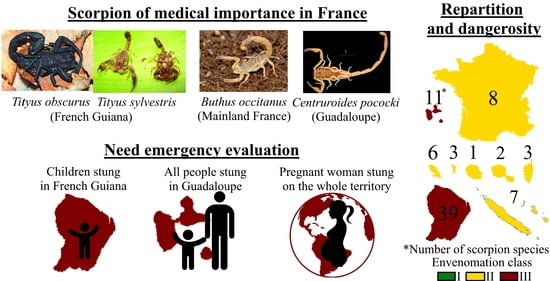
French Scorpionism (Mainland and Oversea Territories): Narrative Review of Scorpion Species, Scorpion Venom, and Envenoming Management
Abstract
1. Epidemiology
| Family or Species | Envenoming Class | Continental France | Corsica | Guadeloupe | French Guiana | French Polynesia | Martinique | Mayotte | New Caledonia | Reunion Island | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Belisariidae | |||||||||||
| Belisarius xambeui (Simon, 1879) | I | [16,18] | |||||||||
| Buthidae | |||||||||||
| Ananteris coineaui (Lourenço, 1982) | - * | [19] | |||||||||
| Ananteris dacostai (Ythier, Chevalier & Lourenço, 2020) | - | [20] | |||||||||
| Ananteris elisabethae (Lourenço, 2003) | - | [19] | |||||||||
| Ananteris guyanensis (Lourenço & Monod, 1999) | - | [19] | |||||||||
| Ananteris intermedia (Lourenço, 2012) | - | [19] | |||||||||
| Ananteris kalina (Ythier, 2018) | - | [19] | |||||||||
| Ananteris mamilihpan (Ythier, Chevalier & Lourenço, 2020) | - | [20] | |||||||||
| Ananteris polleti (Lourenço, 2016) | - | [19] | |||||||||
| Ananteris sabineae (Lourenço, 2001) | - | [19] | |||||||||
| Ananteris sipilili (Ythier, Chevalier & Lourenço, 2020) | - | [20] | |||||||||
| Ananteris tresor (Ythier, Chevalier & Lourenço, 2020) | - | [20] | |||||||||
| Buthus occitanus (Amoreux, 1789) | II | [5,16,18] | |||||||||
| Buthus balmensis (Ythier & Laborieux, 2022) | - | [53] | |||||||||
| Buthus pyrenaeus (Ythier, 2021) | I | [43] | |||||||||
| Centruroides barbudensis (Pocock, 1898) ** | I | [21,22,23] | |||||||||
| Centruroides gracilis (Latreille, 1804) | - | [23] | |||||||||
| Centruroides pococki (Sissom & Francke, 1983) | III | [14,21,23] | |||||||||
| Grosphus goudoti (Lourenço & Goodman, 2006) | - | [24] | |||||||||
| Grosphus mayottensis (Lourenço & Goodman, 2009) | - | [24] | |||||||||
| Isometrus maculatus (De Geer, 1778) | II | [5,9,17,19,21,25] | |||||||||
| Jaguajir pintoi kourounensis (Lourenço, 2008) | - | [19] | |||||||||
| Microananteris abounami (Lourebço & Chevalier 2022) | [58] | ||||||||||
| Microananteris inselberg (Lourenço, 2021) | - | [42] | |||||||||
| Microananteris minor (Lourenço, 2003) | - | [42] | |||||||||
| Microananteris serrulata (Lourenço, 2021) | - | [42] | |||||||||
| Reddyanu heimi (Vachon, 1976) | - | [39] | |||||||||
| Tityus gasci (Lourenço, 1981) | - | [19] | |||||||||
| Tityus mana (Lourenço, 2012) | - | [19] | |||||||||
| Tityus marechali (Lourenço, 2013) | - | [26] | |||||||||
