin. Immunol. Pract. 2021, 9, 3781–3787. [Google Scholar] [CrossRef]
- Blank, S.; Bazon, M.L.; Grosch, J.; Schmidt-Weber, C.B.; Brochetto-Braga, M.R.; Bilò, M.B.; Jakob, T. Antigen 5 Allergens of Hymenoptera Venoms and Their Role in Diagnosis and Therapy of Venom Allergy. Curr. Allergy Asthma Rep. 2020, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.R. Insect Venoms. Chem. Immunol. Allergy. 2010, 95, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Spillner, E.; Blank, S.; Jakob, T. Hymenoptera Allergens: From Venom to “Venome”. Front. Immunol. 2014, 5, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antolín-Amérigo, D.; Ruiz-León, B.; Boni, E.; Alfaya-Arias, T.; Álvarez-Mon, M.; Barbarroja-Escudero, J.; González-de-Olano, D.; Moreno-Aguilar, C.; Rodríguez-Rodríguez, M.; Sánchez-González, M.J.; et al. Component-Resolved Diagnosis in Hymenoptera Allergy. Allergol. Immunopathol. 2018, 46, 253–262. [Google Scholar] [CrossRef]
- Grosch, J.; Hilger, C.; Bilò, M.B.; Kler, S.; Schiener, M.; Dittmar, G.; Bernardin, F.; Lesur, A.; Ollert, M.; Schmidt-Weber, C.B.; et al. Shedding Light on the Venom Proteomes of the Allergy-Relevant Hymenoptera Polistes Dominula (European Paper Wasp) and Vespula Spp. (Yellow Jacket). Toxins 2020, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, R.I.; Gutiérrez, R.; Hoof, I.; Lombardero, M. Purification and Molecular Characterization of Phospholipase, Antigen 5 and Hyaluronidases from the Venom of the Asian Hornet (Vespa Velutina). PLoS ONE 2020, 15, e0225672. [Google Scholar] [CrossRef]
- Vidal, C.; Armisén, M.; Monsalve, R.; Gómez-Rial, J.; González-Fernández, T.; Carballada, F.; Lombardero, M.; González-Quintela, A. Vesp v 5 and Glycosylated Vesp v 1 Are Relevant Allergens in Vespa Velutina Nigrithorax Anaphylaxis. Clin. Exp. Allergy 2020, 50, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Rungsa, P.; Incamnoi, P.; Sukprasert, S.; Uawonggul, N.; Klaynongsruang, S.; Daduang, J.; Patramanon, R.; Roytrakul, S.; Daduang, S. Cloning, Structural Modelling and Characterization of VesT2s, a Wasp Venom Hyaluronidase (HAase) from Vespa Tropica. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 28. [Google Scholar] [CrossRef] [Green Version]
- Seppälä, U.; Selby, D.; Monsalve, R.; King, T.P.; Ebner, C.; Roepstorff, P.; Bohle, B. Structural and Immunological Characterization of the N-Glycans from the Major Yellow Jacket Allergen Ves v 2: The N-Glycan Structures Are Needed for the Human Antibody Recognition. Mol. Immunol. 2009, 46, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, C.; Lord, C.I.; Zhang, C.; Duke-Cohan, J.S.; Letvin, N.L.; Schlossman, S.F. Role of CD26/Dipeptidyl Peptidase IV in Human Immunodeficiency Virus Type 1 Infection and Apoptosis. Proc. Natl. Acad. Sci. USA 1994, 91, 9960–9964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiener, M.; Hilger, C.; Eberlein, B.; Pascal, M.; Kuehn, A.; Revets, D.; Planchon, S.; Pietsch, G.; Serrano, P.; Moreno-Aguilar, C.; et al. The High Molecular Weight Dipeptidyl Peptidase IV Pol d 3 Is a Major Allergen of Polistes Dominula Venom. Sci. Rep. 2018, 8, 1318. [Google Scholar] [CrossRef] [PubMed]
