Cytotoxic Effects of Cannabidiol on Neonatal Rat Cortical Neurons and Astrocytes: Potential Danger to Brain Development
Abstract
1. Introduction
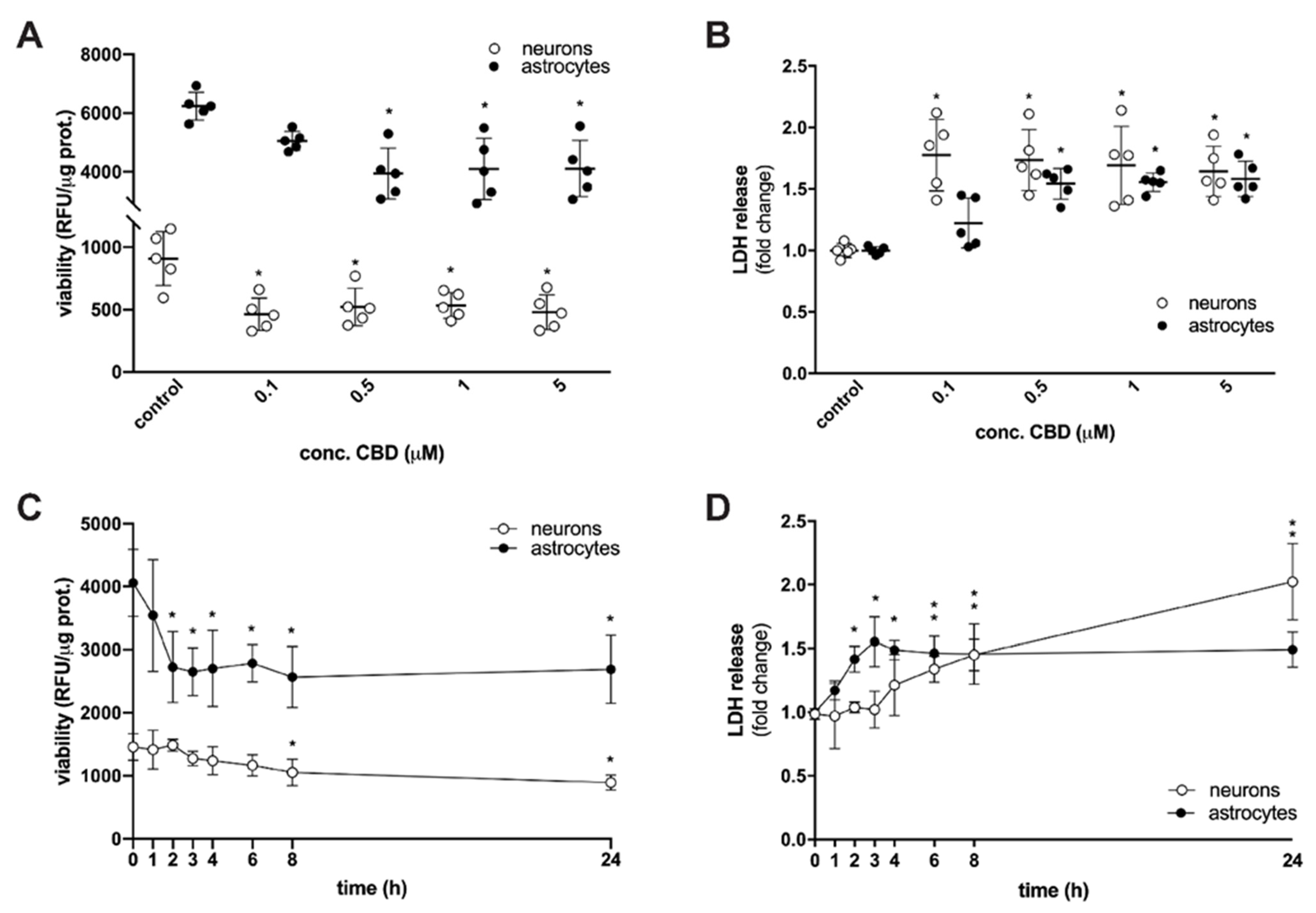
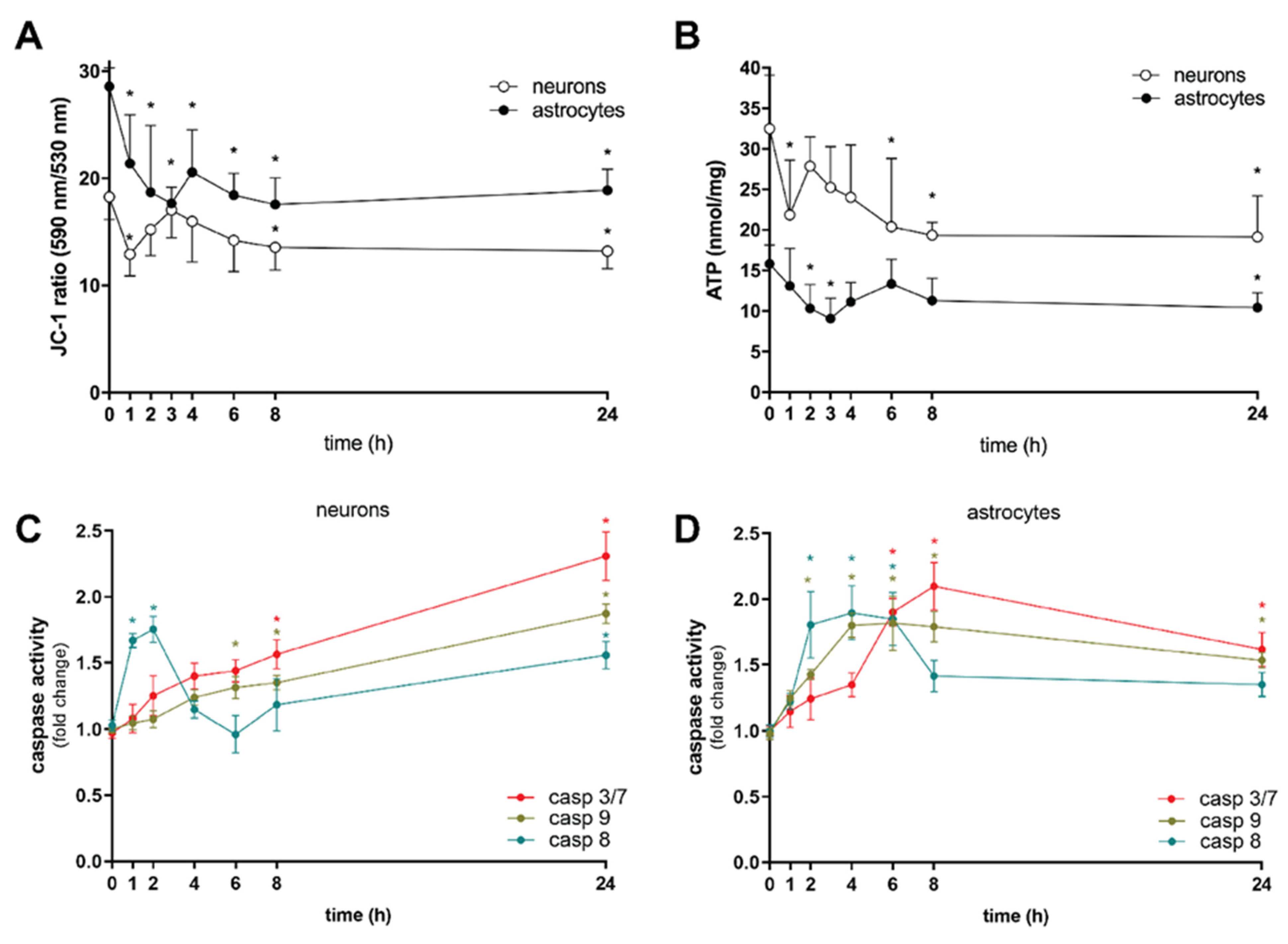
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Primary Cortical Neuron Culture
4.3. Primary Cortical Astrocyte Culture
4.4. Treatment
4.5. Cell Viability Assays
4.5.1. Metabolic Activity
4.5.2. LDH Release
4.5.3. Flow Cytometry Detection of Cell Death
4.6. Change in Mitochondrial Membrane Potential (ΔΨm)
4.7. ATP Measurement
4.8. Detection of Caspase Activity
4.9. Immunocytochemistry
4.10. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cadwell, C.R.; Bhaduri, A.; Mostajo-Radji, M.A.; Keefe, M.G.; Nowakowski, T.J. Development and Arealization of the Cerebral Cortex. Neuron 2019, 103, 980–1004. [Google Scholar] [CrossRef] [PubMed]
- Ullian, E.M.; Sapperstein, S.K.; Christopherson, K.S.; Barres, B.A. Control of synapse number by glia. Science 2001, 291, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural. Dev. 2018, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Alpár, A.; Di Marzo, V.; Harkany, T. At the Tip of an Iceberg: Prenatal Marijuana and Its Possible Relation to Neuropsychiatric Outcome in the Offspring. Biol. Psychiatry 2016, 79, e33–e45. [Google Scholar] [CrossRef]
- Bara, A.; Ferland, J.N.; Rompala, G.; Szutorisz, H.; Hurd, Y.L. Cannabis and synaptic reprogramming of the developing brain. Nat. Rev. Neurosci. 2021, 22, 423–438. [Google Scholar] [CrossRef]
- Ujváry, I.; Hanuš, L. Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy. Cannabis Cannabinoid Res. 2016, 1, 90–101. [Google Scholar] [CrossRef]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- Nelson, K.M.; Bisson, J.; Singh, G.; Graham, J.G.; Chen, S.N.; Friesen, J.B.; Dahlin, J.L.; Niemitz, M.; Walters, M.A.; Pauli, G.F. The Essential Medicinal Chemistry of Cannabidiol (CBD). J. Med. Chem. 2020, 63, 12137–12155. [Google Scholar] [CrossRef] [PubMed]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Z.; Duncan, R.E. Regulatory Effects of Cannabidiol on Mitochondrial Functions: A Review. Cells 2021, 10, 1251. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An overview of some pharmacological aspects. J. Clin. Pharmacol. 2002, 42, 11S–19S. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- di Giacomo, V.; Chiavaroli, A.; Recinella, L.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Ronci, M.; Leone, S.; Brunetti, L.; et al. Antioxidant and Neuroprotective Effects Induced by Cannabidiol and Cannabigerol in Rat CTX-TNA2 Astrocytes and Isolated Cortexes. Int. J. Mol. Sci. 2020, 21, 3575. [Google Scholar] [CrossRef]