| Tityus metuendus (Pocock, 1897) | - | [27] | |||||||||
| Tityus obscurus (Gervais, 1843) | III | [5,9,19] | |||||||||
| Tityus silvestris (Pocock, 1897) | III | [15,19] | |||||||||
| Bothriuridae | |||||||||||
| Cercophonius squama (Gervais, 1843) | - | [41] | |||||||||
| Chactidae | |||||||||||
| Auyantepuia aluku (Ythier, 2018) | - | [19] | |||||||||
| Auyantepuia aurum (Ythier, 2018) | - | [19] | |||||||||
| Auyantepuia fravalae (Lourenço, 1983) | - | [19] | |||||||||
| Auyantepuia gaillardi (Lourenço, 1983) | - | [19] | |||||||||
| Auyantepuia kelleri (Lourenço, 1997) | - | [19] | |||||||||
| Auyantepuia laurae (Ythier, 2015) | - | [19] | |||||||||
| Auyantepuia sissomi (Lourenço, 1983) | - | [19] | |||||||||
| Broteochactas delicatus (Karsch, 1879) | I | [19,28] | |||||||||
| Brotheas gervaisii (Pocock, 1893) | I | [19,28] | |||||||||
| Brotheas granulatus (Simon, 1877) | I | [19,28] | |||||||||
| Guyanochactas flavus (Lourenço & Ythier, 2011) | - | [19] | |||||||||
| Guyanochactas gonzalezspongai (Lourenço, 1983) | - | [19] | |||||||||
| Guyanochactas touroulti (Lourenço, 2018) | - | [29] | |||||||||
| Hadrurochactas cristinae (Ythier, 2018) | - | [19] | |||||||||
| Hadrurochactas schaumii (Kirsch, 1880) | - | [19] | |||||||||
| Spinochactas mitaraka (Lourenço, 2016) | - | [19] | |||||||||
| Diplocentridae | |||||||||||
| Didymocentrus martinicae (Teruel & Questel, 2020) | - | [23,36] | |||||||||
| Oiclus ardens (Ythier, 2019) | - | [31] | |||||||||
| Oiclus cousteaui (Ythier, 2019) | - | [31] | |||||||||
| Oiclus nanus (Teruel & Chazal, 2010) | - | [32] | |||||||||
| Oiclus purvesii purvesii (Becker, 1880) | - | [31] | |||||||||
| Oiclus purvesii sabae (Francke, 1978) | - | [31] | |||||||||
| Oiclus questeli (Teruel, 2008) | - | [31] | |||||||||
| Oiclus tipunch (Ythier, 2019) | - | [31] | |||||||||
| Euscorpiidae | |||||||||||
| Euscorpius concinnus (Koch, 1837) | II | [16,33] | |||||||||
| Euscorpius italicus (Herbst, 1800) | II | [9,18,34,35] | |||||||||
| Euscorpius tergestinus (Koch, 1837) | II | [9,18,34,35] | |||||||||
| Tetratrichobothrius flavicaudis (De Geer, 1778) | II | [9,16,18,34,35] | |||||||||
| Hormuridae | |||||||||||
| Hormurus neocaledonicus (Simon, 1877) | - | [40] | |||||||||
| Opisthacanthus heurtaultae (Lourenço, 1980) | - | [19] | |||||||||
| Liochelidae | |||||||||||
| Liocheles australasiae (Fabricius, 1775) | - | [25,40] | |||||||||
| Liocheles neocaledonicus (Simon, 1877) | - | [38] | |||||||||
