Stability and Chemical Conversion of the Purified Reference Material of Gymnodimine-A under Different Temperature and pH Conditions
Abstract
1. Introduction
2. Results and Discussion
2.1. Homogeneity Assessment
2.2. Stability Assessment
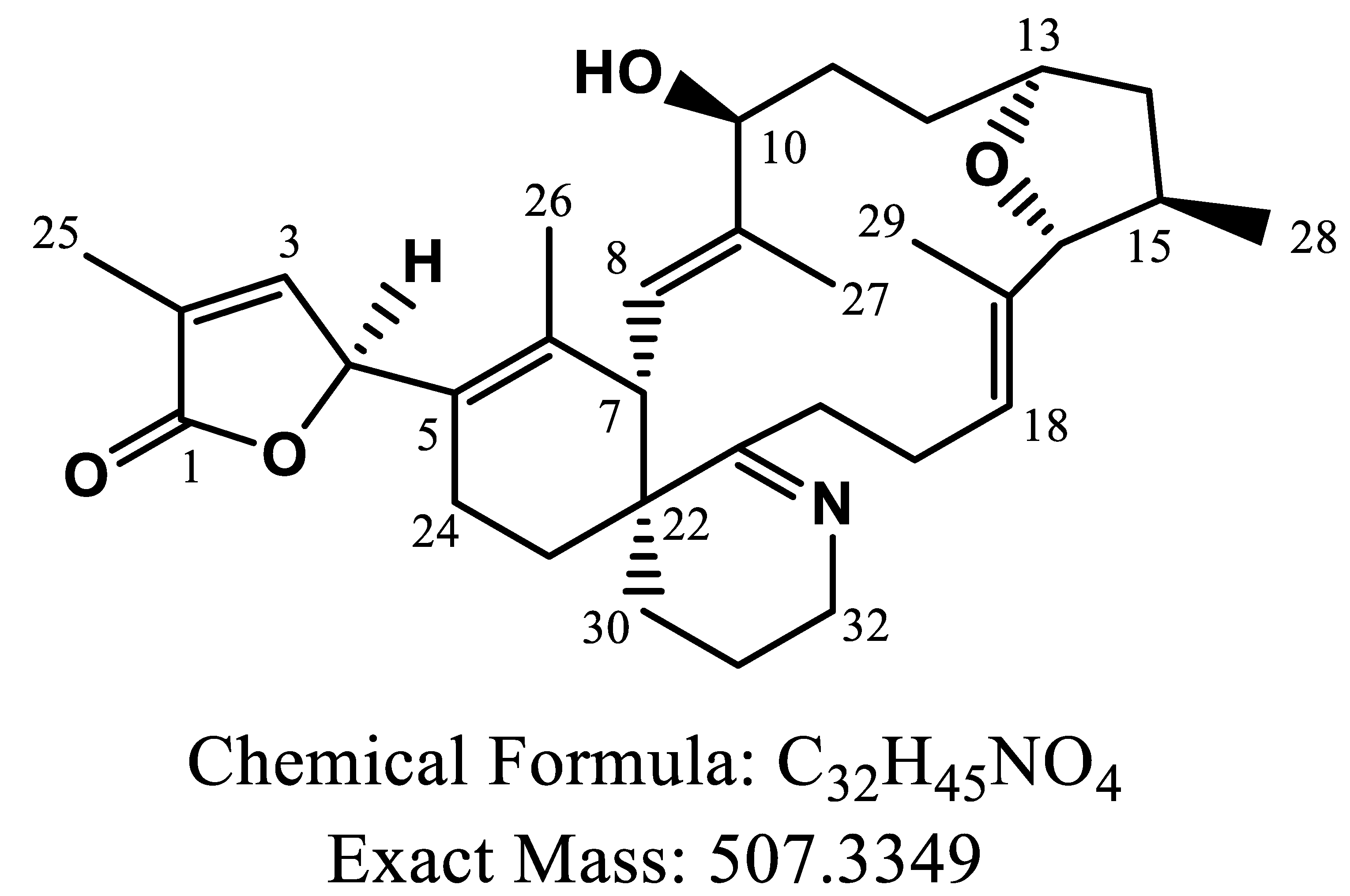
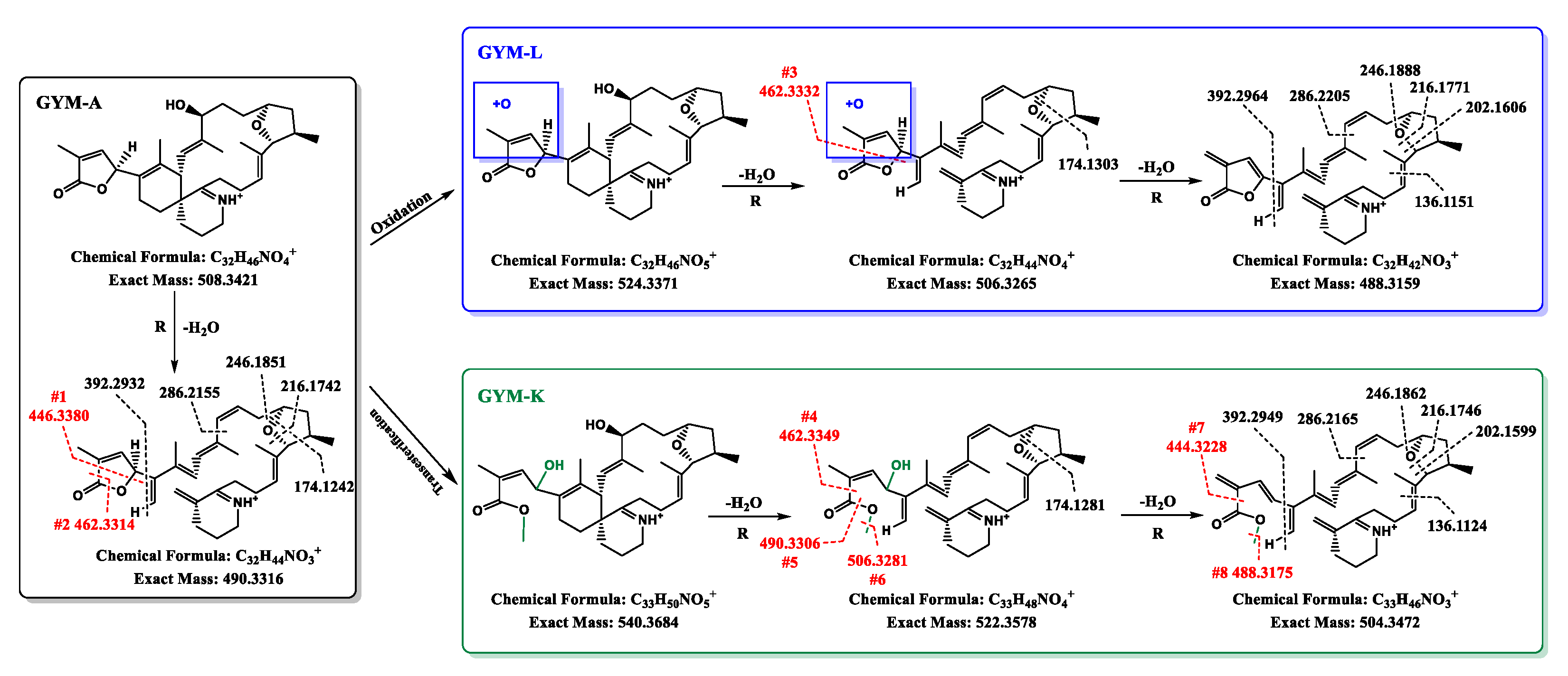
2.3. Hypothetical Structure of the Converting Products of GYM-A
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Biological Source and Preparation of GYM-A
4.2.1. Culture of Karenia Selliformis
4.2.2. Extraction and Purification of GYM-A
4.3. Homogeneity Assessment
4.4. Stability Assessment
4.5. The LC-MS/MS Analysis Method
4.6. The LC-HRMS/MS Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seki, T.; Satake, M.; Mackenzie, L.; Kaspar, H.F.; Yasumoto, T. Gymnodimine, a new marine toxin of unprecedented structure isolated from New Zealand oysters and the dinoflagellate, Gymnodinium sp. Tetrahedron Lett. 1995, 36, 7093–7096. [Google Scholar] [CrossRef]
- Van de Waal, D.B.; Tillmann, U.; Martens, H.; Krock, B.; van Scheppingen, Y.; John, U. Characterization of multiple isolates from an Alexandrium ostenfeldii bloom in The Netherlands. Harmful Algae 2015, 49, 94–104. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, L.; Li, Y.; Zhang, J.; Tan, Z.; Wu, H.; Jiang, T.; Lu, S. Occurrence of marine algal toxins in oyster and phytoplankton samples in Daya Bay, South China Sea. Chemosphere 2017, 183, 80–88. [Google Scholar] [CrossRef]
- Hassoun, A.E.R.; Ujevic, I.; Mahfouz, C.; Fakhri, M.; Roje-Busatto, R.; Jemaa, S.; Nazlic, N. Occurrence of domoic acid and cyclic imines in marine biota from Lebanon-Eastern Mediterranean Sea. Sci. Total Environ. 2021, 755, 142542. [Google Scholar] [CrossRef] [PubMed]
