Biodegradation of Free Gossypol by Helicoverpa armigera Carboxylesterase Expressed in Pichia pastoris
Abstract
1. Introduction
2. Results and Discussion
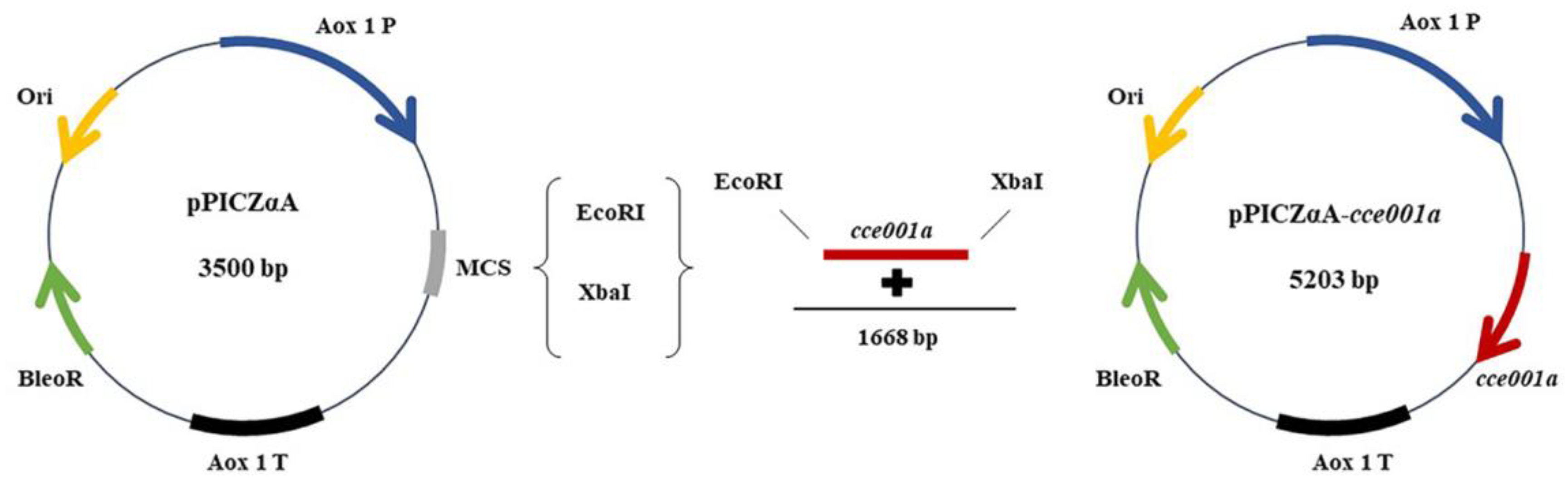
2.1. Source of CarE and Construction of Expression Vector
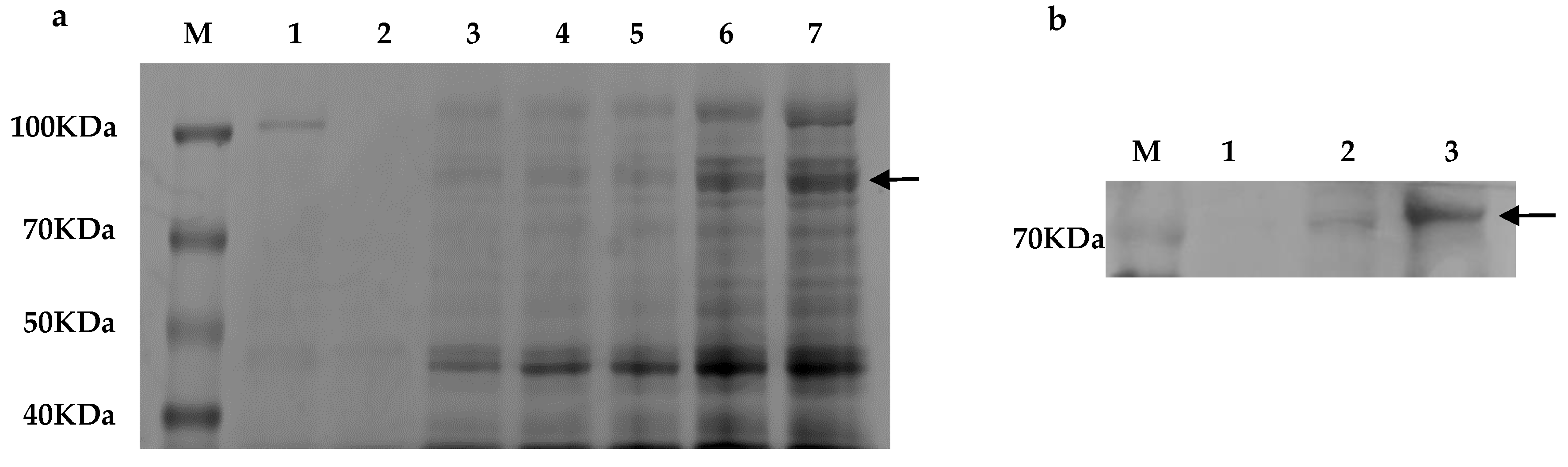
2.2. Expression of Recombinant CarE CCE001a
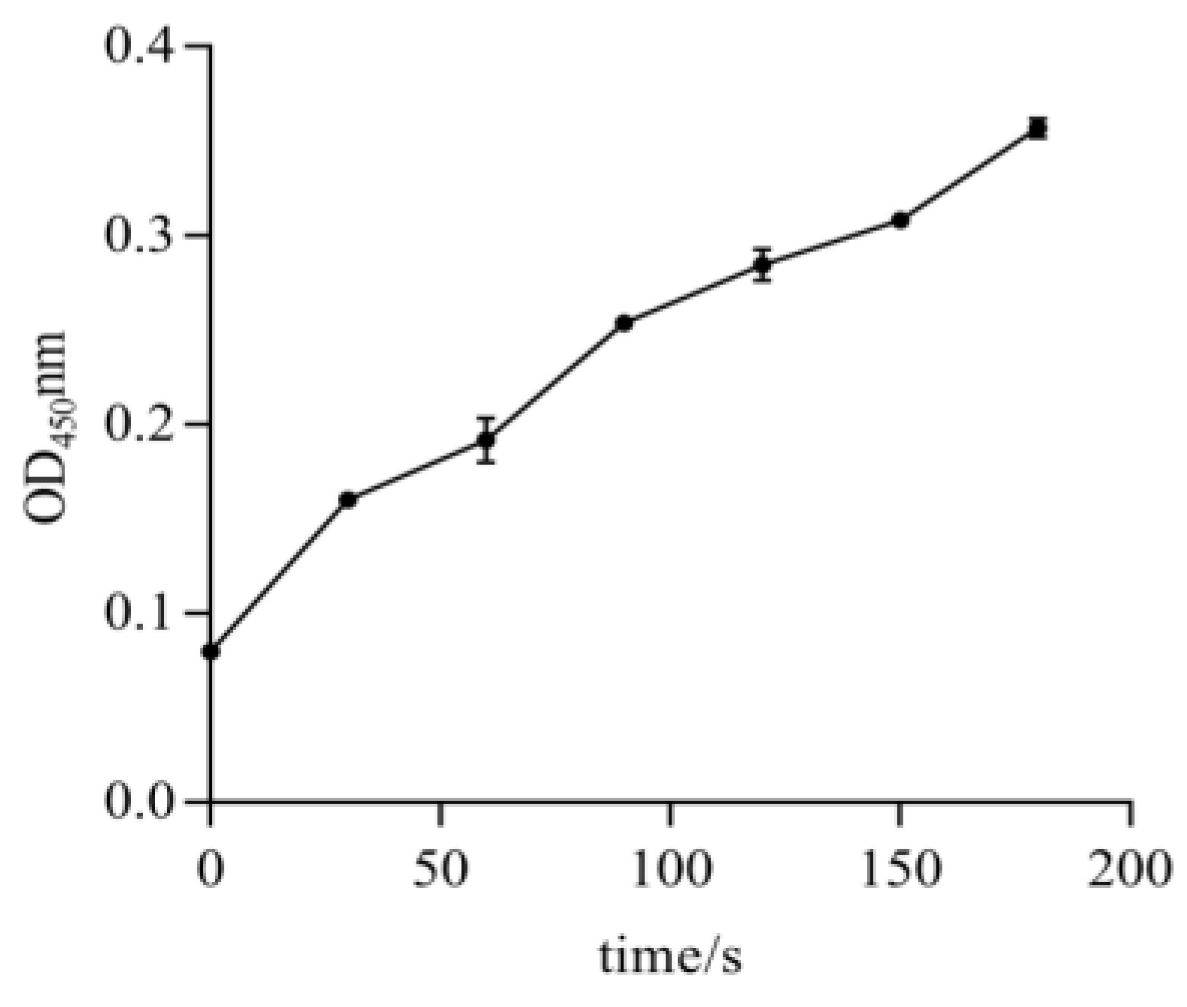
2.3. Exploration of CCE001a’s Activity on Model Substrates
2.4. Gossypol Analysis
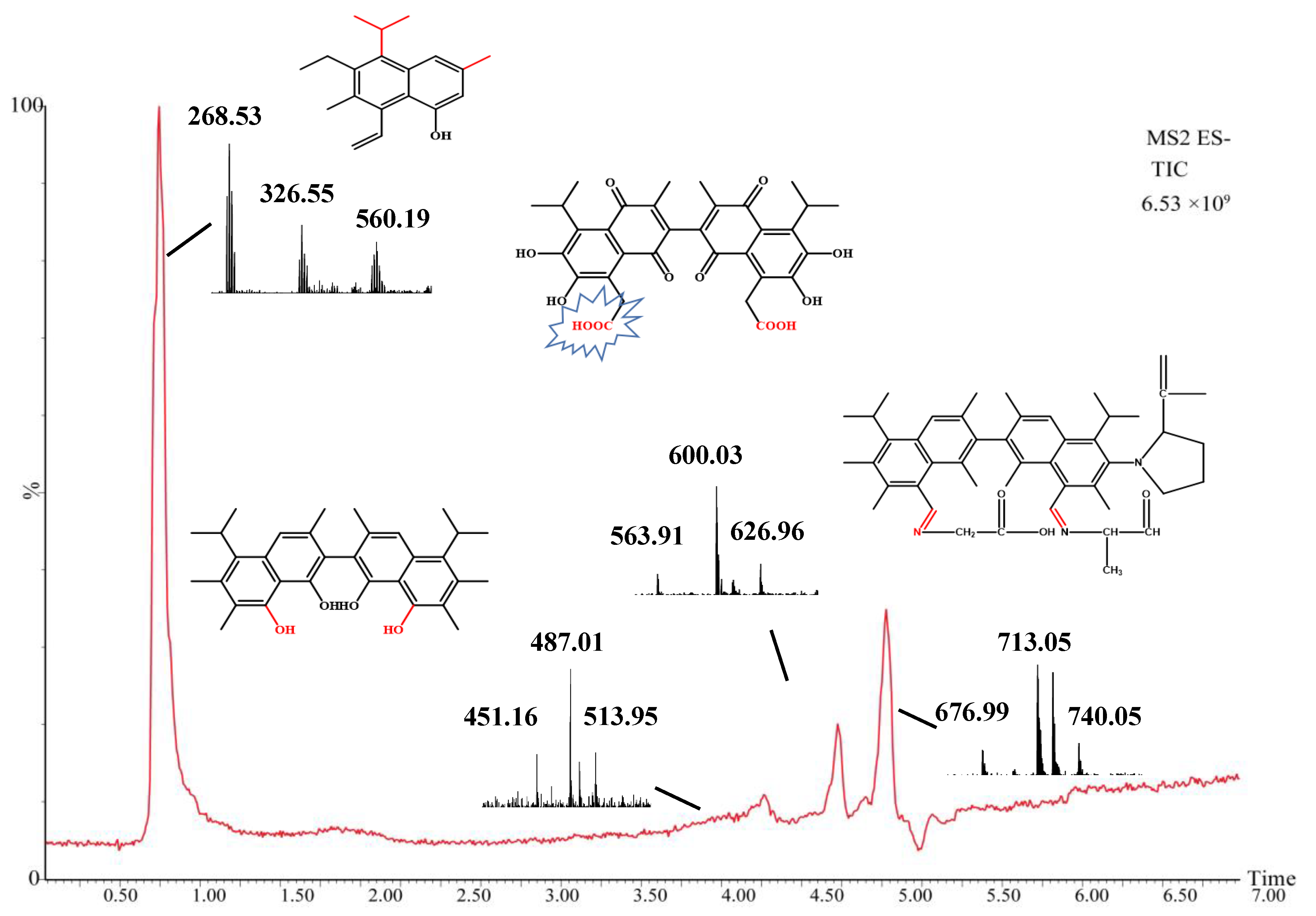
2.5. Mass Spectrometry Analysis of Intermediate Products
3. Conclusions
4. Materials and Methods
4.1. Source of Target Gene Sequence
4.2. cce001a Cloning and Expression Vector Construction
4.3. Electrotransformation, Screening, and Identification of Transformants with High Expression of CCE001a in P. pastoris
4.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blotting
4.5. CarE Activity Measurements
4.6. Ultra-High Performance Liquid Chromatography (UHPLC) Analysis of Gossypol
4.7. UPLC-Quadrupole Time of Flight Mass Spectrometry (UPLC-QTOF/MS) Analysis
4.7.1. UPLC Conditions
4.7.2. MS Conditions
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, T.; Xie, Q.; Li, C.; Li, C.; Mei, L.; Yu, J.Z.; Chen, J.; Zhu, S. Cotton roots are the major source of gossypol biosynthesis and accumulation. BMC Plant Biol. 2020, 20, 88. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.S.; Paulino, M.F.; Marcondes, M.I.; Rennó, L.N.; Mageste de Almeida, D.; Lopes, S.A.; Marquez, D.E.C.; Manso, M.R.; Gomes da Silva, A.; Valente, É.E.L. Cottonseed meal is a suitable replacement for soybean meal in supplements fed to Nellore heifers grazing Brachiaria decumbens. Anim. Prod. Sci. 2016, 57, 1893–1898. [Google Scholar] [CrossRef]
- Gadelha, I.C.; Fonseca, N.B.; Oloris, S.C.; Melo, M.M.; Soto-Blanco, B. Gossypol toxicity from cottonseed products. Sci. World J. 2014, 2014, 231635. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Robinson, E.H. Use of Cottonseed meal in aquatic animal diets: A review. N. Am. J. Aquac. 2006, 68, 14–22. [Google Scholar] [CrossRef]
- Yang, A.; Zhang, C.; Zhang, B.; Wang, Z.; Zhu, L.; Mu, Y.; Wang, S.; Qi, D. Effects of dietary cottonseed oil and cottonseed meal supplementation on liver lipid content, fatty acid profile and hepatic function in laying hens. Animals 2021, 11, 78. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, C.; Wu, Y.; Tang, Y. Metabolic engineering of gossypol in cotton. Appl. Microbiol. Biotechnol. 2013, 97, 6159–6165. [Google Scholar] [CrossRef]
