Cuspidothrix Is the First Genetically Proved Anatoxin A Producer in Bulgarian Lakes and Reservoirs
Abstract
1. Introduction
2. Results
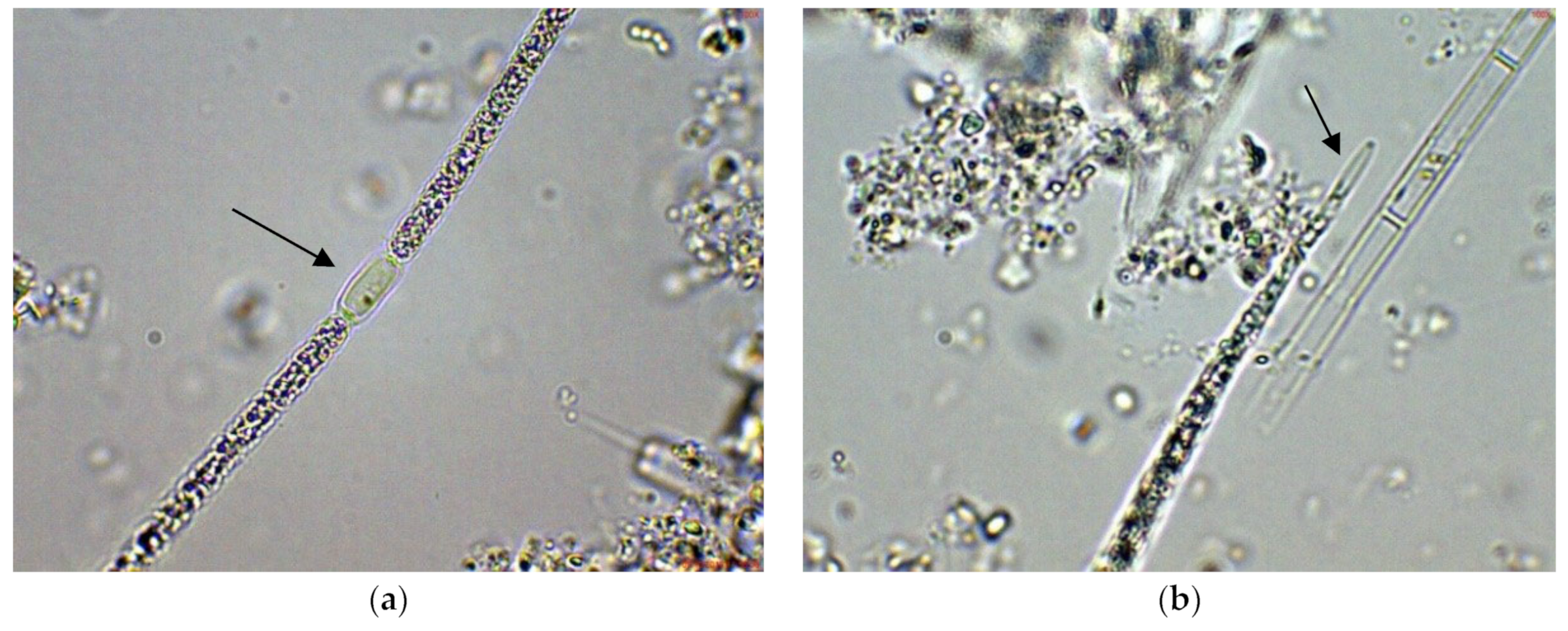
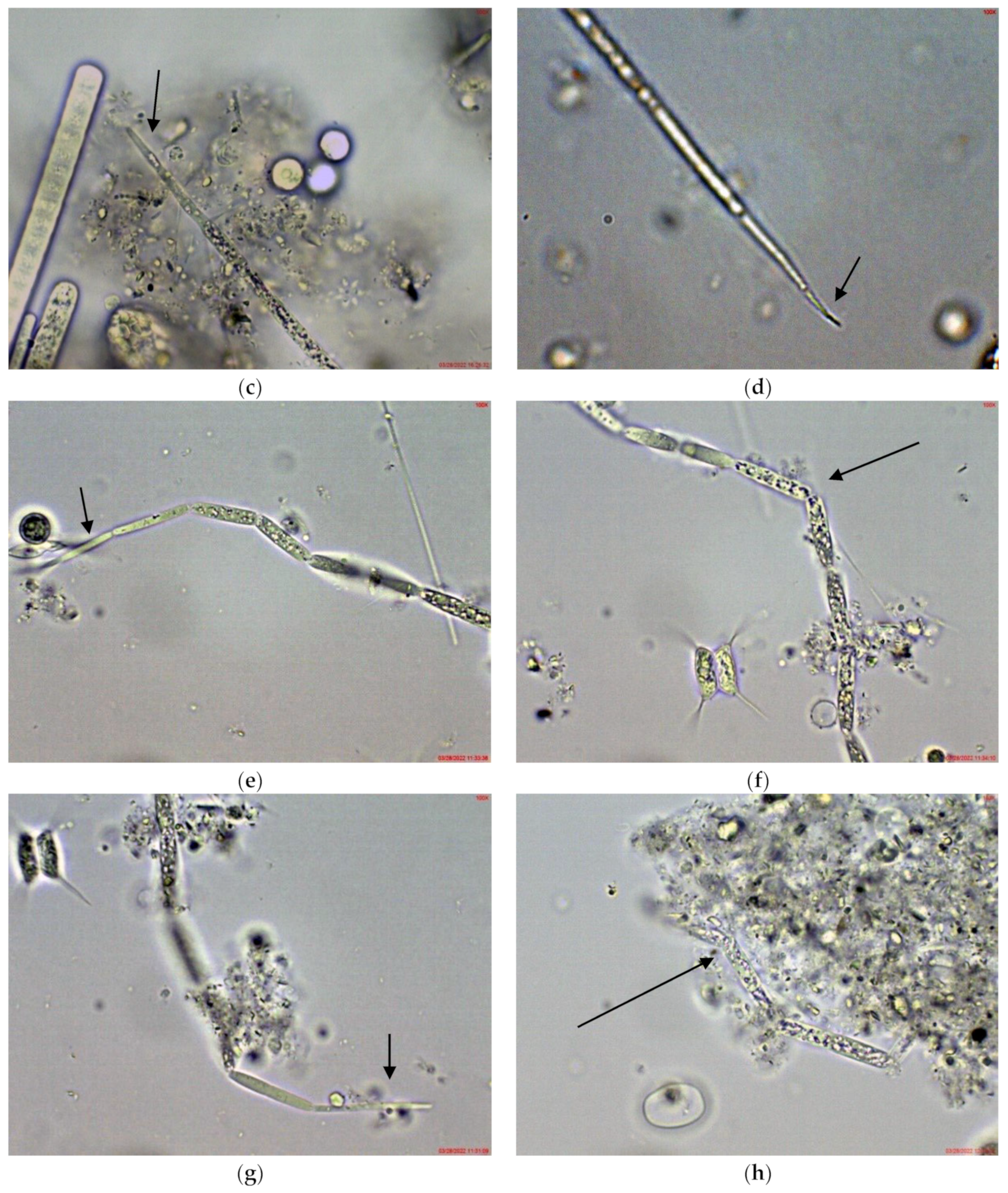
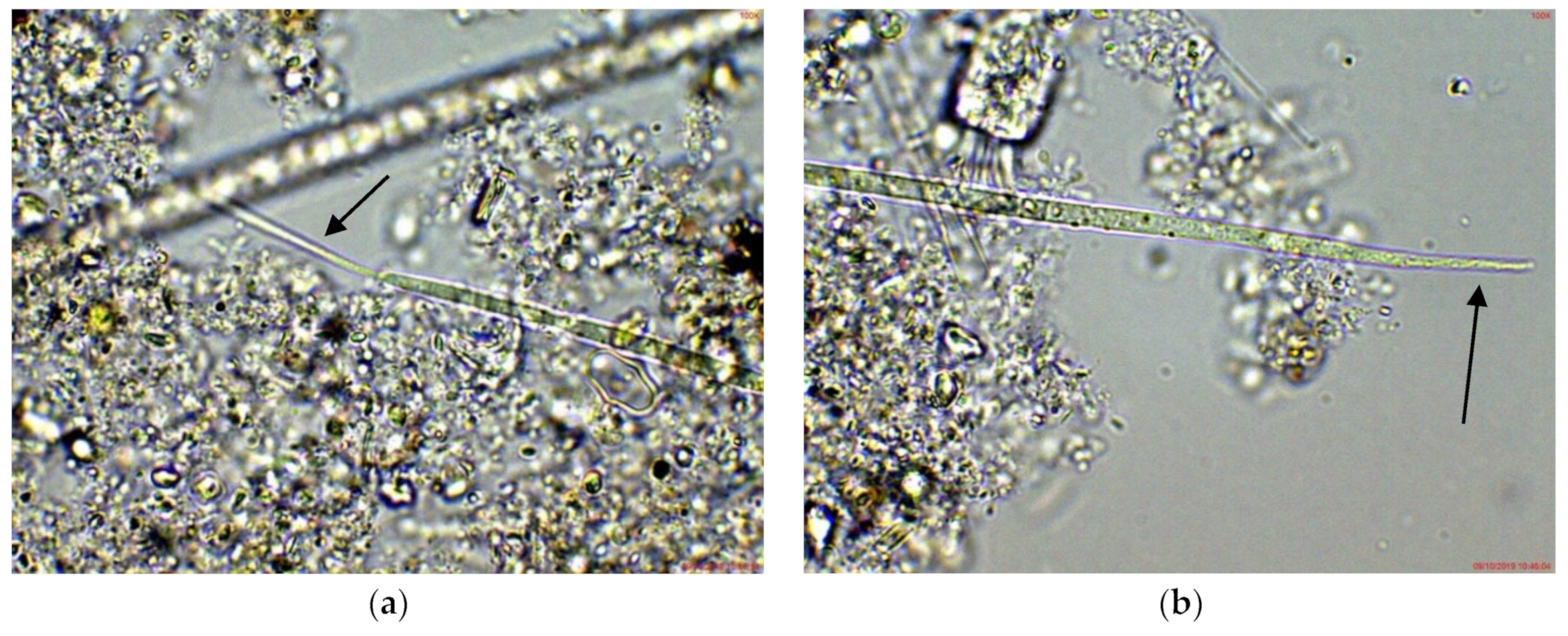
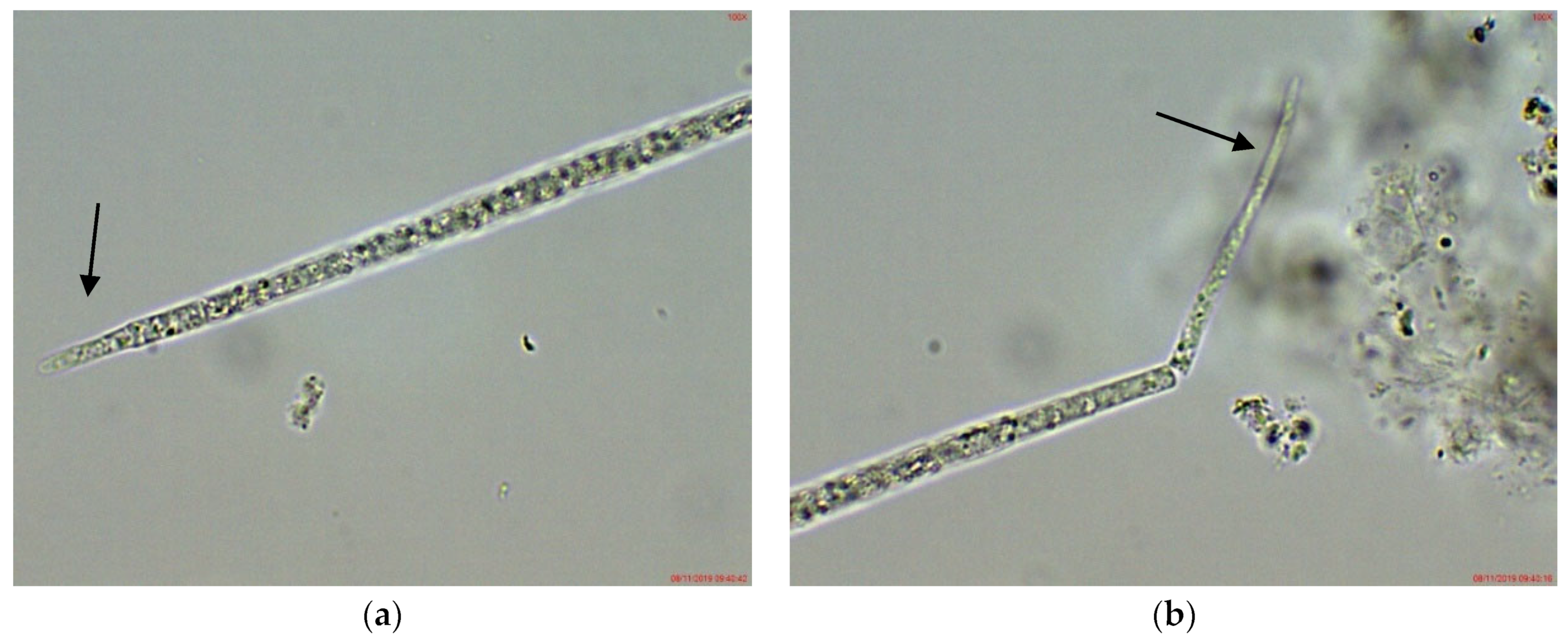
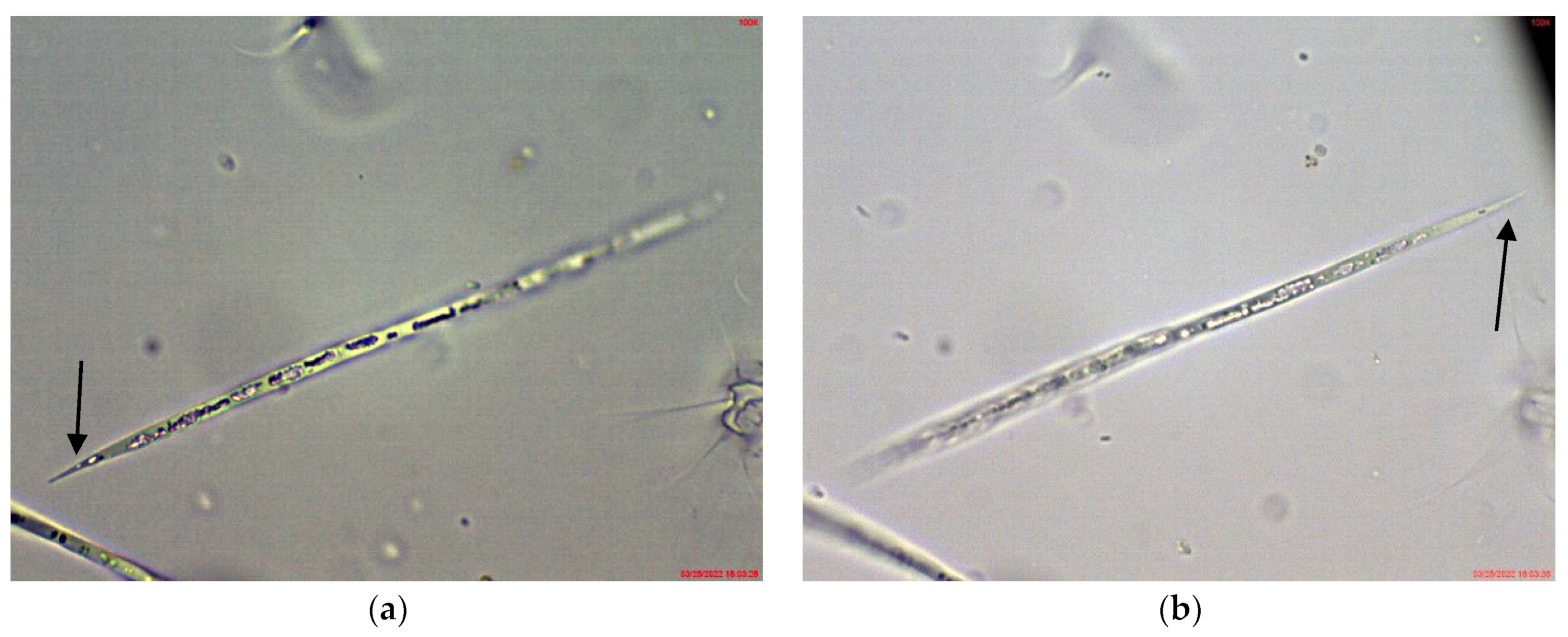
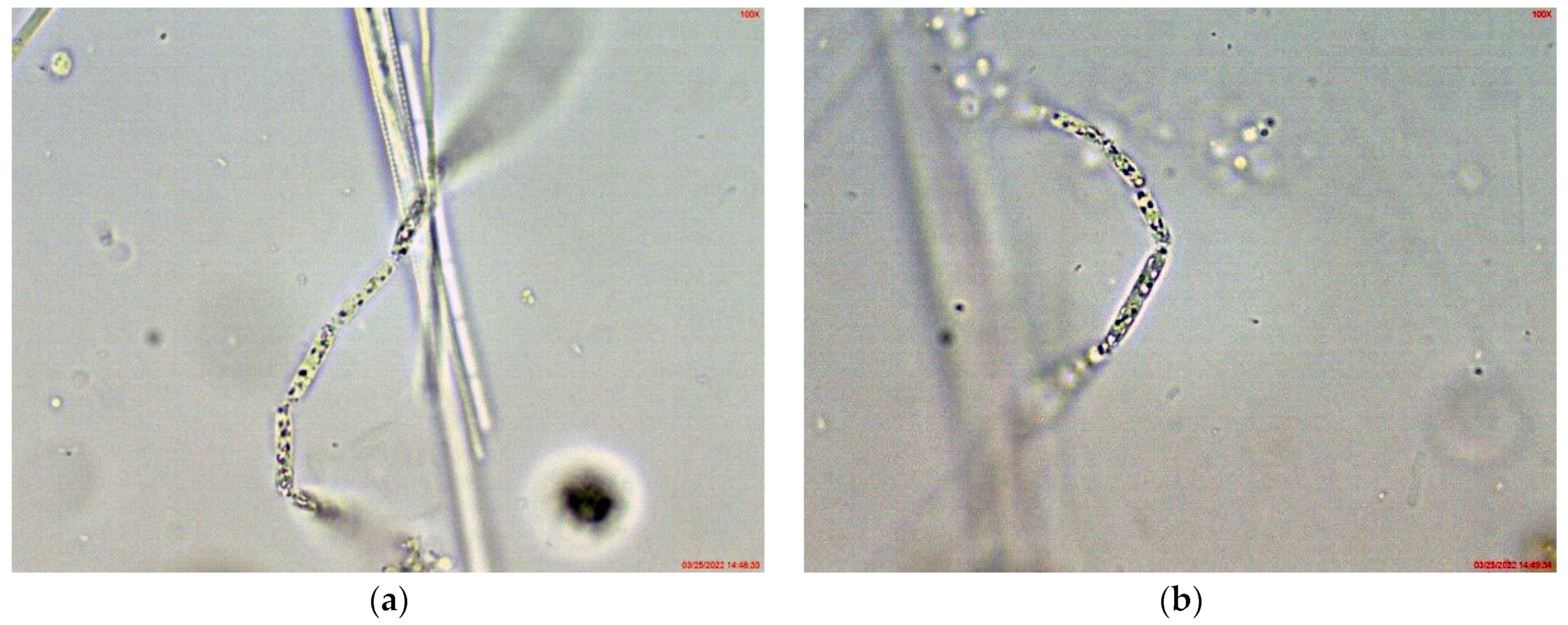
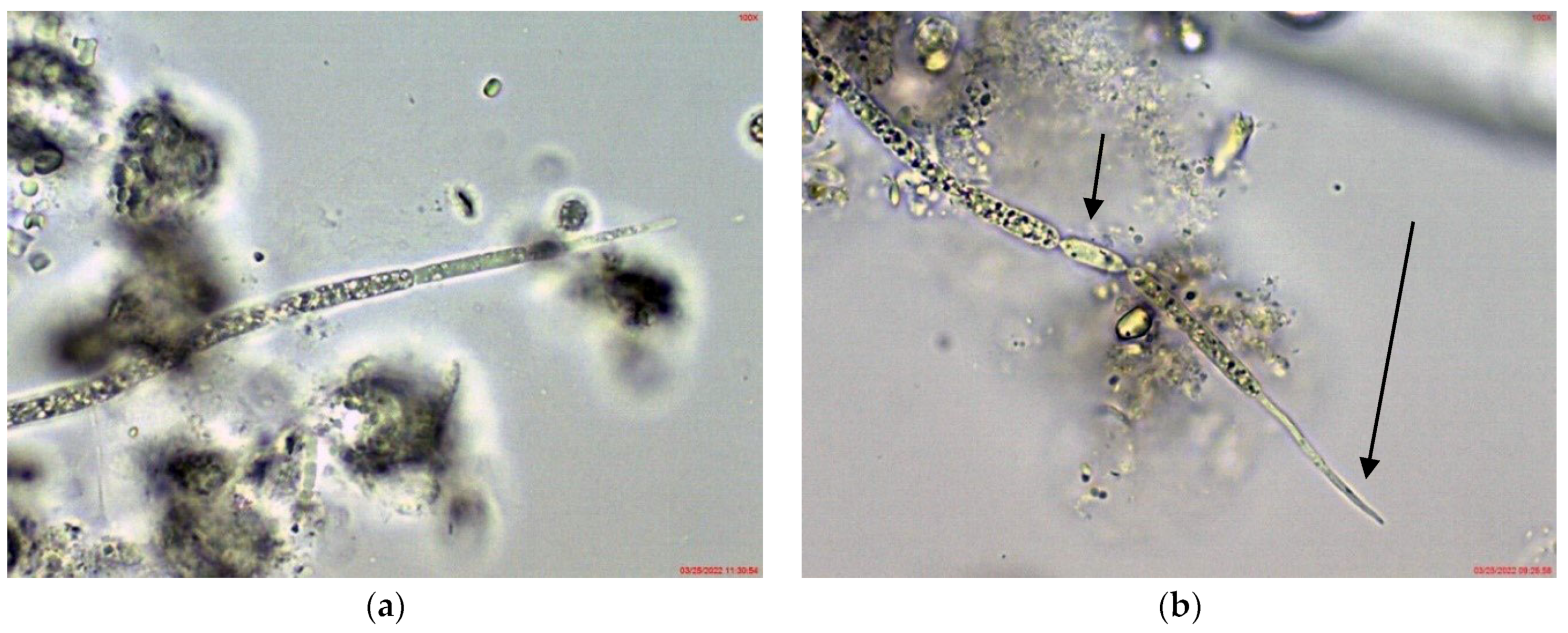
2.1. Results from Light Microscopy Observations
2.2. Results from Genetic-Molecular Studies
3. Discussion
- Our first finding of C. tropicalis in Bulgaria in June 2018 (in Vaya, Durankulak and Poroy) was already documented by its inclusion in the list of potential producers of another important cyanotoxin—cylindrospermopsin [39]. However, there we had not published more detailed LM and the supporting genetic data, which we provide in this paper, extending information on its occurrence by data from samplings conducted in 2019. In addition, here, based on our long expertise in phytoplankton studies in Bulgarian waterbodies, we would like to underline the presumed alien character of this tropical species. This statement is strongly supported by the fact that it was observed mainly in coastal waterbodies along the Black Sea, which provide resting and nesting sites on the important bird migration route Via Pontica, used by waterfowl and other species flying back from tropical Africa [47].