- Blank, S.; Seismann, H.; Bockisch, B.; Braren, I.; Cifuentes, L.; McIntyre, M.; Rühl, D.; Ring, J.; Bredehorst, R.; Ollert, M.W.; et al. Identification, Recombinant Expression, and Characterization of the 100 KDa High Molecular Weight Hymenoptera Venom Allergens Api m 5 and Ves v 3. J. Immunol. 2010, 184, 5403–5413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winningham, K.M.; Fitch, C.D.; Schmidt, M.; Hoffman, D.R. Hymenoptera Venom Protease Allergens. J. Allergy Clin. Immunol. 2004, 114, 928–933. [Google Scholar] [CrossRef]
- Baker, T.W.; Forester, J.P.; Johnson, M.L.; Stolfi, A.; Stahl, M.C. The HIT Study: Hymenoptera Identification Test--How Accurate Are People at Identifying Stinging Insects? Ann. Allergy. Asthma Immunol. 2014, 113, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Bilò, M.B.; Pravettoni, V.; Bignardi, D.; Bonadonna, P.; Mauro, M.; Novembre, E.; Quercia, O.; Cilia, M.; Cortellini, G.; Costantino, M.T.; et al. Hymenoptera Venom Allergy: Management of Children and Adults in Clinical Practice. J. Investig. Allergol. Clin. Immunol. 2019, 29, 180–205. [Google Scholar] [CrossRef] [Green Version]
- Jakob, T.; Spillner, E. Comparing Sensitivity of Hymenoptera Allergen Components on Different Diagnostic Assay Systems: Comparing Apples and Oranges? J. Allergy Clin. Immunol. 2017, 139, 1066–1067. [Google Scholar] [CrossRef] [PubMed]
- Caruso, B.; Bonadonna, P.; Bovo, C.; Melloni, N.; Lombardo, C.; Senna, G.; Lippi, G. Wasp Venom Allergy Screening with Recombinant Allergen Testing. Diagnostic Performance of RPol d 5 and RVes v 5 for Differentiating Sensitization to Vespula and Polistes Subspecies. Clin. Chim. Acta. 2016, 453, 170–173. [Google Scholar] [CrossRef]
- Lambert, C.; Birnbaum, J.; Dzviga, C.; Hutt, N.; Apoil, P.A.; Bienvenu, F.; Drouet, M.; Beauvillain, C.; Brabant, S.; Guilloux, L.; et al. Antigen 5-Spiked Vespula and Polistes Venom Extracts for Vespid Allergy Diagnostics: A French Multicenter Study. Ann. Allergy. Asthma Immunol. 2018, 120, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, P.; Caruso, B.; Labardi, D.; Dama, A.; Senna, G.; Passalacqua, G. Treatment with American Polistes Venom Was Ineffective in an Italian Patient Allergic to European Polistes. Allergy 2007, 62, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Savi, E.; Peveri, S.; Makri, E.; Pravettoni, V.; Incorvaia, C. Comparing the Ability of Molecular Diagnosis and CAP-Inhibition in Identifying the Really Causative Venom in Patients with Positive Tests to Vespula and Polistes Species. Clin. Mol. Allergy 2016, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Korošec, P.; Šilar, M.; Eržen, R.; Čelesnik, N.; Bajrović, N.; Zidarn, M.; Košnik, M. Clinical Routine Utility of Basophil Activation Testing for Diagnosis of Hymenoptera-Allergic Patients with Emphasis on Individuals with Negative Venom-Specific IgE Antibodies. Int. Arch. Allergy Immunol. 2013, 161, 363–368. [Google Scholar] [CrossRef]
