Multifunctional Properties of a Bacillus thuringiensis Strain (BST-122): Beyond the Parasporal Crystal
Abstract
1. Introduction
2. Results
2.1. Selection of a Bt Strain Harboring Previously Described Toxicity Factors against Pest Organisms Belonging to Different Phyla
2.2. The BST-122 Bt Strain as a Biological Control Agent against Newly Hatched Larva of Leptinotarsa decemlineata
2.3. Activity of BST-122 Bt Strain against Tetranychus urticae Protonymphs
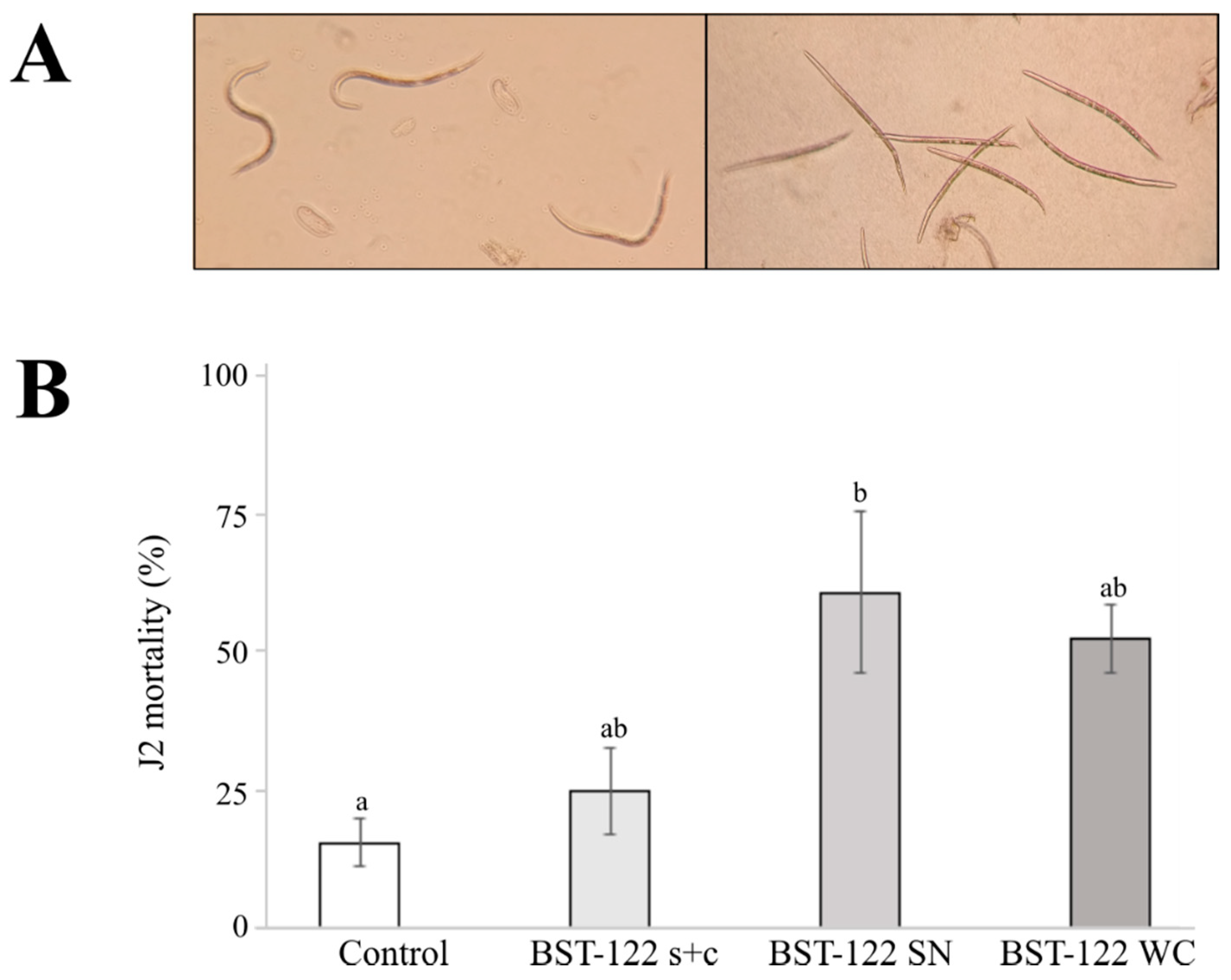
2.4. The BST-122 Bt Strain as a Biological Control Agent against Meloidogyne incognita J2 Juveniles
2.5. Effect of BST-122 in the Growth of Phytopathogenic Fungi: Verticillium dahliae and Fusarium oxysporum
3. Discussion
4. Materials and Methods
4.1. Selection of Bacterial Strains
4.1.1. Bacterial Isolation
4.1.2. Total DNA Extraction and Genomic Sequencing
4.1.3. Identification of Potential Nematocidal/Insecticidal, Acaricidal, and Fungicidal Genes
4.1.4. Production of Spores and Crystals from the Wild Bt Strain
4.1.5. SDS-PAGE
4.2. Detection of β-Exotoxin
4.3. Coleopteran, Acaricidal, Nematocidal, and Fungicidal Activity of BST-122 Bt Strain
4.3.1. Leptinotarsa decemlineata, Tetranychus urticae, and Meloidogyne incognita Rearing
4.3.2. Phytopathogen Fungi Species Maintenance
4.3.3. L. decemlineata Bioassays In Vivo
4.3.4. T. urticae Bioassays In Vivo
4.3.5. Meloidogyne incognita Bioassays In Vivo
J2 Mortality Treated with Spores and Crystal Mixture, Supernatant, and the Whole Culture
J2 Mortality Treated with Solubilized Protein
4.3.6. Assay of Control of M. incognita Infestation in Cucumber Plants
4.3.7. Phytopathogen Fungi Species Bioassays In Vitro
4.4. Recombinant Protein Expression, Purification, and In Vivo Testing
4.4.1. Amplification and Cloning of the cry5-orf2cry_65 Operon Genes
4.4.2. Production, Purification, and Activity Assay against Diverse Target Species of the Recombinant Toxins
4.4.3. Nucleotide Sequence Accession Numbers
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schnepf, E.; Crickmore, N.; Van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and Its Pesticidal Crystal Proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 775–806. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberón, M. Bacillus Thuringensis: A Story of a Sucessful Bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Dulmage, H.T. Insecticidal Activity of Isolated of Bacillus thuringiensis and Their Potential for Pest Control. In Microbial Control of Pests and Plant Diseases 1970–1980; Burges, H.D., Ed.; Academic Press: London, UK, 1981; pp. 193–222. [Google Scholar]
- Ortiz, A.; Sansinenea, E. Bacillus thuringiensis Based Biopesticides for Integrated Crop Management. In Biopesticides; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–6. [Google Scholar]