4. Materials and Methods
4.1. Studied Sites and Field Sampling
4.2. Microscopic (LM) Processing of the Phytoplankton Samples and Species Identification
4.3. Molecular and Genetic Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meriluoto, J.; Spoof, L.; Codd, J. (Eds.) Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons, Ltd.: Chichester, UK, 2017. [Google Scholar] [CrossRef]
- Christensen, V.G.; Khan, E. Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Sci. Total Environ. 2020, 736, 139515. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cyanobacterial Toxins: Anatoxin-a and Analogues. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Wood, S.A.; Puddick, J.; Fleming, R.; Heussner, A.H. Detection of anatoxin-producing Phormidium in a New Zealand farm pond and an associated dog death. New Zealand J. Bot. 2017, 55, 36–46. [Google Scholar] [CrossRef]
- Rantala-Ylinen, A.; Känä, S.; Wang, H.; Rouhiainen, L.; Wahlsten, M.; Rizzi, E.; Berg, K.; Gugger, M.; Sivonen, K. Anatoxin-a synthetase gene cluster of the cyanobacterium Anabaena sp. strain 37 and molecular methods to detect potential producers. Appl Environ. Microbiol. 2011, 77, 7271–7278. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.M.; Mueller, R.S.; Shepardson, J.W.; Landry, Z.C.; Morré, J.T.; Maier, C.S.; Hardy, F.G.; Dreher, T.W. Structural and functional analysis of the finished genome of the recently isolated toxic Anabaena sp. WA102. BMC Genom. 2016, 17, 457. [Google Scholar] [CrossRef] [PubMed]
- Testai, E.; Scardala, S.; Vichi, S.; Buratti, F.M.; Funari, E. Risk to human health associated with the environmental occurrence of cyanobacterial neurotoxic alkaloids anatoxins and saxitoxins. Crit. Rev. Toxicol. 2016, 46, 385–419. [Google Scholar] [CrossRef]
- Ballot, A.; Fastner, J.; Lentz, M.; Wiedner, C. First report of anatoxin-a-producing cyanobacterium Aphanizomenon issatschenkoi in northeastern Germany. Toxicon 2010, 56, 964–971. [Google Scholar] [CrossRef]
- Shams, S.; Capelli, C.; Cerasino, L.; Ballot, A.; Dietrich, D.R.; Sivonen, K.; Salmaso, N. Anatoxin-a producing Tychonema (cyanobacteria) in European waterbodies. Water Res. 2015, 69, 68–79. [Google Scholar] [CrossRef]
- Tao, S.; Wang, S.; Song, L.; Gan, N. Understanding the differences in the growth and toxin production of anatoxin-producing Cuspidothrix issatschenkoi cultured with Inorganic and organic N sources from a new perspective: Carbon/Nitrogen metabolic blance. Toxins 2020, 12, 724. [Google Scholar] [CrossRef]
- Bouma-Gregson, K.; Olm, M.R.; Probst, A.J.; Anantharaman, K.; Power, M.E.; Banfield, J.F. Impacts of microbial assemblage and environmental conditions on the distribution of anatoxin-a producing cyanobacteria within a river network. ISME J. 2019, 13, 1618–1634. [Google Scholar] [CrossRef]
- Park, H.D.; Watanabe, M.F.; Harda, K.; Nagai, H.; Suzuki, M.; Watanabe, M.; Hayashi, H. Hepatotoxin (microcystin) and neurotoxin (anatoxin-a) contained in natural blooms and strains of cyanobacteria from Japanese freshwaters. Nat. Toxins 1993, 1, 353–360. [Google Scholar] [CrossRef]
- Paerl, H.W.; Barnard, M.A. Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human- and climatically-altered world. Harmful Algae 2020, 96, 101845. [Google Scholar] [CrossRef] [PubMed]
- Al-Lay, J.K.; Poon, G.K.; Codd, G.A. Isolation and purification of peptide and alkaloid toxins from Anabaena flos-aquae using high performance thin-layer chromatography. J. Microbiol. Methods 1998, 7, 251–258. [Google Scholar] [CrossRef]
- Harada, K.-I.; Ogawa, K.; Kimura, Y.; Murata, H.; Suzuki, M.; Thorn, P.M.; Evans, W.R.; Carmichael, W.W. Microcystins from Anabaena flos-aquae NRC 525- 17. Chem. Res. Toxicol. 1991, 4, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management; E & FN Spon: London, UK, 1999. [Google Scholar]
- Gugger, M.; Lenoir, S.; Berger, C.; Ledreux, A.; Druart, J.C.; Humbert, J.F.; Guette, K.; Bernard, C. First report in a river in France of the benthic cyanobacterium Phormidium favosum producing anatoxin-a associated with dog neurotoxicosis. Toxicon 2005, 45, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Puschner, B.; Hoff, B.; Tor, E.R. Diagnosis of anatoxin-a poisoning in dogs from North America. J. Vet. Diagn. Invest. 2008, 20, 80–92. [Google Scholar] [CrossRef]
- Edwards, C.; Beattie, K.A.; Scrimgeour, C.M.; Codd, G.A. Identifcation of anatoxin-a in benthic cyanobacteria (blue-green algae) and in associated dog poisonings at Loch Insh, Scotland. Toxicon 1992, 30, 1165–1175. [Google Scholar] [CrossRef]
- Fastner, J.; Beulker, C.; Geiser, B.; Hoffmann, A.; Kröger, R.; Teske, K.; Hoppe, J.; Mundhenk, L.; Neurath, H.; Sagebiel, D.; et al. Fatal neurotoxicosis in dogs associated with tychoplanktic, anatoxin-a producing Tychonema sp. in mesotrophic Lake Tegel, Berlin. Toxins 2018, 10, 60. [Google Scholar] [CrossRef]
- Wood, S.A.; Heath, M.; McGregor, G.; Holland, P.T.; Munday, R.; Ryan, K. Identification of a benthic microcystin producing filamentous cyanobacterium (Oscillatoriales) associated with a dog poisoning in New Zealand. Toxicon 2010, 55, 897–903. [Google Scholar] [CrossRef]
- John, N.; Baker, L.; Ansell, B.R.E.; Newham, S.; Crosbie, N.D.; Jex, A.R. First report of anatoxin-a producing cyanobacteria in Australia illustrates need to regularly up-date monitoring strategies in a shifting global distribution. Sci. Rep. 2019, 9, 10894. [Google Scholar] [CrossRef]