- Eberlein, B.; Krischan, L.; Darsow, U.; Ollert, M.; Ring, J. Double Positivity to Bee and Wasp Venom: Improved Diagnostic Procedure by Recombinant Allergen-Based IgE Testing and Basophil Activation Test Including Data about Cross-Reactive Carbohydrate Determinants. J. Allergy Clin. Immunol. 2012, 130, 155–161. [Google Scholar] [CrossRef]
- Ruiz-Leon, B.; Navas, A.; Serrano, P.; Espinazo, M.; Guler, I.; Alonso, C.; Jurado, A.; Moreno-Aguilar, C. Helios Negative Regulatory T-Cells as a Key Factor of Immune Tolerance in Non-Allergic Beekeepers. J. Investig. Allergol. Clin. Immunol. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.J.; Varga, E.M.; Roberts, G.; Mosbech, H.; Bilò, M.B.; Akdis, C.A.; Antolín-Amérigo, D.; Cichocka-Jarosz, E.; Gawlik, R.; Jakob, T.; et al. EAACI Guidelines on Allergen Immunotherapy: Hymenoptera Venom Allergy. Allergy 2018, 73, 744–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franken, H.H.; Dubois AE, J.; Minkema, H.J.; van der Heide, S.; de Monchy, J.G.R. Lack of Reproducibility of a Single Negative Sting Challenge Response in the Assessment of Anaphylactic Risk in Patients with Suspected Yellow Jacket Hypersensitivity. J. Allergy Clin. Immunol. 1994, 93, 431–436. [Google Scholar] [CrossRef]
- Tracy, J.M.; Golden, D.B.K. Hymenoptera Venom Extracts in Clinical Practice. J. Allergy Clin. Immunol. Pract. 2018, 6, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Severino, M.G.; Caruso, B.; Bonadonna, P.; Labardi, D.; Macchia, D.; Campi, P.; Passalacqua, G. Cross Reactivity between European Hornet and Yellow Jacket Venoms. Eur. Ann. Allergy Clin. Immunol. 2010, 42, 141–145. [Google Scholar] [CrossRef]
- Macchia, D.; Cortellini, G.; Mauro, M.; Meucci, E.; Quercia, O.; Manfredi, M.; Massolo, A.; Valentini, M.; Severino, M.; Passalacqua, G. Vespa Crabro Immunotherapy versus Vespula-Venom Immunotherapy in Vespa Crabro Allergy: A Comparison Study in Field Re-Stings. World Allergy Organ. J. 2018, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, A.; Shefler, I.; Panasoff, J.; Paitan, Y.; Confino-Cohen, R. Immunotherapy with Commercial Venoms Is Efficacious for Anaphylactic Reactions to Vespa Orientalis Stings. Int. Arch. Allergy Immunol. 2013, 161, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Savi, E.; Incorvaia, C.; Boni, E.; Mauro, M.; Peveri, S.; Pravettoni, V.; Quercia, O.; Reccardini, F.; Montagni, M.; Pessina, L.; et al. Which Immunotherapy Product Is Better for Patients Allergic to Polistes Venom? A Laboratory and Clinical Study. PLoS ONE 2017, 12, e0180270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glérant, J.C.; Martinez, P.; Guillaume, C.; Jounieaux, V. Comparison of 2 Maintenance Doses (100 Microg vs 200 Microg) in Hymenoptera Venom Immunotherapy: Influence of the Maintenance Dose on the Immunologic Response. Ann. Allergy. Asthma Immunol. 2005, 94, 451–456. [Google Scholar] [CrossRef]