- Dunham, W.; Trimmer, M. Biological Products around the World, Bioproducts Industry Alliance Spring Meeting & International Symposium (BPIA.Org); 2BMonthly Newsletter: Nairobi, Kenya, 2018. [Google Scholar]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis Toxins: An Overview of Their Biocidal Activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.A.; Burges, H.D. Technology of Formulation and Application. In Formulation of Microbial Biopesticides; Burges, H.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 7–30. [Google Scholar]
- Van Frankenhuyzen, K. Insecticidal Activity of Bacillus thuringiensis Crystal Proteins. J. Invertebr. Pathol. 2009, 101, 1–16. [Google Scholar] [CrossRef]
- Hernández-Martínez, P.; Ferré, J.; Escriche, B. Susceptibility of Spodoptera exigua to 9 Toxins from Bacillus thuringiensis. J. Invertebr. Pathol. 2008, 97, 245–250. [Google Scholar] [CrossRef]
- Ruiz De Escudero, I.; Estela, A.; Escriche, B.; Caballero, P. Potential of the Bacillus thuringiensis Toxin Reservoir for the Control of Lobesia botrana (Lepidoptera: Tortricidae), a Major Pest of Grape Plants. Appl. Environ. Microbiol. 2007, 73, 337–340. [Google Scholar] [CrossRef][Green Version]
- Haffani, Y.Z.; Cloutier, C.; Belzile, F.J. Bacillus thuringiensis Cry3Ca1 Protein Is Toxic to the Colorado Potato Beetle, Leptinotarsa decemlineata (Say). Biotechnol. Prog. 2001, 17, 211–216. [Google Scholar] [CrossRef]
- Krieg, A.; Huger, A.M.; Langenbruch, G.A.; Schnetter, W. Bacillus thuringiensis Var. tenebrionis: Ein Neuer, Gegenüber Larven von Coleopteren Wirksamer Pathotyp. Z. Angew. Entomol. 1983, 96, 500–508. [Google Scholar] [CrossRef]
- Lambert, B.; Hofte, H.; Annys, K.; Jansens, S.; Soetaert, P.; Peferoen, M. Novel Bacillus thuringiensis Insecticidal Crystal Protein with a Silent Activity against Coleopteran Larvae. Appl. Environ. Microbiol. 1992, 58, 2536–2542. [Google Scholar] [CrossRef]
- Domínguez-Arrizabalaga, M.; Villanueva, M.; Escriche, B.; Ancín-Azpilicueta, C.; Caballero, P. Insecticidal Activity of Bacillus thuringiensis Proteins against Coleopteran Pests. Toxins 2020, 12, 430. [Google Scholar] [CrossRef]
- Barloy, F.; Delécluse, A.; Nicolas, L.; Lecadet, M.M. Cloning and Expression of the First Anaerobic Toxin Gene from Clostridium bifermentans subsp. malaysia, Encoding a New Mosquitocidal Protein with Homologies to Bacillus thuringiensis Delta-Endotoxins. J. Bacteriol. 1996, 178, 3099–3105. [Google Scholar] [CrossRef] [PubMed]
- Chilcott, C.N.; Ellar, D.J. Comparative Toxicity of Bacillus thuringiensis Var. israelensis Crystal Proteins in Vivo and in Vitro. J. Gen. Microbiol. 1988, 134, 2551–2558. [Google Scholar] [CrossRef] [PubMed]
- Donovan, W.P.; Dankocsik, C.; Gilbert, M.P. Molecular Characterization of a Gene Encoding a 72-Kilodalton Mosquito-Toxic Crystal Protein from Bacillus thuringiensis subsp. israelensis. J. Bacteriol. 1988, 170, 4732–4738. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Soto, A.; Del Rincón-Castro, M.C.; Espinoza, A.M.; Ibarra, J.E. Parasporal Body Formation via Overexpression of the Cry10Aa Toxin of Bacillus thuringiensis subsp. israelensis, and Cry10Aa-Cyt1Aa Synergism. Appl. Environ. Microbiol. 2009, 75, 4661–4667. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.L.; Delécluse, A. Contribution of the 65-Kilodalton Protein Encoded by the Cloned Gene Cry19A to the Mosquitocidal Activity of Bacillus thuringiensis subsp. jegathesan. Appl. Environ. Microbiol. 1997, 63, 4449–4455. [Google Scholar] [CrossRef]