- Bauer, F.; Fastner, J.; Bartha-Dima, B.; Breuer, W.; Falkenau, A.; Mayer, C.; Raeder, U. Mass occurrence of anatoxin-a- and dihydroanatoxin-a-producing Tychonema sp. in mesotrophic reservoir Mandichosee (River Lech, Germany) as a cause of neurotoxicosis in dogs. Toxins 2020, 12, 726. [Google Scholar] [CrossRef]
- Blahova, L.; Sehnal, L.; Lepsova-Skacelova, O.; Szmucova, V.; Babica, P.; Hilscherova, K.; Teikari, J.; Sivonen, K.; Blaha, L. Occurrence of cylindrospermopsin, anatoxin-a and their homologs in the southern Czech Republic—Taxonomical, analytical, and molecular approaches. Harmful Algae 2021, 108, 102101. [Google Scholar] [CrossRef] [PubMed]
- Gorham, P.R.; McLachlan, J.; Hammer, U.T.; Kim, W.K. Isolation and culture of toxic strains of Anabaena flos-aquae (Lyngb.) de Bréb. SIL Proc. 1964, 15, 796–804. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Biggs, D.F.; Gorham, P.R. Toxicology and pharmacological action of Anabaena flos-aquae toxin. Science 1975, 187, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Gorham, P.R.; Biggs, D.F. Two laboratory case studies on the oral toxicity to calves of the freshwater cyanophyte (blue-green alga) Anabaena flos-aquae NRC-44-1. Can. Vet. J. 1977, 18, 71–75. [Google Scholar]
- Devlin, J.; Edwards, O.; Gorham, P.; Hunter, N.; Pike, R.; Stavric, B. Anatoxin-a, a toxic alkaloid from Anabaena flos-aquae NRC-44h. Can. J. Chem. 1977, 55, 1367–1371. [Google Scholar] [CrossRef]
- Botana, L.M.; James, K.; Crowley, J.; Duphard, J.; Lehane, M.; Furey, A. Anatoxin-a and analogues: Discovery, distribution, and toxicology. In Phycotoxins: Chemistry and Biochemistry, Botana, L., ed; Blackwell Publishing: Oxford, UK, 2007; pp. 141–158. [Google Scholar] [CrossRef]
- Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. “Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a. Environ. Int. 2007, 33, 1070–1089. [Google Scholar] [CrossRef]
- Adamski, M.; Zimolag, E.; Kaminski, A.; Drukała, J.; Bialczyk, J. Effects of cylindrospermopsin, its decomposition products, and anatoxin-a on human keratinocytes. Sci. Total Environ. 2020, 765, 142670. [Google Scholar] [CrossRef]
- Cerasino, L.; Salmaso, N. Co-occurrence of anatoxin-a and microcystins in Lake Garda and other deep subalpine lakes: Co-occurrence of anatoxin-a and microcystins in Lake Garda. Adv. Oceanogr. Limnol. 2020, 11, 11–21. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Stoyneva-Gärtner, M.P.; Uzunov, B.A.; Descy, J.-P.; Gärtner, G.; Draganova, P.H.; Borisova, C.I.; Pavlova, V.; Mitreva, M. Pilot application of drone-observations and pigment marker detection by HPLC in the studies of CyanoHABs in Bulgarian inland waters. Mar. Freshw. Res. 2019, 71, 606–616. [Google Scholar] [CrossRef]
- Michev, T.; Stoyneva, M. (Eds.) Inventory of Bulgarian Wetlands and Their Biodiversity; Elsi-M: Sofia, Bulgaria, 2007. [Google Scholar]
- Stoyneva-Gärtner, M.P.; Descy, J.-P.; Latli, A.; Uzunov, B.; Pavlova, V.; Bratanova, Z.; Babica, P.; Maršálek, B.; Meriluoto, J.; Spoof, L. Assessment of cyanoprokaryote blooms and of cyanotoxins in Bulgaria in a 15-years period (2000-2015). Adv. Oceanogr. Limnol. 2017, 8, 131–152. [Google Scholar] [CrossRef][Green Version]
- Teneva, I.; Mladenov, R.; Belkinova, D.; Dimitrova-Dyulgerova, I.; Dzhambazov, B. Phytoplankon community of the drinking after supply reservoir Borovitsa (South Bulgaria) with an emphasis on cyanotoxins and water quality. Cent. Eur. J. Biol. 2010, 5, 231–239. [Google Scholar] [CrossRef]
- Komárek, J. Cyanoprokaryota. 3rd Part: Heterocytous Genera. In Süßwasserflora von Mitteleuropa; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier, Spektrum Akad. Verl.: Heidelberg, Germany, 2014. [Google Scholar]
- Stefanova, K.; Radkova, M.; Uzunov, B.; Gärtner, G.; Stoyneva-Gärtner, M.P. Pilot search for cylindrospermopsin-producers in nine shallow Bulgarian waterbodies reveals nontoxic strains of Raphidiopsis raciborskii, R. mediterranea and Chrysosporum bergii. Biotechnol. Biotechnol. Equip. 2020, 34, 384–394. [Google Scholar] [CrossRef]
- Radkova, M.; Stefanova, K.; Uzunov, B.; Gärtner, G.; Stoyneva-Gärtner, M. Morphological and molecular identification of microcystin-producing cyanobacteria in nine shallow Bulgarian water bodies. Toxins 2020, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Rajaniemi, P.; Komárek, J.; Hoffmann, L.; Hrouzek, P.; Kastocská, K.; Sivonen, K. Taxonomic consequences from the combined molecular and phenotype evaluation of selected Anabaena and Aphanizomenon strains. Algol. Stud. 2005, 117, 371–391. [Google Scholar] [CrossRef]
- Uzunov, B.; Stefanova, K.; Radkova, M.; Descy, J.-P.; Gärtner, G.; Stoyneva-Gärtner, M. Microcystis species and their toxigenic strains in phytoplankton of ten Bulgarian wetlands (August 2019). Botanica 2021, 27, 77–94. Available online: 09_uzunov_et_al__botanica27_12021_60d3db833f272.pdf(gamtc.lt) (accessed on 7 November 2022). [CrossRef]
- Stoyneva-Gärtner, M.; Stefanova, K.; Descy, J.-P.; Uzunov, B.; Radkova, M.; Pavlova, V.; Mitreva, M.; Gärtner, G. Microcystis aeruginosa and M. wesenbergii were the primary planktonic microcystin producers in several Bulgarian waterbodies (August 2019). Appl. Sci. 2021, 11, 357. [Google Scholar] [CrossRef]
- Méjean, A.; Paci, G.; Gautier, V.; Ploux, O. Biosynthesis of anatoxin-a and analogues (anatoxins) in cyanobacteria. Toxicon 2014, 91, 15–22. [Google Scholar] [CrossRef]
- NCBI: National Centre for Biotechnology Information (NCBI). Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 12 May 2021).
- BLAST: Basic Local Alignment Search Tool (BLAST). Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 12 May 2021).
