Persistence with Botulinum Toxin Treatment for Spasticity Symptoms in Multiple Sclerosis
Abstract
1. Introduction
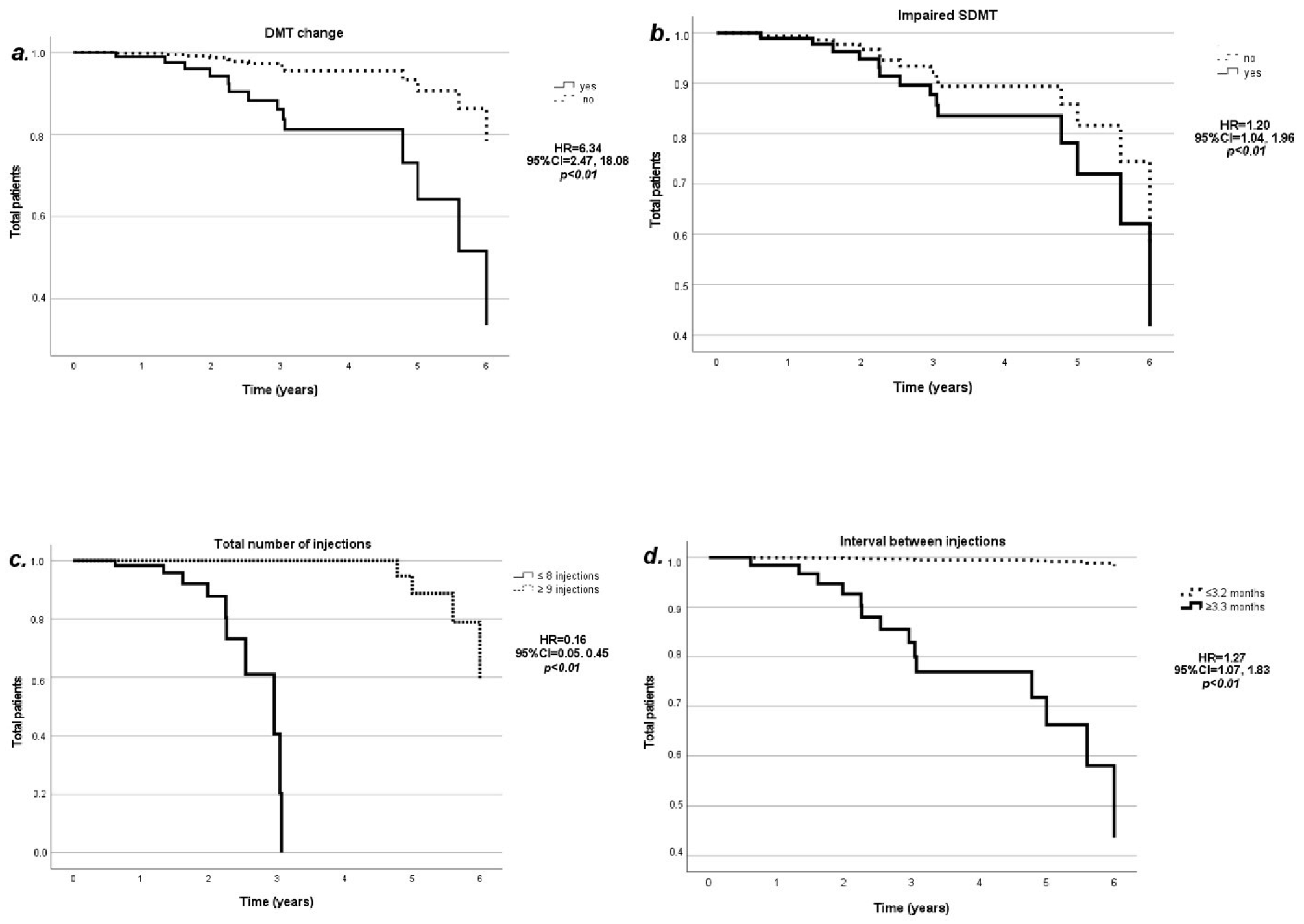
2. Results
3. Discussion
4. Conclusions
5. Methods
5.1. Study Design and Population
5.2. Demographics and MS-Related Clinical Variables
5.3. Spasticity and BT Injection Variables
5.4. Power Calculation
5.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bethoux, F.; Marrie, R.A. A Cross-Sectional Study of the Impact of Spasticity on Daily Activities in Multiple Sclerosis. Patient Patient-Centered Outcomes Res. 2016, 9, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Dolcetti, E.; Centonze, D. Theoretical and Therapeutic Implications of the Spasticity-Plus Syndrome Model in Multiple Sclerosis. Front. Neurol. 2022, 12, 802918. [Google Scholar] [CrossRef] [PubMed]
- Moccia, M.; Frau, J.; Carotenuto, A.; Butera, C.; Coghe, G.; Barbero, P.; Frontoni, M.; Groppo, E.; Giovannelli, M.; Del Carro, U.; et al. Botulinum toxin for the management of spasticity in multiple sclerosis: The Italian botulinum toxin network study. Neurol. Sci. 2020, 41, 2781–2792. [Google Scholar] [CrossRef]
- Sartori, A.; Dinoto, A.; Stragapede, L.; Mazzon, G.; Morelli, M.E.; Pasquin, F.; Bratina, A.; Bosco, A.; Manganotti, P. Nabiximols and botulinum toxin injections for patients with multiple sclerosis: Efficacy on spasticity and spasms in a single-centre experience. Neurol. Sci. 2021, 42, 5037–5043. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, J.H.; Song, H.X.; Dong, Y. Effectiveness of Botulinum Toxin for Lower Limbs Spasticity after Stroke: A Systematic Review and Meta-Analysis. Top. Stroke Rehabilitation 2015, 23, 217–223. [Google Scholar] [CrossRef]
- Intiso, D.; Simone, V.; Bartolo, M.; Santamato, A.; Ranieri, M.; Gatta, M.T.; Di Rienzo, F. High Dosage of Botulinum Toxin Type A in Adult Subjects with Spasticity Following Acquired Central Nervous System Damage: Where Are We at? Toxins 2020, 12, 315. [Google Scholar] [CrossRef]
- Lannin, N.A.; Ada, L.; English, C.; Ratcliffe, J.; Faux, S.; Palit, M.; Gonzalez, S.; Olver, J.; Schneider, E.; Crotty, M.; et al. Long-term effect of additional rehabilitation following botulinum toxin-A on upper limb activity in chronic stroke: The InTENSE randomised trial. BMC Neurol. 2022, 22, 154. [Google Scholar] [CrossRef]