- Ward, E.S.; Ridley, A.R.; Ellar, D.J.; Todd, J.A. Bacillus thuringiensis Var. israelensis δ-Endotoxin. Cloning and Expression of the Toxin in Sporogenic and Asporogenic Strains of Bacillus subtilis. J. Mol. Biol. 1986, 191, 13–22. [Google Scholar] [CrossRef]
- Valtierra-de-Luis, D.; Villanueva, M.; Berry, C.; Caballero, P. Potential for Bacillus thuringiensis and Other Bacterial Toxins as Biological Control Agents to Combat Dipteran Pests of Medical and Agronomic Importance. Toxins 2020, 12, 773. [Google Scholar] [CrossRef]
- Baum, J.A.; Sukuru, U.R.; Penn, S.R.; Meyer, S.E.; Subbarao, S.; Shi, X.; Flasinski, S.; Heck, G.R.; Brown, R.S.; Clark, T.L. Cotton Plants Expressing a Hemipteran-Active Bacillus thuringiensis Crystal Protein Impact the Development and Survival of Lygus hesperus (Hemiptera: Miridae) Nymphs. J. Econ. Entomol. 2012, 105, 616–624. [Google Scholar] [CrossRef]
- Thomson, M.; Knuth, M.; Cardineau, G. Bacillus thuringiensis Toxins with Improved Activity. U.S. Patent 5,874,288 A, 1999. [Google Scholar]
- Li, H.; Olson, M.; Lin, G.; Hey, T.; Tan, S.Y.; Narva, K.E. Bacillus thuringiensis Cry34Ab1/Cry35Ab1 Interactions with Western Corn Rootworm Midgut Membrane Binding Sites. PLoS ONE 2013, 8, e53079. [Google Scholar] [CrossRef]
- Guo, S.; Liu, M.; Peng, D.; Ji, S.; Wang, P.; Yu, Z.; Sun, M. New Strategy for Isolating Novel Nematicidal Crystal Protein Genes from Bacillus thuringiensis Strain YBT-1518. Appl. Environ. Microbiol. 2008, 74, 6997–7001. [Google Scholar] [CrossRef]
- Payne, J.M.; Cannon, R.J.C.; Bagley, A.L. Bacillus thuringiensis Isolates for Controlling Acarides. U.S. Patent 5,211,946, 1993. [Google Scholar]
- Payne, J.M.; Cannon, R.J.C.; Bagley, A.L. Bacillus thuringiensis Isolates for Controlling Acarida. U.S. Patent 5,262,158, 1993. [Google Scholar]
- Payne, J.; Raymond, J.C.C.; Ralph, A.L. Bacillus thuringiensis Isolates for Controlling Acarides. U.S. Patent 5,350,576, 1994. [Google Scholar]
- Reyes-Ramírez, A.; Escudero-Abarca, B.I.; Aguilar-Uscanga, G.; Hayward-Jones, P.M.; Eleazar Barboza-Corona, J. Antifungal Activity of Bacillus thuringiensis Chitinase and Its Potential for the Biocontrol of Phytopathogenic Fungi in Soybean Seeds. J. Food Sci. 2004, 69, 131–134. [Google Scholar] [CrossRef]
- Zhou, Y.; Choi, Y.L.; Sun, M.; Yu, Z. Novel Roles of Bacillus thuringiensis to Control Plant Diseases. Appl. Microbiol. Biotechnol. 2008, 80, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Migeon, A.; Dorkeld, F. Spider Mites Web: A Comprehensive Database for the Tetranychidae. Available online: http://www.montpellier.inra.fr/CBGP/spmweb (accessed on 12 September 2022).