- Michev, T.M.; Profirov, L.A.; Karaivanov, N.P.; Michev, B.T. Migration of soaring birds over Bulgaria. Acta Zool. Bulg. 2012, 64, 33–41. [Google Scholar]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Stoyneva-Gärtner, M.P.; Morana, C.; Borges, A.V.; Okello, W.; Bouillon, S.; Deirmendjian, L.; Lambert, T.; Roland, F.; Nankabirwa, A.; Nabafu, E.; et al. Diversity and ecology of phytoplankton in Lake Edward (East Africa): Present status and long-term changes. JGLR 2020, 46, 741–751. [Google Scholar] [CrossRef]
- Stoyneva, M.P. Development of the phytoplankton of the shallow Srebarna lake (North-Eastern Bulgaria) across the trophic gradient. Hydrobiologia 1998, 369, 259–367. [Google Scholar] [CrossRef]
- Deirmendjian, L.; Descy, J.-P.; Morana, C.; Okello, W.; Stoyneva-Gärtner, M.P.; Bouillon, S.; Borges, A.V. Limnological changes in Lake Victoria since the mid-20th century. Freshw. Biol. 2021, 66, 1630–1648. [Google Scholar] [CrossRef]
- Namikoshi, M.; Murakami, T.; Watanabe, M.F.; Oda, T.; Yamada, J.; Tsujimura, S.; Nagai, H.; Oishi, S. Simultaneous production of homoanatoxin-a, anatoxin-a, and a new non-toxic 4-hydroxyhomoanatoxin-a by the cyanobacterium Raphidiopsis mediterranea Skuja. Toxicon 2003, 42, 533–538. [Google Scholar] [CrossRef]
- Watanabe, M.F.; Tsujimura, S.; Oishi, S.; Niki, T.; Namikoshi, M. Isolation and identification of homoanatoxina from a toxic strain of the cyanobacterium Raphidiopsis mediterranea Skuja isolated from Lake Biwa, Japan. Phycologia 2003, 42, 364–369. [Google Scholar] [CrossRef]
- Hodoki, Y.; Ohbayashi, K.; Kobayashi, Y.; Takasu, H.; Okuda, N.; Nakano, S. Anatoxin-a-producing Raphidiopsis mediterranea Skuja var. grandis Hill is one ecotype of non-heterocytous Cuspidothrix issatschenkoi (Usačev) Rajaniemi et al. in Japanese lakes. Harmful Algae 2013, 21–22, 44–53. [Google Scholar] [CrossRef]
- Gagnon, A.; Pick, F.R. Effect of nitrogen on cellular production and release of the neurotoxin anatoxin-a in a nitrogen-fixing cyanobacterium. Front. Microbiol. 2012, 3, 211. [Google Scholar] [CrossRef]
- Wood, S.A.; Rasmussen, J.P.; Holland, P.T.; Campbell, R.; Crowe, A.L.M. First report of the cyanotoxin anatoxin-a from Aphanizomenon issatschenkoi (cyanobacteria). J. Phycol. 2007, 43, 356–365. [Google Scholar] [CrossRef]
- Uzunov, B.; Stefanova, K.; Radkova, M.; Descy, J.-P.; Gärtner, G.; Stoyneva-Gärtner, M. First report on Microcystis as a potential microviridin producer in Bulgarian waterbodies. Toxins 2021, 13, 448. [Google Scholar] [CrossRef]
- Ballot, A.; Scherer, P.I.; Wood, S.A. Variability in the anatoxin gene clusters of Cuspidothrix issatschenkoi from Germany, New Zealand, China and Japan. PLoS ONE 2018, 13, e0200774. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, G.; Pan, Q.; Yang, Y.; Li, R. Identification of genes for anatoxin-a biosynthesis in Cuspidothrix issatschenkoi. Harmful Algae 2015, 46, 43–48. [Google Scholar] [CrossRef]
- Ryu, H.S.; Shin, R.Y.; Lee, J.H. Morphology and taxonomy of the Aphanizomenon spp. (Cyanophyceae) and related species in the Nakdong River, South Korea. J. Ecol. Environ. 2017, 41, 6. [Google Scholar] [CrossRef]
- Aguilera, A.; Berrendero Gómez, E.; Kaštovský, J.; Echenique, R.O.; Salerno, G.L. The polyphasic analysis of two native Raphidiopsis isolates supports the unification of the genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). Phycologia 2018, 57, 130–146. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Kormas, K.A.; Vardaka, E.; Katsiapi, M.; Gkelis, S. Raphidiopsis mediterranea Skuja represents non-heterocytous lifecycle stages of Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju in Lake Kastoria (Greece), its type locality: Evidence by morphological and phylogenetic analysis. Harmful Algae 2009, 8, 864–872. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. Available online: http://www.algaebase.org/ (accessed on 26 July 2022).
- Moustaka-Gouni, M.; Kormas, K.A.; Polykarpou, P.; Gkelis, S.; Bobori, D.C.; Vardaka, E. Polyphasic evaluation of Aphanizomenon issatschenkoi and Raphidiopsis mediterranea in a Mediterranean lake. J. Plankton. Res. 2010, 32, 927–936. [Google Scholar] [CrossRef][Green Version]
- Vodenicharov, D.; Draganov, S.; Temniskova, D. Flora of Bulgaria. In Algae; Narodna Prosveta: Sofia, Bulgaria, 1971. [Google Scholar]
- Stoyneva, M. 1998a. Algae. In Biodiversity of the Srebarna Biosphere Reserve. Checklist and Bibliography; Michev, T.M., Georgiev, B.B., Petrova, A.V., Stoyneva, M.P., Eds.; Co-publ. Context & Pensoft: Sofia, Bulgaria, 1998; pp. 10–37. [Google Scholar]
- Stoyneva, M.P. Algological studies of Bulgarian coastal wetlands. I. Species composition of the phytoplankton of Durankulak and Shabla-Ezeretz lakes. Ann. Univ. Sof. 2000, 91, 27–48. [Google Scholar]
- Stoyneva, M.P. Contribution to the studies of aero- and hydrobiontic prokaryotic and eukaryotic algae of Bulgaria. Ph.D. Thesis, Sofia University “St Kliment Ohridski”, Faculty of Biology, Sofia, Bulgaria, 2014. [Google Scholar]
- Dimitrova, R.; Nenova, E.; Uzunov, B.; Shishiniova, M.; Stoyneva, M. Phytoplankton composition of Vaya Lake (2004–2006). Bulg. J. Agric. Sci. Suppl. 2014, 20, 165–172. [Google Scholar]