- Willard, S.T.; Neuendorff, D.A.; Lewis, A.W.; Randel, R.D. Effects of free gossypol in the diet of pregnant and postpartum Brahman cows on calf development and cow performance. J. Anim. Sci. 1995, 73, 496–507. [Google Scholar] [CrossRef]
- Reiser, R.; Fu, H.C. The mechanism of gossypol detoxification by ruminant animals. J. Nutr. 1962, 76, 215–218. [Google Scholar] [CrossRef]
- Wang, W.K.; Li, W.J.; Wu, Q.C.; Wang, Y.L.; Li, S.L.; Yang, H.J. Isolation and identification of a rumen Lactobacillus bacteria and its degradation potential of gossypol in cottonseed meal during solid-state fermentation. Microorganisms 2021, 9, 2200. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Dai, L.; Liu, Y.; Cheng, M.; Chen, L. Isolation and characterization of a novel gossypol-degrading bacteria Bacillus subtilis strain Rumen Bacillus Subtilis. Asian-Australas. J. Anim. Sci. 2018, 31, 63–70. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Cockburn, A. Nitrite as undesirable substances in animal feed-Scientific opinion of the panel on contaminants in the food chain. EFSA J. 2009, 7, 1017. [Google Scholar] [CrossRef]
- Lim, W.; Ham, J.; Park, S.; Bae, H.; You, S.; Song, G. Gossypol induces disruption of spermatogenesis and steroidogenesis in male mice. J. Agric. Food Chem. 2019, 67, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, N.B.d.S.; Gadelha, I.C.N.; Oloris, S.C.S.; Soto-Blanco, B. Effectiveness of albumin-conjugated gossypol as an immunogen to prevent gossypol-associated acute hepatotoxicity in rats. Food Chem. Toxicol. 2013, 56, 149–153. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Presence of free gossypol in whole cottonseed. EFSA J. 2017, 15, e04850. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romero, A.C.; Calori-Domingues, M.A.; Abdalla, A.L.; Augusto, P.E.D. Evaluation of ozone technology as an alternative for degradation of free gossypol in cottonseed meal: A prospective study. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Howell, C.P.; Chen, F.; Yin, J.; Jiang, Y. Chapter 6 Gossypol-A polyphenolic compound from cotton plant. Adv. Food Nutr Res. 2009, 58, 215–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Z.; Sun, J.; Yang, X. A Study on the reduction of gossypol levels by mixed culture solid substrate fermentation of cottonseed meal. Asian-Australas. J. Anim. Sci. 2006, 19, 1314–1321. [Google Scholar] [CrossRef]