- Helle, W.; Sabelis, M.W. Spider Mites. Their Biology, Natural Enemies and Control; Helle, W., Sabelis, M.W., Eds.; Elsevier Science Publisher B.V.: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Engelbrecht, G.; Horak, I.; Jansen van Rensburg, P.J.; Claassens, S. Bacillus-Based Bionematicides: Development, Modes of Action and Commercialisation. Biocontrol Sci. Technol. 2018, 28, 629–653. [Google Scholar] [CrossRef]
- Ciancio, A.; Mukerji, K.G. Integrated Management of Fruit Crops and Forest Nematodes; Ciancio, A., Mukerji, K.G., Eds.; Springer Science & Business Media: Bari, Italy, 2009. [Google Scholar]
- Jung, C.; Wyss, U. New Approaches to Control Plant Parasitic Nematodes. Appl. Microbiol. Biotechnol. 1999, 51, 439–446. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Lakra, N.; Marwal, A.; Sudheep, N.M.; Anwar, K. Crop Genetic Engineering: An Approach to Improve Fungal Resistance in Plant System. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: Berlin, Germany, 2017; pp. 581–591. ISBN 9789811065934. [Google Scholar]
- Nauen, R.; Stumpf, N.; Elbert, A.; Zebitz, C.P.W.; Kraus, W. Acaricide Toxicity and Resistance in Larvae of Different Strains of Tetranychus urticae and Panonychus ulmi (Acari: Tetranychidae). Pest Manag. Sci. 2001, 57, 253–261. [Google Scholar] [CrossRef]
- Wei, J.-Z.; Hale, K.; Carta, L.; Platzer, E.; Wong, C.; Fang, S.-C.; Aroian, R. V Bacillus Thuringiensis Crystal Proteins That Target Nematodes. Proc. Natl. Acad. Sci. USA 2003, 100, 2760–2765. [Google Scholar] [CrossRef]
- Guo, Y.; Weng, M.; Sun, Y.; Carballar-Lejarazú, R.; Wu, S.; Lian, C. Bacillus thuringiensis Toxins with Nematocidal Activity against the Pinewood Nematode Bursaphelenchus xylophilus. J. Invertebr. Pathol. 2022, 189, 107726. [Google Scholar] [CrossRef]
- Iatsenko, I.; Boichenko, I.; Sommer, R.J. Bacillus thuringiensis DB27 Produces Two Novel Protoxins, Cry21Fa1 and Cry21Ha1, Which Act Synergistically against Nematodes. Appl. Environ. Microbiol. 2014, 80, 3266–3275. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, J.; Xie, Y.; Lin, H.; Huang, Z.; Xu, L.; Gelbic, I.; Guan, X. Biological Activity of Bacillus thuringiensis (Bacillales: Bacillaceae) Chitinase against Caenorhabditis elegans (Rhabditida: Rhabditidae). J. Econ. Entomol. 2014, 107, 551–558. [Google Scholar] [CrossRef]
- Luo, X.; Chen, L.; Huang, Q.; Zheng, J.; Zhou, W.; Peng, D.; Ruan, L.; Sun, M. Bacillus thuringiensis Metalloproteinase Bmp1 Functions as a Nematicidal Virulence Factor. Appl. Environ. Microbiol. 2013, 79, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Lin, J.; Huang, Q.; Zheng, W.; Liu, G.; Zheng, J.; Zhu, L.; Sun, M. A Novel Metalloproteinase Virulence Factor Is Involved in Bacillus thuringiensis Pathogenesis in Nematodes and Insects. Environ. Microbiol. 2016, 18, 846–862. [Google Scholar] [CrossRef] [PubMed]
- Sick, A.J.; Schwab, G.E.; Payne, J.M. Genes Encoding Nematode-Active Toxins Cloned from Bacillus thuringiensis Isolate PS17. U.S. Patent 5,281,530, 1994. [Google Scholar]
- Xu, C.; Chinte, U.; Chen, L.; Yao, Q.; Meng, Y.; Zhou, D.; Bi, L.J.; Rose, J.; Adang, M.J.; Wang, B.C.; et al. Crystal Structure of Cry51Aa1: A Potential Novel Insecticidal Aerolysin-Type β-Pore-Forming Toxin from Bacillus thuringiensis. Biochem. Biophys. Res. Commun. 2015, 462, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Chai, L.; Wang, F.; Zhang, F.; Ruan, L.; Sun, M. Synergistic Activity between Bacillus thuringiensis Cry6Aa and Cry55Aa Toxins against Meloidogyne incognita. Microb. Biotechnol. 2011, 4, 794–798. [Google Scholar] [CrossRef]