- Stoyanov, P.; Teneva, I.; Mladenov, R.; Belkinova, D. Diversity and ecology of the phytoplankton of filamentous blue-green algae (Cyanoprokaryota, Nostocales) in Bulgarian standing waters. Ecol. Balc. 2013, 5, 1–6. [Google Scholar]
- Dochin, K.; Ivanova, A.; Iliev, I. The phytoplankton of Koprinka Reservoir (Central Bulgaria): Species composition and dynamics. J. BioSci. Biotechnol. 2017, 6, 73–82. [Google Scholar]
- Svirčev, Z.; Lalić, D.; Savić, G.B.; Tokodi, N.; Backović, D.D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Trainer, V.L.; Hardy, F.J. Integrative Monitoring of Marine and Freshwater Harmful Algae in Washington State for Public Health Protection. Toxins 2015, 7, 1206–1234. [Google Scholar] [CrossRef]
- Descy, J.-P.; Stoyneva-Gärtner, M.P.; Uzunov, B.A.; Dimitrova, P.H.; Pavlova, V.T.S.; Gärtner, G. Studies on cyanoprokaryotes of the water bodies along the Bulgarian Black Sea Coast (1890–2017): A review, with special reference to new, rare and harmful taxa. Acta Zool. Bulgar. Suppl. 2018, 11, 43–52. [Google Scholar]
- Stoyneva-Gärtner, M.; Uzunov, B.; Dimitrova, P.; Pavlova, V. Algal toxins—New risk factors for national security in Bulgaria. In Proceedings of the Actual Problems of the Security, Veliko Turnovo, Bulgaria, 26–27 October 2017; Electronic publication, Publishing house complex of NVU “Vasil Levski”: Veliko Turnovo, Bulgaria, 2017; pp. 435–445, ISBN 2367-7473. [Google Scholar]
- Komárek, J.; Komarková, J. Diversity of Aphanizomenon-like cyanobacteria. Czech. Phycol. 2006, 6, 1–32. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 1. Teil: Chroococcales. In Süßwasserflora von Mitteleuropa. Bd. 19/1; Ettl, H., Gärtner, G., Heynig, G., Mollenhauer, D., Eds.; Gustav Fischer: Jena, Germany; Stuttgart, Germany; Lübeck, Germany; Ulm, Germany, 1999. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 2. Teil: Oscillatoriales. In Süßwasserflora von Mitteleuropa. Bd. 19/2; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Elsevier, Spektrum Akad. Verl.: Heidelberg, Germany; München, Germany, 2005. [Google Scholar]
- Catherine, Q.; Wood, S.; Echenigue-Subiabre, I.; Heath, M.; Villeneuve, A.; Humbert, J.-F. A review of current knowledge on toxic benthic freshwater cyanobacteria—Ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef]
- Walther, G.-R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukát, Z.; Bugmann, H.; et al. Alien species in a warmer world: Risks and opportunities. Trends Ecol. Evol. 2009, 24, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Wilk-Woźniak, E.; Najberck, K. Towards clarifying the presence of alien algae in inland waters—Can we predict places of their occurrence? Biologia 2013, 68, 838–844. [Google Scholar] [CrossRef]
- Kaštovský, J.; Hauer, T.; Mareš, J.; Krautová, M.; Bešta, T.; Komárek, J.; Desortová, B.; Heteša, J.; Hindáková, A.; Houk, V.; et al. A review of the alien and expansive species of freshwater cyanobacteria and algae in the Czech Republic. Biol. Invasions 2010, 12, 3599–3625. [Google Scholar] [CrossRef]
- Stoyneva, M.P. Allochtonous planctonic algae recorded in Bulgaria during the last 25 years and their possible dispersal agents. Hydrobiologia 2016, 764, 53–64. [Google Scholar] [CrossRef]
- Savadova, K.; Mazur-Marzec, H.; Karosiene, J.; Kasperoviciene, J.; Vitonyte, I.; Torunska-Sitarz, A.; Koreiviene, J. Effect of increased temperature on native and alien nuisance cyanobacteria from temperate lakes: An experimental approach. Toxins 2018, 10, 445. [Google Scholar] [CrossRef]
- Legrand, B.; Lesobre, J.; Colombet, J.; Latour, D.; Sabart, M. Molecular tools to detect anatoxin-a genes in aquatic ecosystems: Toward a new nested PCR-based method. Harmful Algae 2016, 58, 16–22. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]

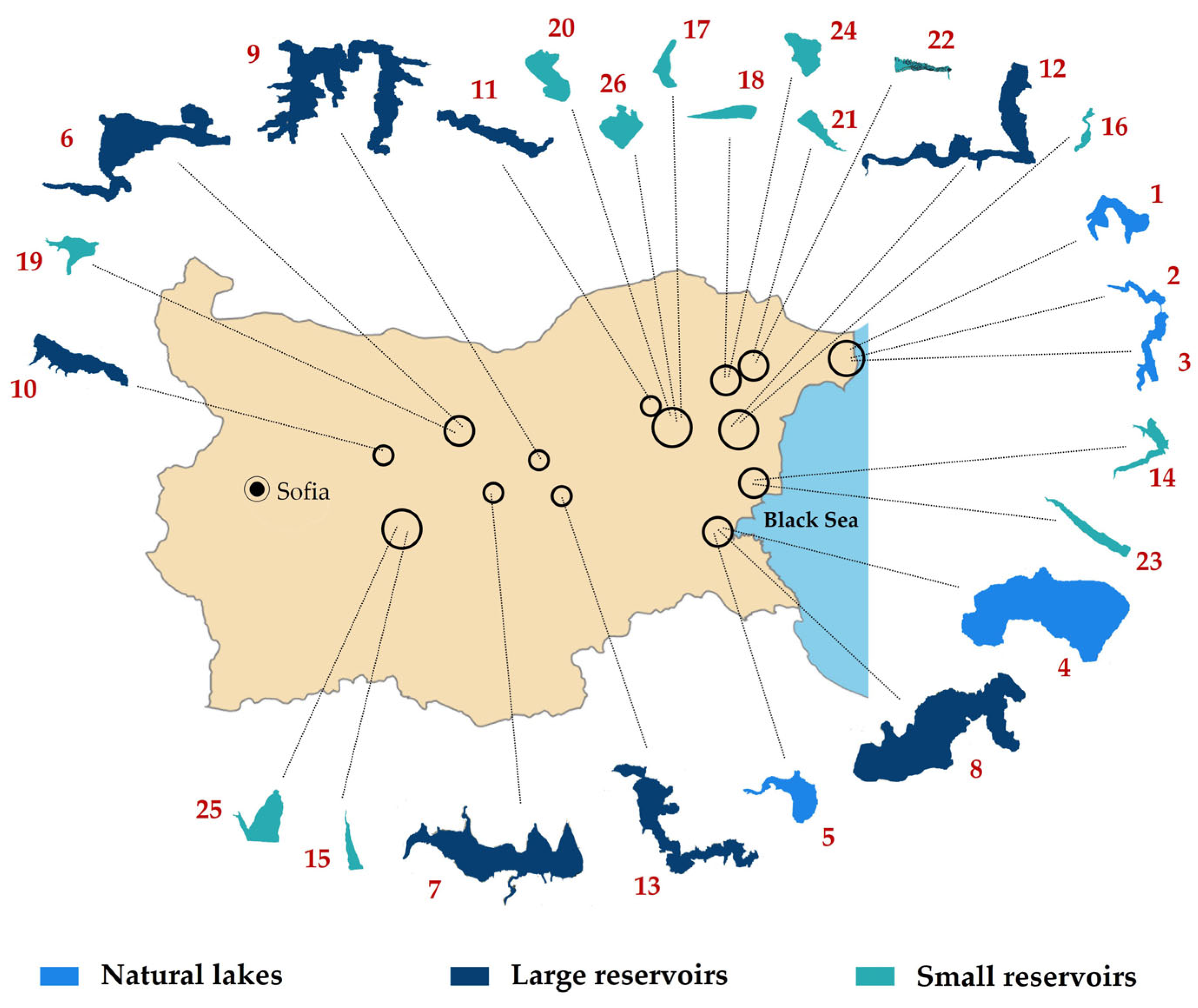
| WBN and IBW | Site | Year | Alt | Latitude | Longitude | WT | pH | SD | CN | TD | DO | TP | TN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Natural Lakes | |||||||||||||
| 1. L. Durankulak (IBW0216) | 1 | 2018 | 6 | 43°40.3240′ | 28°32.0470′ | 24.03 | 8.5 | 1 | 1111 | 722 | 7.35 | 21 | 2.8 |
| 2 | 2018 | 6 | 43°40.3340′ | 28°32.0220′ | 24.7 | 8.2 | 1 | 1094 | 711 | 7.79 | 20 | 4.0 | |
| 3/W | 2018 | 4 | 43°40.5300′ | 28°32.9930′ | 24.6 | 8.5 | 1 | 1075 | 698 | 6.19 | 24 | 3.9 | |
| W | 2019 | 2 | 43°40.0006′ | 29°32.6166′ | 26.5 | 8.9 | 0.6 | 974 | 631 | 7.86 | 0.3 | 0.7 | |
| 4/E | 2018 | 3 | 43°40.6950′ | 28°32.6000′ | 26.5 | 8.5 | 1 | 1087 | 706 | 9.6 | 20 | 3.2 | |
| E | 2019 | 4 | 43°40.5355′ | 28°33.0806′ | 26.7 | 8.9 | 0.6 | 1048 | 680 | 6.04 | 0.3 | 0.6 | |
| 2. L. Ezerets (IBW0233) | 2018 | −2 | 43°35.2770′ | 28°33.2290′ | 26.4 | 8.4 | TTB | 1084 | 0 | 9.94 | 0.5 | 5.3 | |
| 2019 | 6 | 43°35.2681′ | 28°33.2096′ | 25.9 | 8.6 | 1.5 | 1669 | 1739 | 8.58 | 0.1 | 0.1 | ||
| 3. L. Shabla (IBW0219) | 2018 | −2 | 43°33.8180′ | 28°34.1860′ | 27.1 | 8.5 | TTB | 1087 | 706 | 9.97 | 0.1 | 5.1 | |
| 2019 | <1 | 43°33.8212′ | 28°34.8204′ | 25.9 | 8.7 | TTB | 1842 | 1196 | 9.64 | 0.1 | 1.0 | ||