- Khalaf, M.A.; Meleigy, S.A. Reduction of free gossypol levels in cottonseed meal by microbial treatment. Int. J. Agric. Biol. 2008, 10, 185–190. [Google Scholar]
- Mellon, J.E.; Zelaya, C.A.; Dowd, M.K. Inhibitory effects of gossypol-related compounds on growth of Aspergillus flavus. Lett. Appl. Microbiol. 2011, 52, 406–412. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.Y.; Guo, J.L.; Weng, X.Y. Identification and proteomic analysis of a novel gossypol-degrading fungal strain. J. Sci. Food Agric. 2012, 92, 943–951. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Rajarathnam, S.; Shashirekha, M.N.; Bano, Z. Biodegradation of gossypol by the white oyster mushroom, Pleurotus florida, during culturing on rice straw growth substrate, supplemented with cottonseed powder. World J. Microbiol. Biotechnol. 2001, 17, 221–227. [Google Scholar] [CrossRef]
- Banerjee, S.; Maiti, T.K.; Roy, R.N. Enzyme producing insect gut microbes: An unexplored biotechnological aspect. Crit. Rev. Biotechnol. 2022, 42, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Penttinen, L.; Luo, H.; Zhang, Y.; Liu, B.; Yao, B.; Hakulinen, N.; Zhang, W.; Su, X. Patulin detoxification by recombinant manganese peroxidase from Moniliophthora roreri expressed by Pichia pastoris. Toxins 2022, 14, 440. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Cheng, Y.; Guo, X.; Li, M.; Chakrabarty, S.; Liu, K.; Wu, K.; Xiao, Y. Down-regulation of lysosomal protein ABCB6 increases gossypol susceptibility in Helicoverpa armigera. Insect Biochem. Mol. Biol. 2020, 122, 103387. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ruan, J.; Huang, J.; Fang, X.; Mao, Y.; Wang, L.; Chen, X.; Yang, C. Gossypol: Phytoalexin of cotton. Sci. China Life Sci. 2016, 59, 122–129. [Google Scholar] [CrossRef]
- Kulieva, A.M.; Dalimov, D.N.; Dorenskaya, G.M.; Charieva, O.V.; Rozengart, V.I.; Kugusheva, L.I.; Moralev, S.N.; Babaev, B.N.; Abduvakhabov, A.A. Biochemical investigation of cholinesterases and carboxylesterases from the cotton bollwormHeliothis armigera. Chem. Nat. Compd. 1994, 30, 116–120. [Google Scholar] [CrossRef]
- Yang, S.; Wu, H.; Xie, J.; Rantala, M.J. Depressed performance and detoxification enzyme activities of Helicoverpa armigera fed with conventional cotton foliage subjected to methyl jasmonate exposure. Entomol. Exp. Appl. 2013, 147, 186–195. [Google Scholar] [CrossRef]