- Lamas, J.P.; Arevalo, F.; Morono, A.; Correa, J.; Rossignoli, A.E.; Blanco, J. Gymnodimine A in mollusks from the north Atlantic Coast of Spain: Prevalence, concentration, and relationship with spirolides. Environ. Pollut. 2021, 279, 116919. [Google Scholar] [CrossRef]
- Ji, Y.; Yan, G.; Wang, G.; Liu, J.; Tang, Z.; Yan, Y.; Qiu, J.; Zhang, L.; Pan, W.; Fu, Y.; et al. Prevalence and distribution of domoic acid and cyclic imines in bivalve mollusks from Beibu Gulf, China. J. Hazard. Mater. 2022, 423, 127078. [Google Scholar] [CrossRef]
- Munday, R.; Towers, N.R.; Mackenzie, L.; Beuzenberg, V.; Holland, P.T.; Miles, C.O. Acute toxicity of gymnodimine to mice. Toxicon 2004, 44, 173–178. [Google Scholar] [CrossRef]
- Bourne, Y.; Radic, Z.; Araoz, R.; Talley, T.T.; Benoit, E.; Servent, D.; Taylor, P.; Molgo, J.; Marchot, P. Structural determinants in phycotoxins and AChBP conferring high affinity binding and nicotinic AChR antagonism. Proc. Natl. Acad. Sci. USA 2010, 107, 6076–6081. [Google Scholar] [CrossRef]
- Marrouchi, R.; Rome, G.; Kharrat, R.; Molgo, J.; Benoit, E. Analysis of the action of gymnodimine-A and 13-desmethyl spirolide C on the mouse neuromuscular system in vivo. Toxicon 2013, 75, 27–34. [Google Scholar] [CrossRef]
- Nieva, J.A.; Krock, B.; Tillmann, U.; Tebben, J.; Zurhelle, C.; Bickmeyer, U. Gymnodimine A and 13-desMethyl Spirolide C Alter Intracellular Calcium Levels via Acetylcholine Receptors. Toxins 2020, 12, 751. [Google Scholar] [CrossRef]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. New analogue of gymnodimine from a Gymnodinium species. J. Agric. Food Chem. 2000, 48, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. Gymnodimine C, an isomer of gymnodimine B, from Karenia selliformis. J. Agric. Food Chem. 2003, 51, 4838–4840. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, R.M.; Misner, I.; Tomas, C.R.; Wright, J.L.C. Occurrence of 12-methylgymnodimine in a spirolide-producing dinoflagellate Alexandrium peruvianum and the biogenetic implications. Tetrahedron Lett. 2011, 52, 4243–4246. [Google Scholar] [CrossRef]