- Schramm, A.; Ndayisaba, J.P.; auf dem Brinke, M.; Hecht, M.; Herrmann, C.; Huber, M.; Lobsien, E.; Mehnert, S.; Reuter, I.; Stenner, A.; et al. Spasticity treatment with onabotulinumtoxin A: Data from a prospective German real-life patient registry. J. Neural Transm. 2014, 121, 521–530. [Google Scholar] [CrossRef]
- Gracies, J.-M.; Esquenazi, A.; Brashear, A.; Banach, M.; Kocer, S.; Jech, R.; Khatkova, S.; Benetin, J.; Vecchio, M.; McAllister, P.; et al. Efficacy and safety of abobotulinumtoxinA in spastic lower limb: Randomized trial and extension. Neurology 2017, 89, 2245–2253. [Google Scholar] [CrossRef]
- Lanzillo, R.; Prosperini, L.; Gasperini, C.; Moccia, M.; Fantozzi, R.; Tortorella, C.; Nociti, V.; Annovazzi, P.; Cavalla, P.; Radaelli, M.; et al. A multicentRE observational analysiS of PErsistenCe to Treatment in the new multiple sclerosis era: The RESPECT study. J. Neurol. 2018, 265, 1174–1183. [Google Scholar] [CrossRef]
- Moccia, M.; Loperto, I.; Lanzillo, R.; Capacchione, A.; Carotenuto, A.; Triassi, M.; Morra, V.B.; Palladino, R. Persistence, adherence, healthcare resource utilisation and costs for interferon Beta in multiple sclerosis: A population-based study in the Campania region (southern Italy). BMC Health Serv. Res. 2020, 20, 797. [Google Scholar] [CrossRef]
- Latino, P.; Castelli, L.; Prosperini, L.; Marchetti, M.R.; Pozzilli, C.; Giovannelli, M. Determinants of botulinum toxin discontinuation in multiple sclerosis: A retrospective study. Neurol. Sci. 2017, 38, 1841–1848. [Google Scholar] [CrossRef]
- Hara, T.; Abo, M.; Hara, H.; Sasaki, N.; Yamada, N.; Niimi, M.; Shimamoto, Y. The Effect of Repeated Botulinum Toxin A Therapy Combined with Intensive Rehabilitation on Lower Limb Spasticity in Post-Stroke Patients. Toxins 2018, 10, 349. [Google Scholar] [CrossRef]
- Srpova, B.; Sobisek, L.; Novotna, K.; Uher, T.; Friedova, L.; Vaneckova, M.; Krasensky, J.; Havrdova, E.K.; Horakova, D. The clinical and paraclinical correlates of employment status in multiple sclerosis. Neurol. Sci. 2021, 43, 1911–1920. [Google Scholar] [CrossRef]
- Trompetto, C.; Marinelli, L.; Mori, L.; Puce, L.; Pelosin, E.; Serrati, C.; Fattapposta, F.; Rinalduzzi, S.; Abbruzzese, G.; Currà, A. Do flexible inter-injection intervals improve the effects of botulinum toxin A treatment in reducing impairment and disability in patients with spasticity? Med. Hypotheses 2017, 102, 28–32. [Google Scholar] [CrossRef][Green Version]