- Mulrooney, J.E.; Parrott, W.L.; Jenkins, J.N. Tolerance of pyrethroid-resistant tobacco budworm (Lepidoptera: Noctuidae) larvae to gossypol and piperonyl butoxide. J. Econ. Entomol. 1993, 86, 1014–1018. [Google Scholar] [CrossRef]
- Despres, L.; David, J.P.; Gallet, C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef]
- Kim, K.K.; Song, H.K.; Shin, D.H.; Hwang, K.Y.; Choe, S.; Yoo, J.O.; Suh, S.W. Crystal structure of carboxylesterase from Pseudomonas fluorescens, an a/b hydrolase with broad substrate specificity. Structure 1997, 5, 1571–1584. [Google Scholar] [CrossRef]
- Heidel-Fischer, H.M.; Vogel, H. Molecular mechanisms of insect adaptation to plant secondary compounds. Curr. Opin. Insect Sci. 2015, 8, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Daud, M.K.; Zhu, S. Effects of pigment glands and gossypol on growth, development and insecticide-resistance of cotton bollworm (Heliothis armigera (Hübner)). Crop Prot. 2010, 29, 813–819. [Google Scholar] [CrossRef]
- Wu, G.; Harris, M.K.; Guo, J.; Wan, F. Temporal allocation of metabolic tolerance in the body of beet armyworm in response to three gossypol-cotton cultivars. Sci. China C Life Sci. 2009, 52, 1140–1147. [Google Scholar] [CrossRef]
- Yang, S.; Cao, Q.; Peng, K.; Xie, J. Jasmonic acid-treated cotton plant leaves impair larvae growth performance, activities of detoxification enzymes, and insect humoral immunity of cotton bollworm. Neotrop. Entomol. 2022, 51, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Liao, C.; Fu, X.; Holdbrook, R.; Wu, K.; Xiao, Y. Adaptive regulation of detoxification enzymes in Helicoverpa armigera to different host plants. Insect Mol. Biol. 2019, 28, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, N.; Ma, J.; Huang, L.; Liu, X. Effect of silencing CYP6B6 of Helicoverpa armigera (Lepidoptera: Noctuidae) on its growth, development, and insecticide tolerance. J. Econ. Entomol. 2016, 109, 2506–2516. [Google Scholar] [CrossRef]
- Kang, Z.; Huang, H.; Zhang, Y.; Du, G.; Chen, J. Recent advances of molecular toolbox construction expand Pichia pastoris in synthetic biology applications. World J. Microbiol. Biotechnol. 2017, 33, 19. [Google Scholar] [CrossRef]