- Strangman, W.; Anttila, M.; Tomas, C.; Wright, J. (5S)-5-[(4aR,8aS,9E,11S,13R,14S,16R,17R,19S)-11,19-Dihydroxy-8,10,13,16-tetramethyl-18-methylidene-3,4,5,6,8a,11,12,13,14,15,16,17,18,19,20,21-hexadecahydro-2H-14,17-epoxybenzo [2,3]cyclohexadeca [1,2-b]pyridine-7-yl]-3-methylfuran-2(5H)-one (12-Methylgymnodimine B). Molbank 2016, 2016, M896. [Google Scholar] [CrossRef]
- Harju, K.; Koskela, H.; Kremp, A.; Suikkanen, S.; de la Iglesia, P.; Miles, C.O.; Krock, B.; Vanninen, P. Identification of gymnodimine D and presence of gymnodimine variants in the dinoflagellate Alexandrium ostenfeldii from the Baltic Sea. Toxicon 2016, 112, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zurhelle, C.; Nieva, J.; Tillmann, U.; Harder, T.; Krock, B.; Tebben, J. Identification of Novel Gymnodimines and Spirolides from the Marine Dinoflagellate Alexandrium ostenfeldii. Mar. Drugs 2018, 16, 446. [Google Scholar] [CrossRef]
- Haywood, A.J.; Steidinger, K.A.; Truby, E.W.; Bergquist, P.R.; Bergquist, P.L.; Adamson, J.; Mackenzie, L. Comparative morphology and molecular phylogenetic analysis of three new species of the genus Karenia (Dinophyceae) from New Zealand. J. Phycol. 2004, 40, 165–179. [Google Scholar] [CrossRef]
- Medhioub, A.; Medhioub, W.; Amzil, Z.; Sibat, M.; Bardouil, M.; Ben Neila, I.; Mezghani, S.; Hamza, A.; Lassus, P. Influence of environmental parameters on Karenia selliformis toxin content in culture. Cah. Biol. Mar. 2009, 50, 333–342. [Google Scholar]
- Li, S.; Cen, J.; Wang, J.; Lv, S. Study on acute toxicity of two Karenia (Dinophyceae) species to rotifer Brachionus plicatilis. Mar. Environ. Sci. 2018, 37, 61–66. [Google Scholar]