| Liocheles longimanus (Werner, 1939) | - | [40] | |||||||||
| Total: 67 species | 8 | 3 | 11 | 39 | 2 | 6 | 3 | 7 | 1 |
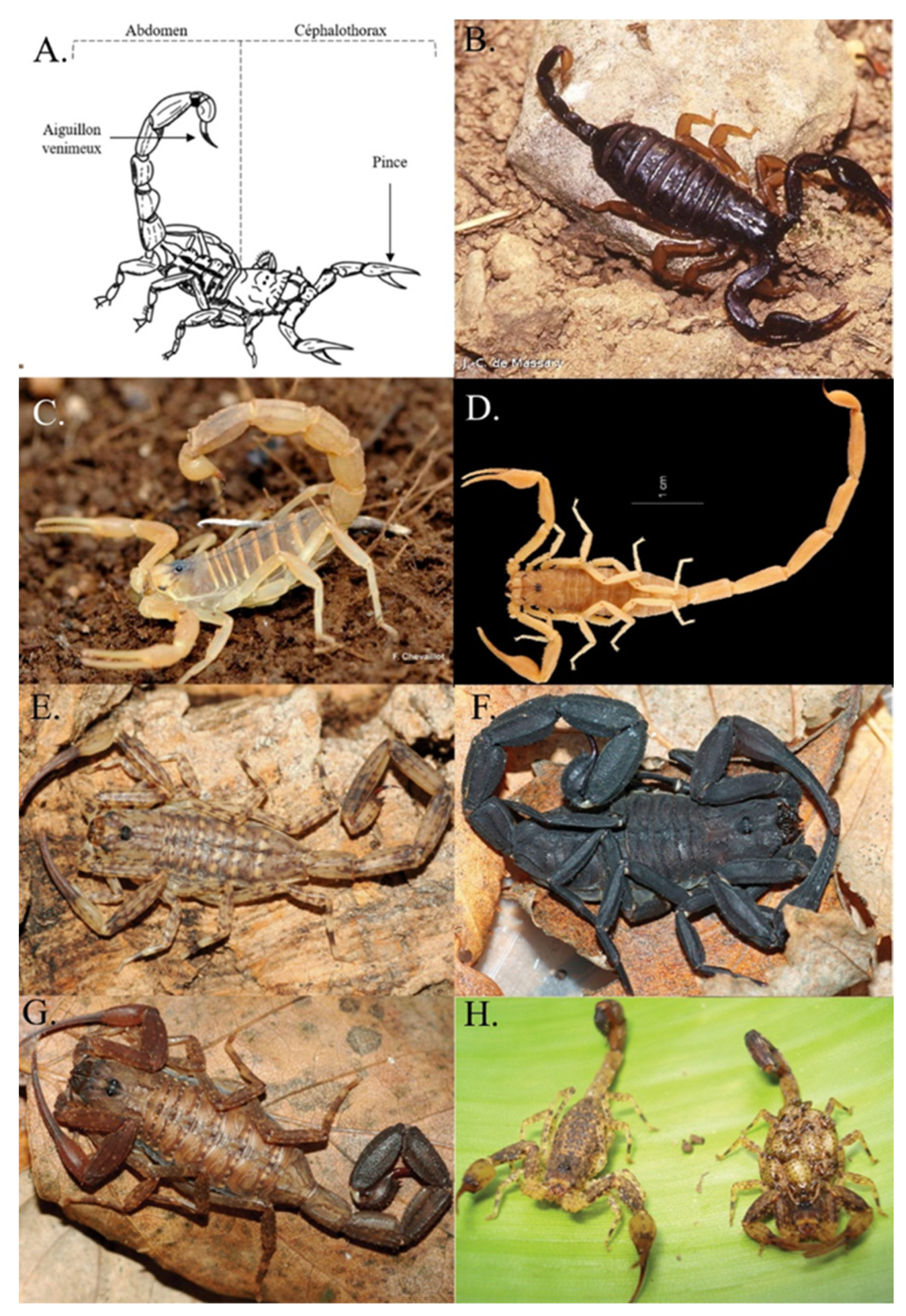
2. Visual Determination of Scorpion Species for Medical Management
2.1. Metropolitan France (Continental France and Corsica)
2.2. The French West Indies
2.3. The islands of the Indian and Pacific Oceans
2.4. French Guiana
3. Scorpion Venom
3.1. Generality
3.2. French Scorpion Venom
4. Clinical Presentation of Scorpionic Envenomation
4.1. Physiopathological Action
4.2. Classification of Scorpionic Envenomation
5. Management of Scorpionic Envenomation
5.1. General Measures for Scorpion Stings
5.2. Specific Measures of Envenomation
5.3. Specificities of Management of Envenoming by Scorpions Responsible of Severe Envenoming
5.4. Specific Care of Pregnant and Breastfeeding Women
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chippaux, J.-P. Emerging options for the management of scorpion stings. Drug Des. Dev. Ther. 2012, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.-P.; Goyffon, M. Epidemiology of scorpionism: A global appraisal. Acta Trop. 2008, 107, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Fabresse, N.; Alvarez, J.-C. Épidémiologie des intoxications aiguës. Toxicol. Anal. Clin. 2020, 32, 291–300. [Google Scholar] [CrossRef]
- Chakroun-Walha, O.; Karray, R.; Jerbi, M.; Nasri, A.; Issaoui, F.; Amine, B.R.; Bahloul, M.; Bouaziz, M.; Ksibi, H.; Rekik, N. Update on the Epidemiology of Scorpion Envenomation in the South of Tunisia. Wilderness Environ. Med. 2018, 29, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.J.; Ellsworth, S.A.; Nystrom, G.S. A global accounting of medically significant scorpions: Epidemiology, major toxins, and comparative resources in harmless counterparts. Toxicon 2018, 151, 137–155. [Google Scholar] [CrossRef]
- Kerdoun, M.A. Epidemiological aspects of scorpion stings in Algeria: A monocentric retrospective study. Toxicol. Anal. Clin. 2021, 34, 4–9. [Google Scholar] [CrossRef]
- Touloun, O.; Boumezzough, A.; Slimani, T. Scorpion envenomation in the region of Marrakesh Tensift Alhaouz (Morocco): Epidemiological characterization and therapeutic approaches. Serket 2012, 13, 38–50. [Google Scholar]
- Sousa, P.; Arnedo, M.; Harris, D.J. Updated catalogue and taxonomic notes on the Old-World scorpion genus Buthus Leach, 1815 (Scorpiones, Buthidae). ZooKeys 2017, 686, 15–84. [Google Scholar] [CrossRef]