- Duan, G.; Ding, L.; Wei, D.; Zhou, H.; Chu, J.; Zhang, S.; Qian, J. Screening endogenous signal peptides and protein folding factors to promote the secretory expression of heterologous proteins in Pichia pastoris. J. Biotechnol. 2019, 306, 193–202. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Vu, V.H.; Le, P.D.; Chu, H.M. High-level expression, purification and properties of an Endochitinase gene without signal peptide from Lecanicillium lecanii 43H in Pichia pastoris. Mol. Biol. Rep. 2018, 45, 1067–1075. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Molina, M.A.; Winnik, D.L.; Busi, M.V.; Farina, J.I.; Villalba, L.L.; Zapata, P.D. Isolation of a laccase-coding gene from the lignin-degrading fungus Phlebia brevispora BAFC 633 and heterologous expression in Pichia pastoris. J. Appl. Microbiol. 2018, 124, 1454–1468. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Farajnia, S.; Ghasemi, Y.; Mortazavi, M.; Zarghami, N.; Samadi, N. New developments in Pichia pastoris expression system, review and update. Curr. Pharm. Biotechnol. 2018, 19, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.S.; Zhao, C.X.; Xu, J.J.; Feng, C.; Li, Y.Q.; Dong, Y.L.; Ma, Z.Q. Identification and biochemical characterization of carboxylesterase 001G associated with insecticide detoxification in Helicoverpa armigera. Pestic. Biochem. Physiol. 2019, 157, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Teese, M.G.; Farnsworth, C.A.; Li, Y.; Coppin, C.W.; Devonshire, A.L.; Scott, C.; East, P.; Russell, R.J.; Oakeshott, J.G. Heterologous expression and biochemical characterisation of fourteen esterases from Helicoverpa armigera. PLoS ONE 2013, 8, e65951. [Google Scholar] [CrossRef]
- Xu, Z.; Kong, J.; Zhang, S.; Wang, T.; Liu, X. Comparison of Enzyme Secretion and Ferulic Acid Production by Escherichia coli Expressing Different Lactobacillus Feruloyl Esterases. Front. Microbiol. 2020, 1, 1. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, C.; Liu, L.; Huang, H. Effects of N-glycosylation on the biochemical properties of recombinant bEKL expressed in Pichia pastoris. Enzym. Microb. Technol. 2018, 114, 40–47. [Google Scholar] [CrossRef]
- Han, M.; Wang, X.; Ding, H.; Jin, M.; Yu, L.; Wang, J.; Yu, X. The role of N-glycosylation sites in the activity, stability, and expression of the recombinant elastase expressed by Pichia pastoris. Enzym. Microb. Technol. 2014, 54, 32–37. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Lu, M.; Ma, Z.; Cai, C.; Wang, Y.; Zhang, X. Bacterial expression and kinetic analysis of carboxylesterase 001D from Helicoverpa armigera. Int. J. Mol. Sci. 2016, 17, 493. [Google Scholar] [CrossRef]