- Ji, Y.; Che, Y.; Wright, E.J.; McCarron, P.; Hess, P.; Li, A. Fatty acid ester metabolites of gymnodimine in shellfish collected from China and in mussels (Mytilus galloprovincialis) exposed to Karenia selliformis. Harmful Algae 2020, 92, 101774. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Ji, Y.; Qiu, J.; Wang, G.; Tang, Z.; Li, A. Comparative study on the esterification of gymnodimine in different shellfish exposed to the dissolved toxin in seawater. Harmful Algae 2022, 115, 102233. [Google Scholar] [CrossRef] [PubMed]
- De la Iglesia, P.; McCarron, P.; Diogene, J.; Quilliam, M.A. Discovery of gymnodimine fatty acid ester metabolites in shellfish using liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Varriale, F.; Tartaglione, L.; Cinti, S.; Milandri, A.; Dall’Ara, S.; Calfapietra, A.; Dell’Aversano, C. Development of a data dependent acquisition-based approach for the identification of unknown fast-acting toxins and their ester metabolites. Talanta 2021, 224, 121842. [Google Scholar] [CrossRef]
- Indrasena, W.; Gill, T. Storage stability of paralytic shellfish poisoning toxins. Food Chem. 2000, 71, 71–77. [Google Scholar] [CrossRef]
- Beach, D.G.; Crain, S.; Lewis, N.; LeBlanc, P.; Hardstaff, W.R.; Perez, R.A.; Giddings, S.D.; Martinez-Farina, C.F.; Stefanova, R.; Burton, I.W.; et al. Development of Certified Reference Materials for Diarrhetic Shellfish Poisoning Toxins, Part 1: Calibration Solutions. J. AOAC Int. 2016, 99, 1151–1162. [Google Scholar] [CrossRef]
- Alfonso, C.; Rehmann, N.; Hess, P.; Alfonso, A.; Wandscheer, C.B.; Abuin, M.; Vale, C.; Otero, P.; Vieytes, M.R.; Botana, L.M. Evaluation of various pH and temperature conditions on the stability of azaspiracids and their importance in preparative isolation and toxicological studies. Anal. Chem. 2008, 80, 9672–9680. [Google Scholar] [CrossRef] [PubMed]