- Moreno, C.; Barasona, M.J.; Serrano, P.; Justicia, J.L.; Ruz, J.M.; Guerra, F. Alternating Polistes-Vespula Venom Immunotherapy: A Therapeutic Strategy to Resolve a Diagnostic Deficiency. J. Investig. Allergol. Clin. Immunol. 2011, 21, 28–33. [Google Scholar] [PubMed]


| Allergens | % Sensitization [23] | kDa | Isoforms | Glycosylation | Sequence Homology | Eukaryotic Expression |
|---|---|---|---|---|---|---|
| Ves v 1Phospholipase A1 | 33.3–54 | 35 | 1 | no | Ves v 1 y Ves f 1, Ves g 1, Ves m 1, Ves s 1, Pol a 1, Pol d 1, Dol m 1, Ves p 1, Vesp c 1, Poly p 1 y Sol i 1 | yes |
| Ves v 2Hyaluronidase | 5–25 | 44 | 2 | yes | Api c 2, Api m 2, Dol m 2, Pol a 2 y Ves g 2 | yes |
| Ves v 3Dipeptidylpeptidase IV | 50–62.8 | 100 | 1 | yes | Pol d 3, Api m 5 | yes |
| Ves v 5Antigen 5 | 84.5–100 | 23 | 1 | no | Dol a 5, Dol m 5, Pac c 3, Pol a 5, Pol d 5, Pol e 5, Pol f 5, Pol g 5, Poly s 5, Sol i 3, Sol r 3, Ves f 5, Ves g 5, Ves m 5, Ves p 5, Ves s 5, Ves vi 5, Vesp c 5, Vesp m 5. | yes |
| Ves v 6Vitellogenin | 39 | 200 | 1 | yes | Api m 12 | yes |
| Pol d 1Phospholipase A1 | 87 | 33 | 4 | no | Dol a 5, Dol m 5, Pac c 3, Pol a 5, Pol d 5, Pol e 5, Pol f 5, Pol g 5, Poly s 5, Sol i 3, Sol r 3, Ves f 5, Ves g 5, Ves m 5, Ves p 5, Ves s 5, Ves vi 5, Vesp c 5, Vesp m 5. | no |
| Pol d 2Hyaluronidase | ? | 50 | 1 | yes | yes | |
| Pol d 3Dipeptidylpeptidase IV | 66.7 | 100 | 1 | yes | yes | |
| Pol d 4 Serinprotease | ? | 30 | 1 | yes | Api m 7, Bom ig 4, Bom p 4, Bom t 4 | no |
| Pol d 5Antigen 5 | 69–72 | 23 | 1 | no | Dol a 5, Pac c 3, Poly s 5, Sol i 3, Ves v 5, Vesp c 5 | no |
| Ves G 1 | Ves G 2 | Ves G 3 | Ves G 4 * | Ves G 5 | |
|---|---|---|---|---|---|
| Pol d 1 | 54% | ||||
| Pol d 2 | 74.1% | ||||
| Pol d 3 | 73.7% | ||||
| Pol d 4 | 51.9% | ||||
| Pol d 5 | 59.6% |
| Ves v 1 | Ves g 1 | Ves m 1 | Ves s 1 | Pol a 1 | Pol d 1 | Dol m 1 | Vesp c 1 | Vesp v 1 | Poly p 1 |
| 94.7 | 95.7 | 71.1 | 53.8 | 52.8 | 67.2 | 72.1 | 62.5 | 61.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Leon, B.; Serrano, P.; Vidal, C.; Moreno-Aguilar, C. Management of Double Sensitization to Vespids in Europe. Toxins 2022, 14, 126. https://doi.org/10.3390/toxins14020126
Ruiz-Leon B, Serrano P, Vidal C, Moreno-Aguilar C. Management of Double Sensitization to Vespids in Europe. Toxins. 2022; 14(2):126. https://doi.org/10.3390/toxins14020126
Chicago/Turabian StyleRuiz-Leon, Berta, Pilar Serrano, Carmen Vidal, and Carmen Moreno-Aguilar. 2022. "Management of Double Sensitization to Vespids in Europe" Toxins 14, no. 2: 126. https://doi.org/10.3390/toxins14020126
APA StyleRuiz-Leon, B., Serrano, P., Vidal, C., & Moreno-Aguilar, C. (2022). Management of Double Sensitization to Vespids in Europe. Toxins, 14(2), 126. https://doi.org/10.3390/toxins14020126