- Luo, S.; Shu, C.; Xu, C.; Wang, R. Molecular cloning and expression in vitro of a carboxylesterase gene from the Glanville fritillary butterfly (Melitaea cinxia). Gene 2013, 524, 275–281. [Google Scholar] [CrossRef]
- Wang, Z.G.; Jiang, S.S.; Mota-Sanchez, D.; Wang, W.; Li, X.R.; Gao, Y.L.; Lu, X.P.; Yang, X.Q. Cytochrome P450-mediated lambda-cyhalothrin-resistance in a field strain of Helicoverpa armigera from northeast China. J. Agric. Food Chem. 2019, 67, 3546–3553. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Yuan, G.; Campbell, P.M.; Teese, M.G.; Russell, R.J.; Oakeshott, J.G.; Wu, Y. Overexpressed esterases in a fenvalerate resistant strain of the cotton bollworm, Helicoverpa armigera. Insect Biochem. Mol. Biol. 2011, 41, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Huang, J.Q.; Fang, X.; Tian, X.; Chen, P.; Lin, J.L.; Guo, X.X.; Li, J.X.; Fan, Z.; Song, W.M.; Chen, F.Y.; et al. Aromatization of natural products by a specialized detoxification enzyme. Nat. Chem. Biol. 2020, 16, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Hron, R.J.; Kuk, M.S.; Abraham, G. Determination of free and total gossypol by high performance liquid chromatography. J. Am. Oil Chem. Soc. 1990, 67, 182–187. [Google Scholar] [CrossRef]
- Cheng, C.; Cun-Xi, N.; Jing, L.; Yong-Qiang, W.; Yan-Feng, L.; Wen-Xia, G.; Wen-Ju, Z. Validated method to determine (±)-gossypol inCandida tropicalisculture by high-performance liquid chromatography. Acta Chromatogr. 2018, 30, 269–273. [Google Scholar] [CrossRef]
- Dowd, M.K. Stability of the gossypol-amine adducts used for chromatographic measurement of total and isomeric gossypol. J. Am. Oil Chem. Soc. 2020, 97, 671–675. [Google Scholar] [CrossRef]
- Chen, C.; Pi, W.; Zhang, Y.; Nie, C.X.; Liang, J.; Ma, X.; Wang, Y.; Ge, W.; Zhang, W.J. Effect of a functional recombinant cytochrome P450 enzyme of Helicoverpa armigera on gossypol metabolism co-expressed with NADPH-cytochrome P450 reductase in Pichia pastoris. Pestic. Biochem. Physiol. 2019, 155, 15–25. [Google Scholar] [CrossRef]
- Beyazit, N.; Cakran, H.S.; Cabir, A.; Akiscan, Y.; Demetgul, C. Synthesis, characterization and antioxidant activity of chitosan Schiff base derivatives bearing (−)-gossypol. Carbohydr. Polym. 2020, 240, 116333. [Google Scholar] [CrossRef]