- Ni, H.; Zeng, S.; Qin, X.; Sun, X.; Zhang, S.; Zhao, X.; Yu, Z.; Li, L. Molecular Docking and Site-Directed Mutagenesis of a Bacillus thuringiensis Chitinase to Improve Chitinolytic, Synergistic Lepidopteran-Larvicidal and Nematicidal Activities. Int. J. Biol. Sci. 2015, 11, 304–3015. [Google Scholar] [CrossRef]
- Tang, Y.; Zou, J.; Zhang, L.; Li, Z.; Ma, C.; Ma, N. Anti-Fungi Activities of Bacillus thuringiensis H3 Chitinase and Immobilized Chitinase Particles and Their Effects to Rice Seedling Defensive Enzymes. J. Nanosci. Nanotechnol. 2012, 12, 8081–8086. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Brévault, T.; Carrière, Y. Insect Resistance to Bt Crops: Lessons from the First Billion Acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar] [CrossRef]
- Petzold-Maxwell, J.L.; Alves, A.P.; Estes, R.E.; Gray, M.E.; Meinke, L.J.; Shields, E.J.; Thompson, S.D.; Tinsley, N.A.; Gassmann, A.J. Applying an Integrated Refuge to Manage Western Corn Rootworm (Coleoptera: Chrysomelidae): Effects on Survival, Fitness, and Selection Pressure. J. Econ. Entomol. 2013, 106, 2195–2207. [Google Scholar] [CrossRef][Green Version]
- Jeppson, L.R.; Keifer, H.H.; Baker, E.W. Mites Injurious to Economic Plants; Jeppson, L.R., Keifer, H.H., Baker, E.W., Eds.; California Univ. Press: Berkeley, CA, USA, 1975. [Google Scholar]
- Alquisira-Ramírez, E.V.; Paredes-Gonzalez, J.R.; Hernández-Velázquez, V.M.; Ramírez-Trujillo, J.A.; Peña-Chora, G. In Vitro Susceptibility of Varroa destructor and Apis mellifera to Native Strains of Bacillus thuringiensis. Apidologie 2014, 45, 707–718. [Google Scholar] [CrossRef]
- Dunstand-Guzmán, E.; Peña-Chora, G.; Hallal-Calleros, C.; Pérez-Martínez, M.; Hernández-Velazquez, V.M.; Morales-Montor, J.; Flores-Pérez, F.I. Acaricidal Effect and Histological Damage Induced by Bacillus thuringiensis Protein Extracts on the Mite Psoroptes cuniculi. Parasites Vectors 2015, 8, 285. [Google Scholar] [CrossRef]
- Dunstand-Guzmán, E.; Hallal-Calleros, C.; Morales-Montor, J.; Hernández-Velázquez, V.M.; Zárate-Ramos, J.J.; Hoffman, K.L.; Peña-Chora, G.; Flores-Pérez, F.I. Therapeutic Use of Bacillus thuringiensis in the Treatment of Psoroptic Mange in Naturally Infested New Zealand Rabbits. Vet. Parasitol. 2017, 238, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Neethu, K.B.; Priji, P.; Unni, K.N.; Sajith, S.; Sreedevi, S.; Ramani, N.; Anitha, K.; Rosana, B.; Girish, M.B.; Benjamin, S. New Bacillus thuringiensis Strain Isolated from the Gut of Malabari Goat Is Effective against Tetranychus macfarlanei. J. Appl. Entomol. 2016, 140, 187–198. [Google Scholar] [CrossRef]
- Krieg, A. Effectiveness of Bacillus thuringiensis Exotoxin on Tetranychus telarius (Acarina: Tetranychidae). J. Invertebr. Pathol. 1968, 12, 478. [Google Scholar] [CrossRef]
- Hall, I.M.; Hinter, D.K.; Arakawa, K.Y. The Effect of the B-Exotoxin Fraction Bacillus thuringiensis on the Citrus Red Mite. J. Invertebr. Pathol. 1971, 18, 359–362. [Google Scholar] [CrossRef]
- Hoy, M.A.; Ouyang, Y.-L. Toxicity of the B-Exotoxin of Bacillus thuringiensis to Tetranychus pacificus and Metaseiulus occidentalis (Acari: Tetranychidae and Phytoseiidae). J. Econ. Entomol. 1987, 80, 507–511. [Google Scholar] [CrossRef]
- Royalty, R.N.; Hall, F.R.; Taylor, R.A.J. Effects of Thuringiensin on Tetranychus urticae (Acari: Tetranychidae) Mortality, Fecundity, and Feeding. J. Econ. Entomol. 1990, 83, 792–798. [Google Scholar] [CrossRef]