- Sun, S.; Hu, F.; Wu, J.; Zhang, S. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox. Biol. 2017, 11, 577–585. [Google Scholar] [CrossRef]
- Mato, S.; Sánchez-Gómez, V.M.; Matute, C. Cannabidiol induces intracellular calcium elevation and cytotoxicity in oligodendrocytes. Glia 2010, 58, 1739–1747. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.Y.; Seo, J.; Choi, I.S. Neuroprotective Effect of Cannabidiol against Hydrogen Peroxide in Hippocampal Neuron Culture. Cannabis Cannabinoid Res. 2021, 6, 40–47. [Google Scholar] [CrossRef]
- Wu, H.Y.; Goble, K.; Mecha, M.; Wang, C.C.; Huang, C.H.; Guaza, C.; Jan, T.R. Cannabidiol-induced apoptosis in murine microglial cells through lipid raft. Glia 2012, 60, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, X. A new kind of cell suicide: Mechanisms and functions of programmed necrosis. Trends Biochem. Sci. 2014, 39, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021, 28, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Šimenc, J.; Lipnik-Štangelj, M. Staurosporine induces apoptosis and necroptosis in cultured rat astrocytes. Drug Chem. Toxicol. 2012, 35, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Simenc, J.; Lipnik-Stangelj, M. Staurosporine induces different cell death forms in cultured rat astrocytes. Radiol. Oncol. 2012, 46, 312–320. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef]
- Fritsch, M.; Günther, S.D.; Schwarzer, R.; Albert, M.C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef]
- Fricker, M.; Vilalta, A.; Tolkovsky, A.M.; Brown, G.C. Caspase inhibitors protect neurons by enabling selective necroptosis of inflamed microglia. J. Biol. Chem. 2013, 288, 9145–9152. [Google Scholar] [CrossRef]
- Kim, S.J.; Li, J. Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis. 2013, 4, e716. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhang, K.; Shan, L.; Kuang, F.; Chen, K.; Zhu, K.; Ma, H.; Ju, G.; Wang, Y.Z. Reactive astrocytes undergo M1 microglia/macrohpages-induced necroptosis in spinal cord injury. Mol. Neurodegener. 2016, 11, 14. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sørgård, M.; Di Marzo, V.; Julius, D.; Högestätt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–457. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007, 152, 576–582. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–2910. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Mezey, E.; Tóth, Z.E.; Cortright, D.N.; Arzubi, M.K.; Krause, J.E.; Elde, R.; Guo, A.; Blumberg, P.M.; Szallasi, A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA 2000, 97, 3655–3660. [Google Scholar] [CrossRef]