- Giddings, S.D.; Crain, S.; Thomas, K.; Quilliam, M.A.; McCarron, P. CRM-GYM-b: Certified calibration solution for gymnodimine. In Biotoxin Metrology Technical Report CRM-GYM-b-20120712; National Research Council of Canada: Halifax, NS, Canada, 2013. [Google Scholar]
- Che, Y.; Ding, L.; Qiu, J.; Ji, Y.; Li, A. Conversion and Stability of New Metabolites of Paralytic Shellfish Toxins under Different Temperature and pH Conditions. J. Agric. Food Chem. 2020, 68, 1427–1435. [Google Scholar] [CrossRef]
- Wright, E.J.; Beach, D.G.; McCarron, P. Non-target analysis and stability assessment of reference materials using liquid chromatographyhigh-resolution mass spectrometry. Anal. Chim. Acta 2022, 1201, 339622. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Qiu, J.; Wang, G.; Ji, Y.; Hess, P.; Li, A. Development of an Efficient Extraction Method for Harvesting Gymnodimine-A from Large-Scale Cultures of Karenia selliformis. Toxins 2021, 13, 793. [Google Scholar] [CrossRef]
- Mok, J.-S.; Lee, T.-S.; Oh, E.-G.; Son, K.-T.; Hwang, H.-J.; Kim, J.-H. Stability of domoic acid at different temperature, pH and light. Korean J. Fish. Aquat. Sci. 2009, 42, 8–14. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Qiu, J.; Hu, H.; Meng, F.; Li, A. Performance of different extraction methods for paralytic shellfish toxins and toxin stability in shellfish during storage. Anal. Bioanal. Chem. 2021, 413, 7597–7607. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.J.; Stivala, C.E.; Iorga, B.I.; Molgo, J.; Zakarian, A. Stability of cyclic imine toxins: Interconversion of pinnatoxin amino ketone and pinnatoxin A in aqueous media. J. Org. Chem. 2012, 77, 10435–10440. [Google Scholar] [CrossRef] [PubMed]
- Nagata, D.; Nishiwaki, N. Are acetic acid derivatives really negative to the iodoform test? SN Appl. Sci. 2021, 3, 788. [Google Scholar] [CrossRef]