- Royalty, R.N.; Hall, F.R.; Lucius, B.A. Effects of Thuringiensin on European Red Mite (Acarina: Tetranychidae) Mortality, Oviposition Rate and Feeding. Pestic. Sci. 1991, 33, 383–391. [Google Scholar] [CrossRef]
- Böckenhoff, A.; Grundler, F.M.W. Studies on the Nutrient Uptake by the Beet Cyst Nematode Heterodera schachtii by in Situ Microinjection of Fluorescent Probes into the Feeding Structures in Arabidopsis thaliana. Parasitology 1994, 109, 249–255. [Google Scholar] [CrossRef]
- Zhang, F.; Peng, D.; Ye, X.; Yu, Z.; Hu, Z.; Ruan, L.; Sun, M. In Vitro Uptake of 140 KDa Bacillus thuringiensis Nematicidal Crystal Proteins by the Second Stage Juvenile of Meloidogyne hapla. PLoS ONE 2012, 7, e38534. [Google Scholar] [CrossRef]
- Zheng, D.; Zeng, Z.; Xue, B.; Deng, Y.; Sun, M.; Tang, Y.J.; Ruan, L. Bacillus thuringiensis Produces the Lipopeptide Thumolycin to Antagonize Microbes and Nematodes. Microbiol. Res. 2018, 215, 22–28. [Google Scholar] [CrossRef]
- Baghaee-Ravari, S.; Mahdikani-Moghaddam, E. Efficacy of Bacillus thuringiensis Cry14 Toxin against Root Knot Nematode, Meloidogyne javanica. Plant Prot. Sci. 2015, 51, 46–51. [Google Scholar] [CrossRef]
- Mohammed, S.H.; El Saedy, M.A.; Enan, M.R.; Ibrahim, N.E.; Ghareeb, A.; Moustafa, S.A. Biocontrol Efficiency of Bacillus Thuringiensis Toxins against Root-Knot Nematode, Meloidogyne incognita. J. Cell Mol. Biol. 2008, 7, 57–66. [Google Scholar]
- Prasad, S.S.V.; Tilak, K.V.B.R.; Gollakota, R.G. Role of Bacillus thuringiensis Var. thuringiensis on the Larval Survivability and Egg Hatching of Meloidogyne spp., the Causative Agent of Root Knot Disease. J. Invertebr. Pathol. 1972, 20, 377–378. [Google Scholar] [CrossRef]
- Devidas, P.; Rehberger, L.A. The Effect of Exotoxin (Thuringiensin) from Bacillus thuringiensis on Meloidogyne incognita and Caenorhabditis elegans. Plant Soil 1992, 145, 115–120. [Google Scholar] [CrossRef]
- Ignoffo, C.M.; Dropkin, V.H. Deleterious Effects of the Thermostable Toxin of Bacillus thuringiensis on Species of Soil-Inhabiting, Myceliophagus, and Plant-Parasitic Nematodes. J. Kansas Entomol. Soc. 1977, 50, 394–398. [Google Scholar]
- Liu, X.; Ruan, L.; Peng, D.; Li, L.; Sun, M.; Yu, Z. Thuringiensin: A Thermostable Secondary Metabolite from Bacillus thuringiensis with Insecticidal Activity against a Wide Range of Insects. Toxins 2014, 6, 2229–2238. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological Control of Plant Pathogens by Bacillus Species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef]
- Slama, H.B.; Cherif-Silini, H.; Bouket, A.C.; Qader, M.; Silini, A.; Yahiaoui, B.; Alenezi, F.N.; Luptakova, L.; Triki, M.A.; Vallat, A.; et al. Screening for Fusarium Antagonistic Bacteria from Contrasting Niches Designated the Endophyte Bacillus halotolerans as Plant Warden against Fusarium. Front. Microbiol. 2019, 9, 3236. [Google Scholar] [CrossRef]
- He, C.N.; Ye, W.Q.; Zhu, Y.Y.; Zhou, W.W. Antifungal Activity of Volatile Organic Compounds Produced by Bacillus methylotrophicus and Bacillus Thuringiensis against Five Common Spoilage Fungi on Loquats. Molecules 2020, 25, 3360. [Google Scholar] [CrossRef]
- Kim, P.I.; Bai, H.; Bai, D.; Chae, H.; Chung, S.; Kim, Y.; Park, R.; Chi, Y.T. Purification and Characterization of a Lipopeptide Produced by Bacillus thuringiensis CMB26. J. Appl. Microbiol. 2004, 97, 942–949. [Google Scholar] [CrossRef]
- Roy, A.; Mahata, D.; Paul, D.; Korpole, S.; Franco, O.L.; Mandal, S.M. Purification, Biochemical Characterization and Self-Assembled Structure of a Fengycin-like Antifungal Peptide from Bacillus thuringiensis Strain SM1. Front. Microbiol. 2013, 4, 332. [Google Scholar] [CrossRef] [PubMed]