- Roberts, J.C.; Davis, J.B.; Benham, C.D. [3H] Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004, 995, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Berghuis, P.; Rajnicek, A.M.; Morozov, Y.M.; Ross, R.A.; Mulder, J.; Urbán, G.M.; Monory, K.; Marsicano, G.; Matteoli, M.; Canty, A.; et al. Hardwiring the brain: Endocannabinoids shape neuronal connectivity. Science 2007, 316, 1212–1216. [Google Scholar] [CrossRef]
- Vitalis, T.; Lainé, J.; Simon, A.; Roland, A.; Leterrier, C.; Lenkei, Z. The type 1 cannabinoid receptor is highly expressed in embryonic cortical projection neurons and negatively regulates neurite growth in vitro. Eur. J. Neurosci. 2008, 28, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, A.M.; Vlachou, S. Effects of Cannabinoid Exposure during Neurodevelopment on Future Effects of Drugs of Abuse: A Preclinical Perspective. Int. J. Mol. Sci. 2021, 22, 9989. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Contreras, R.; Méndez-Reséndiz, K.A.; Rosenbaum, T.; González-Ramírez, R.; Morales-Lázaro, S.L. TRPV1 Channel: A Noxious Signal Transducer that Affects Mitochondrial Function. Int. J. Mol. Sci. 2020, 21, 8882. [Google Scholar] [CrossRef] [PubMed]
- Gibson, H.E.; Edwards, J.G.; Page, R.S.; Van Hook, M.J.; Kauer, J.A. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 2008, 57, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Alter, B.J.; Gereau, R.W. Hotheaded: TRPV1 as mediator of hippocampal synaptic plasticity. Neuron 2008, 57, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Nazıroğlu, M.; Taner, A.N.; Balbay, E.; Çiğ, B. Inhibitions of anandamide transport and FAAH synthesis decrease apoptosis and oxidative stress through inhibition of TRPV1 channel in an in vitro seizure model. Mol. Cell. Biochem. 2019, 453, 143–155. [Google Scholar] [CrossRef]
- Sappington, R.M.; Sidorova, T.; Long, D.J.; Calkins, D.J. TRPV1: Contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressure. Investig. Ophthalmol. Vis. Sci. 2009, 50, 717–728. [Google Scholar] [CrossRef]