- Cornu, D.; Guesmi, H.; Laugel, G.; Krafft, J.M.; Lauron-Pernot, H. On the relationship between the basicity of a surface and its ability to catalyze transesterification in liquid and gas phases: The case of MgO. Phys. Chem. Chem. Phys. 2015, 17, 14168–14176. [Google Scholar] [CrossRef]
- Quilliam, M.A.; Hardstaff, W.R.; Ishida, N.; McLachlan, J.L.; Reeves, A.R.; Ross, N.W.; Windust, A.J. Production of diarrhetic shellfish poisoning (DSP) toxins by Prorocentrum lima in culture and development of analytical methods. Harmful Toxic Algal Bloom. 1996, 289–292. [Google Scholar]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO Guide 35: Reference Materials—Guidance for Characterization and Assessment of Homogeneity and Stability; ISO: Geneva, Switzerland, 2017. [Google Scholar]
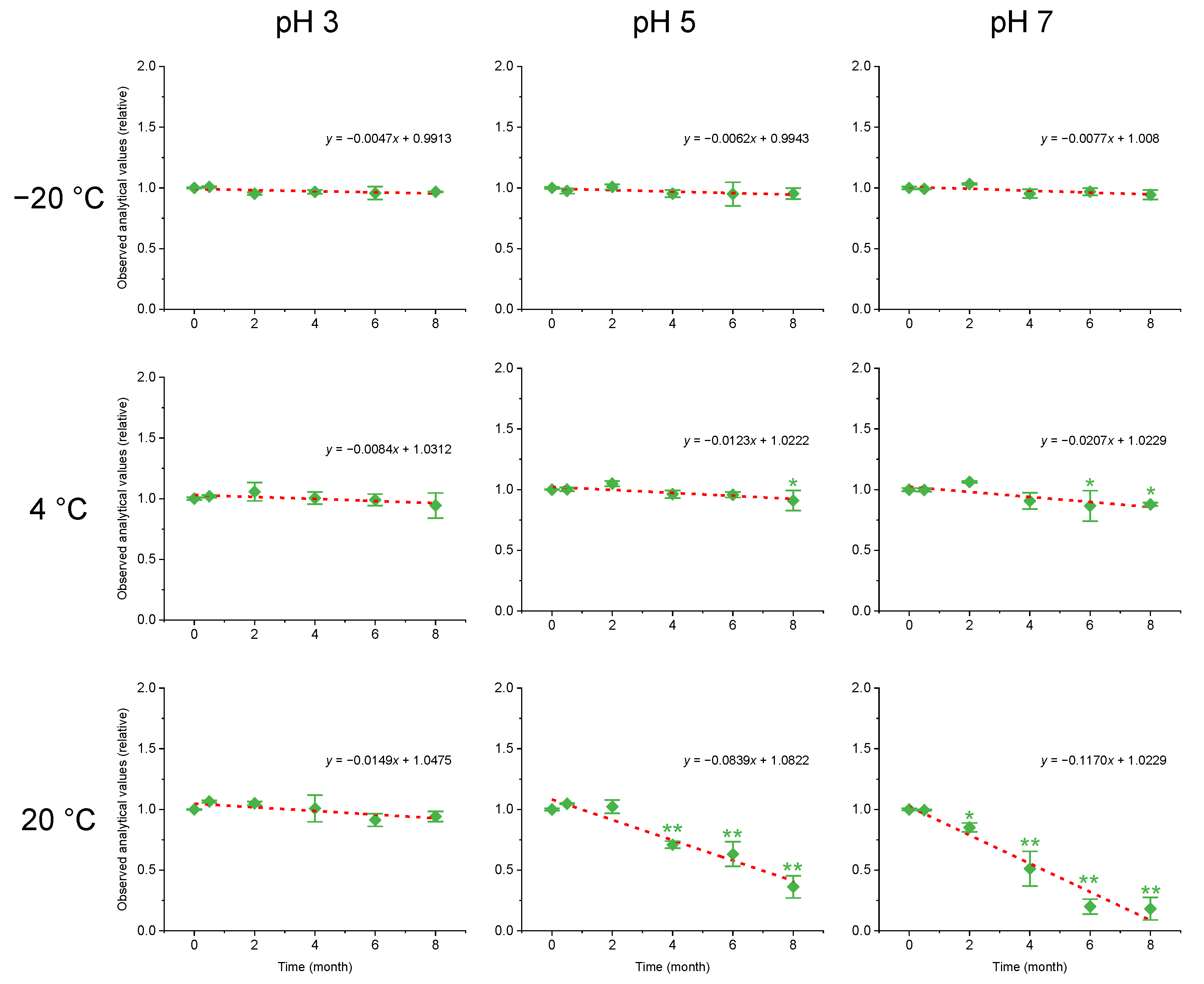



| Treatments | Slope | Standard Error | t | p | Stability | |
|---|---|---|---|---|---|---|
| −20 ℃ | pH 3 | −0.0047 | 0.0028 | −1.685 | 0.167 | Stable |
| pH 5 | −0.0062 | 0.0026 | −2.346 | 0.079 | Stable | |
| pH 7 | −0.0077 | 0.0035 | −2.226 | 0.090 | Stable | |
| 4 ℃ | pH 3 | −0.0084 | 0.0041 | −2.023 | 0.113 | Stable |
| pH 5 | −0.0123 | 0.0043 | −2.845 | 0.047 | Unstable | |
| pH 7 | −0.0207 | 0.0071 | −2.925 | 0.043 | Unstable | |
| 20 ℃ | pH 3 | −0.0149 | 0.0057 | −2.600 | 0.060 | Stable |
| pH 5 | −0.0839 | 0.0112 | −7.501 | 0.002 | Unstable | |
| pH 7 | −0.1170 | 0.0124 | −9.462 | < 0.001 | Unstable | |
| Fragment Ions | Molecular Formula | Theoretical Exact Mass (m/z) | Measured Exact Mass (m/z) | Δ ppm | Relative Abundance (%) |
|---|---|---|---|---|---|
| [M + H]+ | C33H50O5N+ | 540.3684 | 540.3698 | 2.59 | 12.3 |
| [M + H − H2O]+ | C33H48O4N+ | 522.3578 | 522.3591 | 2.49 | 100.0 |
| [M + H − 2H2O]+ | C32H46O3N+ | 504.3472 | 504.3491 | 3.77 | 3.4 |
| C8H12N+ | 122.0964 | 122.0966 | 1.64 | 54.0 | |
| C9H14N+ | 136.1121 | 136.1124 | 2.20 | 52.9 | |
| C11H14N+ | 162.1277 | 162.1281 | 2.47 | 32.9 | |
| C12H16N+ | 174.1277 | 174.1281 | 2.30 | 31.5 | |