- Béchet, M.; Caradec, T.; Hussein, W.; Abderrahmani, A.; Chollet, M.; Leclére, V.; Dubois, T.; Lereclus, D.; Pupin, M.; Jacques, P. Structure, Biosynthesis, and Properties of Kurstakins, Nonribosomal Lipopeptides from Bacillus spp. Appl. Microbiol. Biotechnol. 2012, 95, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Iriarte, J.; Bel, Y.; Ferrandis, M.D.; Andrew, R.; Murillo, J.; Ferré, J.; Caballero, P. Environmental Distribution and Diversity of Bacillus thuringiensis in Spain. Syst. Appl. Microbiol. 1998, 21, 97–106. [Google Scholar] [CrossRef]
- Stewart, G.S.A.B.; Johnstone, K.; Hagelberg, E.; Ellar, D.J. Commitment of Bacterial Spores to Germinate. Biochem. J. 1981, 198, 101–106. [Google Scholar] [CrossRef]
- Borodovsky, M.; McIninch, J. GENMARK: Parallel Gene Recognition for Both DNA Strands. Comput. Chem. 1993, 17, 123–133. [Google Scholar] [CrossRef]
- Crickmore, N.; Zeigler, D.R.; Feitelson, J.; Schnepf, E.; Van Rie, J.; Lereclus, D.; Baum, J.; Dean, D.H. Revision of the Nomenclature for the Bacillus thuringiensis Pesticidal Crystal Proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 807–813. [Google Scholar] [CrossRef]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. Bacterial Pesticidal Protein Resource Center. Available online: https://www.bpprc-db.org (accessed on 9 October 2022).
- Malovichko, Y.V.; Nizhnikov, A.A.; Antonets, K.S. Repertoire of the Bacillus thuringiensis Virulence Factors Unrelated to Major Classes of Protein Toxins and Its Role in Specificity of Host-Pathogen Interactions. Toxins 2019, 11, 347. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional Classification of Proteins via Subfamily Domain Architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and Sequence Analysis Tools Services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantification of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.S.; Ferre, J.; Larget-Thiery, I. Update on the Detection of Beta-Exotoxin in Bacillus thuringiensis Strains by HPLC Analysis. J. Appl. Microbiol. 2001, 90, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Hussey, R.S.; Barker, K.R. A Comparison of Methods of Collecting Inocula of Meloidogyne spp., Including a New Technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Domínguez-Arrizabalaga, M.; Villanueva, M.; Fernandez, A.B.; Caballero, P. A Strain of Bacillus thuringiensis Containing a Novel Cry7Aa2 Gene That Is Toxic to Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Insects 2019, 10, 259. [Google Scholar] [CrossRef]
- Griffitts, J.S.; Whitacre, J.L.; Stevens, D.E.; Aroian, R.V. Bt Toxin Resistance from Loss of a Putative Carbohydrate-Modifying Enzyme. Science 2001, 293, 860–864. [Google Scholar] [CrossRef]
- Terefe, M.; Tefera, T.; Sakhuja, P.K. Effect of a Formulation of Bacillus Firmus on Root-Knot Nematode Meloidogyne incognita Infestation and the Growth of Tomato Plants in the Greenhouse and Nursery. J. Invertebr. Pathol. 2009, 100, 94–99. [Google Scholar] [CrossRef]
- Sharma, R.D. Bacillus thuringiensis: A Biocontrol Agent of Meloidogyne incognita on Barley. Nematol. Bras. 1994, 18, 79–84. [Google Scholar]
- Yu, Z.; Xiong, J.; Zhou, Q.; Luo, H.; Hu, S.; Xia, L.; Sun, M.; Li, L.; Yu, Z. The Diverse Nematicidal Properties and Biocontrol Efficacy of Bacillus thuringiensis Cry6A against the Root-Knot Nematode Meloidogyne hapla. J. Invertebr. Pathol. 2015, 125, 73–80. [Google Scholar] [CrossRef]