- Sugimoto, T.; Takeyama, A.; Xiao, C.; Takano-Yamamoto, T.; Ichikawa, H. Electron microscopic demonstration of nick end-labeled DNA fragments during capsaicin-induced apoptosis of trigeminal primary neurons in neonatal rats. Brain Res. 1999, 818, 147–152. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, D.Y.; Chung, E.S.; Oh, U.T.; Kim, S.U.; Jin, B.K. Transient receptor potential vanilloid subtype 1 mediates cell death of mesencephalic dopaminergic neurons in vivo and in vitro. J. Neurosci. 2005, 25, 662–671. [Google Scholar] [CrossRef]
- Kim, S.R.; Bok, E.; Chung, Y.C.; Chung, E.S.; Jin, B.K. Interactions between CB(1) receptors and TRPV1 channels mediated by 12-HPETE are cytotoxic to mesencephalic dopaminergic neurons. Br. J. Pharmacol. 2008, 155, 253–264. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, S.U.; Oh, U.; Jin, B.K. Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+-mediated mitochondrial damage and cytochrome c release. J. Immunol. 2006, 177, 4322–4329. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Yamaoka, T.; Sanpei, K.; Sasaoka, H.; Nakagawa, T.; Kaneko, S. TRPV1 stimulation triggers apoptotic cell death of rat cortical neurons. Biochem. Biophys. Res. Commun. 2008, 377, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lee, J.H.; Lee, S.H.; Park, K.A.; Lee, W.T.; Lee, J.E. TRPV1 Activation in Primary Cortical Neurons Induces Calcium-Dependent Programmed Cell Death. Exp. Neurobiol. 2013, 22, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Movsesyan, V.A.; Stoica, B.A.; Yakovlev, A.G.; Knoblach, S.M.; Lea, P.M.; Cernak, I.; Vink, R.; Faden, A.I. Anandamide-induced cell death in primary neuronal cultures: Role of calpain and caspase pathways. Cell Death Differ. 2004, 11, 1121–1132. [Google Scholar] [CrossRef]
- Shin, C.Y.; Shin, J.; Kim, B.M.; Wang, M.H.; Jang, J.H.; Surh, Y.J.; Oh, U. Essential role of mitochondrial permeability transition in vanilloid receptor 1-dependent cell death of sensory neurons. Mol. Cell Neurosci. 2003, 24, 57–68. [Google Scholar] [CrossRef]
- Cernak, I.; Vink, R.; Natale, J.; Stoica, B.; Lea, P.M.; Movsesyan, V.; Ahmed, F.; Knoblach, S.M.; Fricke, S.T.; Faden, A.I. The “dark side” of endocannabinoids: A neurotoxic role for anandamide. J. Cereb. Blood. Flow. Metab. 2004, 24, 564–578. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar]
- Covelo, A.; Eraso-Pichot, A.; Fernández-Moncada, I.; Serrat, R.; Marsicano, G. CB1R-dependent regulation of astrocyte physiology and astrocyte-neuron interactions. Neuropharmacology 2021, 195, 108678. [Google Scholar] [CrossRef]