| 4. L. Vaya (IBW0191) | 1 | 2018 | −2 | 42°30.5940′ | 27°22.075′ | 26.9 | 9.7 | 0.25 | 2588 | 1682 | 12.51 | 13 | 5.4 |
| 2/W | 2018 | 0 | 42°28.4540′ | 27°25.482 | 28.28 | 8.9 | 0.25 | 1183 | 768 | 11.94 | 11 | 3.7 | |
| W | 2019 | −2 | 42°30.5940′ | 27°22.075′ | 27.9 | 9.2 | 0.15 | 490 | 17 | 7.69 | 0.5 | 0.3 | |
| 3 | 2018 | 6 | 42°29.1850′ | 27°26.531 | 23.7 | 9.5 | 0.25 | 1024 | 665 | 7.01 | 12 | 4.6 | |
| 5. L. Uzungeren (IBW0710) | 2018 | 7 | 42°26.1782′ | 27°27.1998′ | 25.9 | 8.1 | 0.40 | 1458 | 9351 | 7.83 | 5.0 | 2.8 | |
| 2019 | −3 | 42°26.1551′ | 27°27.2235′ | 27.6 | 8.5 | 0.45 | 1748 | 1132 | 9.7 | 0.4 | 0.3 | ||
| Large reservoirs | |||||||||||||
| 6. Res. Al. Stamboliyski (IBW2056) | 2019 | 190 | 43°07.0000′ | 25°07.3936′ | 29.4 | 8.9 | 2.00 | 670 | 4.33 | 9.82 | 1.4 | 3.5 | |
| 7. Res. Koprinka (IBW2062) | 2019 | 450 | 42°37.0172′ | 25°19.4795′ | 27.2 | 8.2 | 2.5 | 250 | 163 | 7.21 | 0.1 | 0.2 | |
| 8. Res. Mandra (IBW1720) | 1 | 2018 | 12 | 42°24.0643′ | 27°26.1120′ | 25.9 | 8.3 | 0.4 | 649 | 421 | 6.81 | 3.0 | 3.0 |
| 2/W | 2018 | 13 | 42°24.0670′ | 27°19.1310′ | 26.2 | 8.2 | 0.2 | 663 | 461 | 5.89 | 6.0 | 4.0 | |
| W | 2019 | 7 | 42°24.0295′ | 27°19.1194′ | 25.88 | 7.9 | 0.45 | 676 | 436 | 7.93 | 0.7 | 0.5 | |
| 3/E | 2018 | 9 | 42 26.1420′ | 27°26.5860′ | 24.9 | 8.5 | 0.3 | 639 | 415 | 7.91 | 4.0 | 3.3 | |
| E | 2019 | 8 | 42°25.9303′ | 27°26.7652′ | 27.2 | 8.5 | 0.45 | 578 | 375 | 7.87 | 1.5 | 1.8 | |
| 9. Res. Shilkovtsi (IBW2105) | 2019 | 410 | 42°55.2320′ | 25°47.6743′ | 27.2 | 8.9 | 0.5 | 746 | 479 | 7.48 | 0.03 | 0.1 | |
| 10. Res. Sopot (IBW1437) | 2019 | 376 | 40°00.7017′ | 24°52.6045′ | 29.0 | 8.3 | 2.0 | 779 | 490 | 3.44 | 0.1 | 0.1 | |
| 11. Res. Suedinenie (IBW2642) | 2019 | 133 | 43°20.0734′ | 26°33.6368′ | 28.1 | 7.6 | 0.5 | 739 | 481 | 6.77 | 0.1 | 0.3 | |
| 12. Res. Tsonevo (IBW3022) | 2019 | 75 | 43°01.8055′ | 27°24.3965′ | 24.8 | 8.8 | 4.2 | 355 | 231 | 8.2 | 0.1 | 0.1 | |
| 13. Res. Zhrebchevo (IBW2545) | 2019 | 253 | 42°36.6024′ | 25°51.2345′ | 27.6 | 7.7 | 0.7 | 358 | 233 | 8.01 | 0.1 | 0.2 | |
| Small reservoirs | |||||||||||||
| 14. Res. Aheloy (IBW3032) | 2018 | 144 | 42°42.8230′ | 27°30.9740′ | 25.4 | 8.5 | 1.10 | 614 | 399 | 8.92 | 1 | 4.1 | |
| 15. Res. Duvanli (IBW1483) | 2019 | 250 | 42°23.1851′ | 24°43.1000′ | 26.3 | 8.8 | 0.4 | 4050 | 291 | 7.09 | 0.1 | 0.3 | |
| 16. Res. Eleshnitsa (IBW3023) | 2019 | 44 | 43°00.3344′ | 27°28. 0744′ | 26.7 | 8.4 | 2.00 | 532 | 347 | 6.78 | 0.1 | 0.3 | |
| 17. Res. Fisek (IBW2674) | 2019 | 182 | 43°18.8453′ | 26°44. 3765′ | 27.2 | 8.7 | 0.5 | 690 | 3.97 | 7.52 | 0.2 | 0.1 | |
| 18. Rez. Izvornik 2 (IBW3082) | 2019 | 255 | 43°27.3838′ | 27°21.111′ | 24.5 | 9.4 | 0.15 | 389 | 253 | 13.26 | 9.0 | 4.8 | |
| 19. Res. Krapets (IBW2000) | 2019 | 410 | 43°04.0316′ | 24°52.3835′ | 28.7 | 8.3 | 5.0 | 870 | 564 | 7.74 | 0.1 | 1.0 | |
| 20. Res. Kriva Reka (IBW3071) | 2019 | 133 | 43°22.6573′ | 27°10.9807′ | 23.7 | 8.4 | 0.3 | 662 | 428 | 6.24 | 1.0 | 9.0 | |
| 21. Res. Malka Smolnitsa (IBW3107) | 2019 | 211 | 43°36.2606′ | 27°44.5367′ | 25.2 | 9.1 | 0.3 | 755 | 490 | 7.05 | 0.6 | 0.6 | |
| 22. Res. Plachidol 2 (IBW5073) | 2019 | 220 | 43°33.3504′ | 27°52.6338′ | 24.6 | 9.0 | 0.5 | 1225 | 793 | 9.13 | 0.2 | 0.4 | |
| 23. Res. Poroy (IBW3038) | 2018 | 41 | 42°43.0190′ | 27°37.3160′ | 25.10 | 8.3 | 1.2 | 762 | 495 | 9.45 | 1.0 | 2.8 | |
| 2019 | 43 | 42°43.3403′ | 27°37.5255′ | 27.5 | 8.1 | 0.4 | 644 | 416 | 7.6 | 0.1 | 0.3 | ||
| 24. Res. Preselka (IBW3078) | 2019 | 281 | 43°25.3767′ | 27°16.6214′ | 24.1 | 9.0 | 0.5 | 138 | 282 | 10.05 | 0.6 | 2.8 | |
| 25. Res. Sinyata Reka (IBW1890) | 2018 | 317 | 42°28.1480′ | 24°42.2170 | 27.4 | 9.7 | 0.5 | 470 | 305 | 9.36 | 25 | 4.8 | |
| 2018 | 317 | 42°28.1473′ | 24°42.2175 | 26.7 | 9.4 | 0.6 | 468 | 306 | 9.21 | 27 | 4.3 | ||
| 2019 | 317 | 42°28.1518′ | 24°42.0159′ | 28.2 | 10.4 | 0.4 | 490 | 317 | 14.76 | 1.0 | 0.2 | ||
| 26. Res. Shumensko Ezero (IBW2754) | 2019 | 152 | 43°14.8140′ | 26°57.5675′ | 25.2 | 8.5 | 1.0 | 471 | 445 | 6.32 | 0.2 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyneva-Gärtner, M.; Stefanova, K.; Uzunov, B.; Radkova, M.; Gärtner, G. Cuspidothrix Is the First Genetically Proved Anatoxin A Producer in Bulgarian Lakes and Reservoirs. Toxins 2022, 14, 778. https://doi.org/10.3390/toxins14110778
Stoyneva-Gärtner M, Stefanova K, Uzunov B, Radkova M, Gärtner G. Cuspidothrix Is the First Genetically Proved Anatoxin A Producer in Bulgarian Lakes and Reservoirs. Toxins. 2022; 14(11):778. https://doi.org/10.3390/toxins14110778
Chicago/Turabian StyleStoyneva-Gärtner, Maya, Katerina Stefanova, Blagoy Uzunov, Mariana Radkova, and Georg Gärtner. 2022. "Cuspidothrix Is the First Genetically Proved Anatoxin A Producer in Bulgarian Lakes and Reservoirs" Toxins 14, no. 11: 778. https://doi.org/10.3390/toxins14110778
APA StyleStoyneva-Gärtner, M., Stefanova, K., Uzunov, B., Radkova, M., & Gärtner, G. (2022). Cuspidothrix Is the First Genetically Proved Anatoxin A Producer in Bulgarian Lakes and Reservoirs. Toxins, 14(11), 778. https://doi.org/10.3390/toxins14110778