- Vu, V.V.; Nhung, T.T.; Thanh, N.T.; Chinh, L.V.; Tien, V.D.; Thuy, V.T.; Thi Thao, D.; Nam, N.H.; Koeckritz, A.; Vu, T.K. Synthesis and biological evaluation of new (−)-gossypol-derived Schiff bases and hydrazones. J. Chem. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.; Liu, Y.; Wang, Q. Antiviral mechanism study of gossypol and its Schiff base derivatives based on reactive oxygen species (ROS). RSC Adv. 2016, 6, 87637–87648. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Luo, X.; Fan, Y.; Zheng, Z.; He, Z.; Yin, R.; Meng, T.; Xu, S.; Pan, Y.; et al. Intramolecular annulation of gossypol by laccase to produce safe cottonseed protein. Front. Chem. 2020, 8, 583176. [Google Scholar] [CrossRef] [PubMed]
- Merali, Z.; Ross, S.; Pare, G. The pharmacogenetics of carboxylesterases: CES1 and CES2 genetic variants and their clinical effect. Drug Metabol. Drug Interact. 2014, 29, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Hosokawa, M. Structure, function and regulation of carboxylesterases. Chem. Biol. Interact. 2006, 162, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.W.; Jin, Q.; Wang, D.D.; Qian, Q.K.; Hao, D.C.; Ge, G.B.; Yang, L. Carboxylesterase inhibitors: An update. Curr. Med. Chem. 2018, 25, 1627–1649. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef]
- Wang, D.D.; Zou, L.W.; Jin, Q.; Hou, J.; Ge, G.B.; Yang, L. Recent progress in the discovery of natural inhibitors against human carboxylesterases. Fitoterapia 2017, 117, 84–95. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Microbial carboxyl esterases: Classification, properties and application in biocatalysis. FEMS Microbiol. Rev. 2002, 26, 73–81. [Google Scholar] [CrossRef]
- Katoh, N.; Kimura, K. Inhibition by gossypol of cyclic nucleotide-independent phosvitin kinase from pig testis. Nihon Juigaku Zasshi 1989, 51, 105–109. [Google Scholar] [CrossRef]
- Gamboa, D.A.; Calhoun, M.C.; Kuhlmann, S.W.; Haq, A.U.; Bailey, C.A. Tissue distribution of gossypol enantiomers in broilers fed various cottonseed meals. Poult. Sci. 2001, 80, 920–925. [Google Scholar] [CrossRef]
- Abou-Donia, M.B. Physiological effects and metabolism of gossypol. Am. J. Physiol. Endocrinol. Metab. 1976, 61, 6. [Google Scholar] [CrossRef]