- Stella, N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 2010, 58, 1017–1030. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.; Bonilla-Del Río, I.; Puente, N.; Gómez-Urquijo, S.M.; Fontaine, C.J.; Egaña-Huguet, J.; Elezgarai, I.; Ruehle, S.; Lutz, B.; Robin, L.M.; et al. Localization of the cannabinoid type-1 receptor in subcellular astrocyte compartments of mutant mouse hippocampus. Glia 2018, 66, 1417–1431. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Blasco, D.; Busquets-Garcia, A.; Hebert-Chatelain, E.; Serrat, R.; Vicente-Gutierrez, C.; Ioannidou, C.; Gómez-Sotres, P.; Lopez-Fabuel, I.; Resch-Beusher, M.; Resel, E.; et al. Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature 2020, 583, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.J.; Finch, P.; Müller-Taubenberger, A.; Leung, K.Y.; Warren, E.C.; Damstra-Oddy, J.; Sharma, D.; Patra, P.H.; Glyn, S.; Boberska, J.; et al. A new mechanism for cannabidiol in regulating the one-carbon cycle and methionine levels in Dictyostelium and in mammalian epilepsy models. Br. J. Pharmacol. 2020, 177, 912–928. [Google Scholar] [CrossRef] [PubMed]
- Mele, T.; Jurič, D.M. Indentification and pharmacological characterization of the histamine H3 receptor in cultured rat astrocytes. Eur. J. Pharmacol. 2013, 720, 198–204. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Jurič, D.M.; Finderle, Ž.; Šuput, D.; Brvar, M. The effectiveness of oxygen therapy in carbon monoxide poisoning is pressure- and time-dependent: A study on cultured astrocytes. Toxicol. Lett. 2015, 233, 16–23. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurič, D.M.; Bulc Rozman, K.; Lipnik-Štangelj, M.; Šuput, D.; Brvar, M. Cytotoxic Effects of Cannabidiol on Neonatal Rat Cortical Neurons and Astrocytes: Potential Danger to Brain Development. Toxins 2022, 14, 720. https://doi.org/10.3390/toxins14100720
Jurič DM, Bulc Rozman K, Lipnik-Štangelj M, Šuput D, Brvar M. Cytotoxic Effects of Cannabidiol on Neonatal Rat Cortical Neurons and Astrocytes: Potential Danger to Brain Development. Toxins. 2022; 14(10):720. https://doi.org/10.3390/toxins14100720
Chicago/Turabian StyleJurič, Damijana Mojca, Klara Bulc Rozman, Metoda Lipnik-Štangelj, Dušan Šuput, and Miran Brvar. 2022. "Cytotoxic Effects of Cannabidiol on Neonatal Rat Cortical Neurons and Astrocytes: Potential Danger to Brain Development" Toxins 14, no. 10: 720. https://doi.org/10.3390/toxins14100720
APA StyleJurič, D. M., Bulc Rozman, K., Lipnik-Štangelj, M., Šuput, D., & Brvar, M. (2022). Cytotoxic Effects of Cannabidiol on Neonatal Rat Cortical Neurons and Astrocytes: Potential Danger to Brain Development. Toxins, 14(10), 720. https://doi.org/10.3390/toxins14100720