- Lyousfi, N.; Lahlali, R.; Letrib, C.; Belabess, Z.; Ouaabou, R.; Ennahli, S.; Blenzar, A.; Barka, E.A. Improving the Biocontrol Potential of Bacterial Antagonists with Salicylic Acid against Brown Rot Disease and Impact on Nectarine Fruits Quality. Agronomy 2021, 11, 209. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]



| Target Database | Pairwise Identity (%) | MW (kDa) | Length (No. Amino Acid Residues) | Amino Acids Overlap in Global Alignment | Accession Number of Reference | Accession Number |
|---|---|---|---|---|---|---|
| Crystal proteins | ||||||
| Mpp51Aa2 | 98 | 34.5 | 312 | 312 | ADK94873.1 | OP696897 |
| Cry5Ad1 | 36 | 119.2 | 1092 | 1148 | ABQ82087.1 | OP604599 |
| Orf2_cry65Aa | 96 | 58.4 | 512 | 490 | AEB52308.1 | OP722690 |
| Mpp2Aa6 | 30 | 46.2 | 412 | 136 | U41822.1 | OP722691 |
| Secreted factors | ||||||
| Exochitinase | 99 | 39.4 | 360 | 360 | AIE34993.1 | OP722692 |
| ColB (metalloprotease) | 77 | 48.3 | 426 | 428 | ACZ37253.1 | OP722693 |
| Bmp1 (metalloprotease) | 34 | 65.3 | 592 | 367 | AFZ77001.1 | OP722694 |
| LC | Concentration (μg/mL) | Lower Limits | Upper Limits | χ2 | df | Slope | SE Slope | Intercept |
|---|---|---|---|---|---|---|---|---|
| LC50 | 10.5 | 6.99 | 15.0 | 1.96 | 5 | 0.947 | 0.111 | −0.969 |
| Treatment | Mortality (%) |
|---|---|
| BMB171-Cry5-orf65 | 53.3 ± 16.3 a |
| BMB171-pSTAB | 11.7 ± 3.4 b |
| Treatment | J2 Mortality (%) Mean ± SE |
|---|---|
| Control | 15.5 ± 4.3 a |
| BST-122 s + c | 24.7 ± 7.9 ab |
| BST-122 SN | 61.1 ± 14.7 b |
| BST-122 WC | 52.5 ± 6.3 ab |
| Treatment | No. of Galls/ Plant 1 | % Reduction of Galls/Plant |
|---|---|---|
| Control | 257.6 ± 13.9 a | - |
| BST-122 s + c | 209.2 ± 17.1 a | 18.8% |
| BST-122 SN | 112.4 ± 14.1 b | 56.4% |
| BST-122 WC | 76.0 ± 14.8 b | 70.5% |
| Fungal Pathogen | Incubation Time (Days) | Biomass Surface | % Growth Inhibition | Significative Differences | |
|---|---|---|---|---|---|
| Control | Bt Treated | ||||
| Verticillium dahliae | 6 | 5.17 ± 0.26 | 3.89 ± 0.24 | 24.76% | ** |
| 12 | 10.46 ± 0.70 | 3.92 ± 0.42 | 63.90% | *** | |
| Fusarium oxysporum lycopersici | 4 | 17.69 ± 0.89 | 14.96 ± 0.50 | 15.43% | * |
| 5 | 24.77 ± 1.07 | 18.48 ± 0.51 | 25.39% | *** | |
| 6 | 35.82 ± 2.43 | 24.08 ± 1.18 | 32.77% | ** | |
| 7 | 39.2 ± 1.96 | 25.98 ± 0.97 | 33.72% | *** | |
| Fusarium oxysporum melonis | 4 | 19.32 ± 0.51 | 15.88 ± 0.29 | 17.80% | *** |
| 5 | 27.30 ± 0.78 | 20.61 ± 0.46 | 24.50% | *** | |
| 6 | 37.34 ± 2.15 | 25.89 ± 1.34 | 30.66% | *** | |
| 7 | 39.70 ± 1.92 | 27.66 ± 1.47 | 30.33% | *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unzue, A.; Caballero, C.J.; Villanueva, M.; Fernández, A.B.; Caballero, P. Multifunctional Properties of a Bacillus thuringiensis Strain (BST-122): Beyond the Parasporal Crystal. Toxins 2022, 14, 768. https://doi.org/10.3390/toxins14110768
Unzue A, Caballero CJ, Villanueva M, Fernández AB, Caballero P. Multifunctional Properties of a Bacillus thuringiensis Strain (BST-122): Beyond the Parasporal Crystal. Toxins. 2022; 14(11):768. https://doi.org/10.3390/toxins14110768
Chicago/Turabian StyleUnzue, Argine, Carlos J. Caballero, Maite Villanueva, Ana Beatriz Fernández, and Primitivo Caballero. 2022. "Multifunctional Properties of a Bacillus thuringiensis Strain (BST-122): Beyond the Parasporal Crystal" Toxins 14, no. 11: 768. https://doi.org/10.3390/toxins14110768
APA StyleUnzue, A., Caballero, C. J., Villanueva, M., Fernández, A. B., & Caballero, P. (2022). Multifunctional Properties of a Bacillus thuringiensis Strain (BST-122): Beyond the Parasporal Crystal. Toxins, 14(11), 768. https://doi.org/10.3390/toxins14110768