- Baccouche, I.; Bensmail, D.; Leblong, E.; Fraudet, B.; Aymard, C.; Quintaine, V.; Pottier, S.; Lansaman, T.; Malot, C.; Gallien, P.; et al. Goal-Setting in Multiple Sclerosis-Related Spasticity Treated with Botulinum Toxin: The GASEPTOX Study. Toxins 2022, 14, 582. [Google Scholar] [CrossRef]
- Blaszczyk, I.; Foumani, N.P.; Ljungberg, C.; Wiberg, M. Questionnaire about the Adverse Events and Side Effects Following Botulinum Toxin A Treatment in Patients with Cerebral Palsy. Toxins 2015, 7, 4645–4654. [Google Scholar] [CrossRef]
- Cinone, N.; Santoro, L.; Spina, S.; Facciorusso, S.; Battaglia, M.; Baricich, A.; Marcogiuseppe, P.; Santamato, A. Reasons and Determinants of BoNT-A Treatment Discontinuation in Patients Living with Spasticity: A 10-Year Retrospective Analysis. Toxins 2022, 14, 675. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Moccia, M.; Lanzillo, R.; Palladino, R.; Chang, K.C.-M.; Costabile, T.; Russo, C.; De Rosa, A.; Carotenuto, A.; Sacca, F.; Maniscalco, G.T.; et al. Cognitive impairment at diagnosis predicts 10-year multiple sclerosis progression. Mult. Scler. 2016, 22, 659–667. [Google Scholar] [CrossRef]
- Saccà, F.; Costabile, T.; Carotenuto, A.; Lanzillo, R.; Moccia, M.; Pane, C.; Russo, C.V.; Barbarulo, A.M.; Casertano, S.; Rossi, F.; et al. The EDSS integration with the Brief International Cognitive Assessment for Multiple Sclerosis and orientation tests. Mult. Scler. J. 2016, 23, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Kalincik, T.; Cutter, G.; Spelman, T.; Jokubaitis, V.; Havrdova, E.K.; Horáková, D.; Trojano, M.; Izquierdo, G.; Girard, M.; Duquette, P.; et al. Defining reliable disability outcomes in multiple sclerosis. Brain 2015, 138, 3287–3298. [Google Scholar] [CrossRef] [PubMed]
- Baude, M.; Nielsen, J.B.; Gracies, J.-M. The neurophysiology of deforming spastic paresis: A revised taxonomy. Ann. Phys. Rehabilitation Med. 2018, 62, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Comi, G.; Solari, A.; Leocani, L.; Centonze, D.; Otero-Romero, S.; Amadeo, R.; Amato, M.P.; Bertolotto, A.; Boffa, L.; Brichetto, G.; et al. Italian consensus on treatment of spasticity in multiple sclerosis. Eur. J. Neurol. 2019, 27, 445–453. [Google Scholar] [CrossRef]
- Albrecht, P.; Jansen, A.; Lee, J.-I.; Moll, M.; Ringelstein, M.; Rosenthal, D.; Bigalke, H.; Aktas, O.; Hartung, H.-P.; Hefter, H. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology 2019, 92, e48–e54. [Google Scholar] [CrossRef]
| BT Continuation (n = 108) | BT Discontinuation (n = 14) | p-Value | ||
|---|---|---|---|---|