| C14H20N+ | 202.1590 | 202.1599 | 4.45 | 25.4 | |
| C15H22N+ | 216.1747 | 216.1746 | −0.46 | 4.7 | |
| C16H24ON+ | 246.1853 | 246.1862 | 3.66 | 7.7 | |
| C19H30O2N+ | 304.2271 | 304.2269 | −0.66 | 3.3 | |
| C27H38ON+ | 392.2948 | 392.2949 | 0.25 | 8.3 | |
| C27H40O2N+ | 410.3054 | 410.3054 | 0.00 | 2.2 | |
| C31H42ON+ | 444.3261 | 444.3228 | −7.43 | 0.4 | |
| C31H44O2N+ | 462.3367 | 462.3349 | −3.89 | 2.8 | |
| C32H42O3N+ | 488.3159 | 488.3175 | 3.28 | 0.9 | |
| C32H44O3N+ | 490.3316 | 490.3306 | −2.04 | 1.7 | |
| C32H44O4N+ | 506.3265 | 506.3281 | 3.16 | 1.3 |
| ESI mode | Precursor ions [M + H]+ (m/z) | Product ions (m/z) | Fragmentor (V) | Collision Energy (eV) |
|---|---|---|---|---|
| ESI+ | 508.3 | 490.3 | 55 | 45 |
| 392.2 | 55 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Qiu, J.; Li, A.; Ji, Y.; Tang, Z.; Hess, P. Stability and Chemical Conversion of the Purified Reference Material of Gymnodimine-A under Different Temperature and pH Conditions. Toxins 2022, 14, 744. https://doi.org/10.3390/toxins14110744
Wang G, Qiu J, Li A, Ji Y, Tang Z, Hess P. Stability and Chemical Conversion of the Purified Reference Material of Gymnodimine-A under Different Temperature and pH Conditions. Toxins. 2022; 14(11):744. https://doi.org/10.3390/toxins14110744
Chicago/Turabian StyleWang, Guixiang, Jiangbing Qiu, Aifeng Li, Ying Ji, Zhixuan Tang, and Philipp Hess. 2022. "Stability and Chemical Conversion of the Purified Reference Material of Gymnodimine-A under Different Temperature and pH Conditions" Toxins 14, no. 11: 744. https://doi.org/10.3390/toxins14110744
APA StyleWang, G., Qiu, J., Li, A., Ji, Y., Tang, Z., & Hess, P. (2022). Stability and Chemical Conversion of the Purified Reference Material of Gymnodimine-A under Different Temperature and pH Conditions. Toxins, 14(11), 744. https://doi.org/10.3390/toxins14110744