- Krempl, C.; Sporer, T.; Reichelt, M.; Ahn, S.J.; Heidel-Fischer, H.; Vogel, H.; Heckel, D.G.; Joussen, N. Potential detoxification of gossypol by UDP-glycosyltransferases in the two Heliothine moth species Helicoverpa armigera and Heliothis virescens. Insect Biochem. Mol. Biol. 2016, 71, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.A.; Stipanovic, R.D.; Howell, C.R.; Fryxell, P.A. Antimicrobial terpenoids of Gossypium: Hemigossypol, 6-methoxyhemigossypol and 6-deoxyhemigossypol. Phytochemistry 1975, 14, 225–231. [Google Scholar] [CrossRef]
- Heywood, R. The toxicology of gossypol acetic acid and (−)-gossypol. Contraception 1988, 37, 185–190. [Google Scholar] [CrossRef]
- Abou-Donia, M.B.; Dieckert, J.W. Metabolic fate of gossypol: The metabolism of [14C]gossypol in swine. Toxicol. Appl. Pharmacol. 1975, 31, 32–46. [Google Scholar] [CrossRef]




| Grouping | Gossypol Content (μg/mL) | Detoxification Rate (%) | ||
|---|---|---|---|---|
| TG | FG | TG | FG | |
| Bl-1 | 406.62 ± 0.09 a | 258.63 ± 0.01 a | 10 | 42 |
| Co-1 | 85.76 ± 0.07 b | 48.15 ± 0.07 b | 80 | 81 |
| MY-1 | 42.34 ± 0.05 c | 27.43 ± 0.03 c | 90 | 89 |
| Compound | Experimental Mass (m/z) | Theoretical Mass (m/z) | Retention Time (min) | Molecular Formula |
|---|---|---|---|---|
| Gossypol | 517.18 | 518.19 | 19.7 | C30H30O8 |
| M0 | 268.18 | 269.19 | 0.75 | C19H24O |
| M1 | 488.22 | 489.26 | 4.97 | C31H36O5 |
| M2 | 600.25 | 601.25 | 4.97 | C32H30O12 |
| M3 | 713.46 | 714.46 | 5.28 | C47H59N3O3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yang, X.; Huang, R.; Nie, C.; Niu, J.; Chen, C.; Zhang, W. Biodegradation of Free Gossypol by Helicoverpa armigera Carboxylesterase Expressed in Pichia pastoris. Toxins 2022, 14, 816. https://doi.org/10.3390/toxins14120816
Zhang L, Yang X, Huang R, Nie C, Niu J, Chen C, Zhang W. Biodegradation of Free Gossypol by Helicoverpa armigera Carboxylesterase Expressed in Pichia pastoris. Toxins. 2022; 14(12):816. https://doi.org/10.3390/toxins14120816
Chicago/Turabian StyleZhang, Li, Xiaolong Yang, Rongzheng Huang, Cunxi Nie, Junli Niu, Cheng Chen, and Wenju Zhang. 2022. "Biodegradation of Free Gossypol by Helicoverpa armigera Carboxylesterase Expressed in Pichia pastoris" Toxins 14, no. 12: 816. https://doi.org/10.3390/toxins14120816
APA StyleZhang, L., Yang, X., Huang, R., Nie, C., Niu, J., Chen, C., & Zhang, W. (2022). Biodegradation of Free Gossypol by Helicoverpa armigera Carboxylesterase Expressed in Pichia pastoris. Toxins, 14(12), 816. https://doi.org/10.3390/toxins14120816