| Age, years | 50.1 ± 9.4 | 44.0 ± 10.6 | 0.02 * | |
| Sex, females | 46 (42.6%) | 6 (42.8%) | 0.98 | |
| Follow-up duration, years | 2.7 ± 1.5 | 3.0 ± 1.5 | 0.47 | |
| Disease duration, years | 14.4 ± 8.3 | 13.1 ± 9.1 | 0.58 | |
| EDSS | 5.8 ± 1.2 | 5.4 ± 1.3 | 0.26 | |
| DMT | None | 9 (8.3%) | 0 (0%) | 0.30 |
| Low/Medium efficacy | 35 (32.4%) | 3 (21.4%) | ||
| High efficacy | 64 (59.3%) | 11 (78.6%) | ||
| DMT change | 29 (26.8%) | 2 (14.3%) | 0.54 | |
| SDMT, adjusted score | 38.0 ± 11.3 | 35.4 ± 17.5 | 0.37 | |
| SDMT, impaired | 40 (37.0%) | 6 (42.8%) | ||
| MAS, highest score | 1.8 ± 0.5 | 1.9 ± 0.5 | 0.77 | |
| Concomitant spasticity treatments | 48 (44.4%) | 6 (42.8%) | 0.91 | |
| BT formulation | Botox | 39 (36.2%) | 7 (50.0%) | 0.56 |
| Dysport | 45 (41.6%) | 4 (28.6%) | ||
| Xeomin | 24 (22.2%) | 3 (21.4%) | ||
| BT dose, uDU | 263.4 ± 157.0 | 225.0 ± 131.19 | 0.38 | |
| BT changes | 28 (25.9%) | 4 (28.6%) | 0.83 | |
| Total number of BT injections | 10.3 ± 5.5 | 8.1 ± 5.6 | 0.17 | |
| Interval between BT injections, months | 3.1 ± 0.4 | 4.9 ± 1.3 | <0.01 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novarella, F.; Carotenuto, A.; Cipullo, P.; Iodice, R.; Cassano, E.; Spiezia, A.L.; Capasso, N.; Petracca, M.; Falco, F.; Iacovazzo, C.; et al. Persistence with Botulinum Toxin Treatment for Spasticity Symptoms in Multiple Sclerosis. Toxins 2022, 14, 774. https://doi.org/10.3390/toxins14110774
Novarella F, Carotenuto A, Cipullo P, Iodice R, Cassano E, Spiezia AL, Capasso N, Petracca M, Falco F, Iacovazzo C, et al. Persistence with Botulinum Toxin Treatment for Spasticity Symptoms in Multiple Sclerosis. Toxins. 2022; 14(11):774. https://doi.org/10.3390/toxins14110774
Chicago/Turabian StyleNovarella, Federica, Antonio Carotenuto, Paolo Cipullo, Rosa Iodice, Emanuele Cassano, Antonio Luca Spiezia, Nicola Capasso, Maria Petracca, Fabrizia Falco, Carmine Iacovazzo, and et al. 2022. "Persistence with Botulinum Toxin Treatment for Spasticity Symptoms in Multiple Sclerosis" Toxins 14, no. 11: 774. https://doi.org/10.3390/toxins14110774
APA StyleNovarella, F., Carotenuto, A., Cipullo, P., Iodice, R., Cassano, E., Spiezia, A. L., Capasso, N., Petracca, M., Falco, F., Iacovazzo, C., Servillo, G., Lanzillo, R., Brescia Morra, V., & Moccia, M. (2022). Persistence with Botulinum Toxin Treatment for Spasticity Symptoms in Multiple Sclerosis. Toxins, 14(11), 774. https://doi.org/10.3390/toxins14110774