- Vaucel, J.-A.; Gil-Jardine, C.; Labadie, M.; Larréché, S.; Paradis, C.; Nardon, A.; Courtois, A.; Langrand, J.; Kallel, H. Epidemiology of scorpionism in France: Nationwide scorpion exposure. Clin. Toxicol. 2021, 59, 888–895. [Google Scholar] [CrossRef]
- Benmosbah, M.; Guegueniat, P.; Mayence, C.; Egmann, G.; Narcisse, E.; Gonon, S.; Hommel, D.; Kallel, H. Epidemiological and clinical study on scorpionism in French Guiana. Toxicon 2013, 73, 56–62. [Google Scholar] [CrossRef]
- Chippaux, J.P.; Galtier, J.; Lefait, J.F. Epidemiology of envenomation in French Guiana. Bull. Soc. Pathol. Exot. Filiales 1984, 77, 206–215. [Google Scholar]
- de Haro, L. Envenimations par les nouveaux animaux de compagnie en France métropolitaine. Réanimation 2009, 18, 617–625. [Google Scholar] [CrossRef]
- Riffard, O.; Gault, G. Les nouveaux animaux de compagnie: Un danger toxicologique? Toxicol. Anal. Clin. 2014, 26, 229–230. [Google Scholar] [CrossRef]
- Caré, W.; Larréché, S.; Busser, P.; Dufayet, L.; Vodovar, D.; de Haro, L.; Langrand, J. Envenomation by Centruroides pococki scorpion with neuromuscular toxicity. Toxicon 2021, 190, 39–40. [Google Scholar] [CrossRef]
- Monteiro, W.M.; de Oliveira, S.S.; Pivoto, G.; Alves, E.C.; de Almeida Gonçalves Sachett, J.; Alexandre, C.N.; Fé, N.F.; Guerra, M.D.G.V.B.; da Silva, I.M.; Tavares, A.M.; et al. Scorpion envenoming caused by Tityus cf. silvestris evolving with severe muscle spasms in the Brazilian Amazon. Toxicon Off. J. Int. Soc. Toxinol. 2016, 119, 266–269. [Google Scholar] [CrossRef]
- Vaucel, J.; Le Blond du Plouy, N.; Courtois, A.; Bragança, C.; Labadie, M. Euscorpius flavicaudis sting is not lethal but not harmless either: First record of neurological symptoms in child after sting. Toxicol. Anal. Clin. 2020, 32, 85–88. [Google Scholar] [CrossRef]
- Delbarre, N.; de Haro, L. Piqûre de scorpion Isometrus maculatus à la Réunion. Ann. Françaises Méd. Urgence 2014, 4, 191–193. [Google Scholar] [CrossRef]
- Fet, V. Scorpions of Europe. Acta Zool. Bulg. 2010, 62, 3–12. [Google Scholar]
- Ythier, E. A synopsis of the scorpion fauna of French Guiana, with description of four new species. ZooKeys 2018, 764, 27–90. [Google Scholar] [CrossRef]
- Ythier, E.; Chevalier, J.; Lourenço, W.R. A synopsis of the genus Ananteris Thorell, 1891 (Scorpiones: Buthidae) in French Guiana, with description of four new species. Arachnida 2020, 28, 2–33. [Google Scholar]
- Lourenço, W.R. Les peuplements des scorpions des Antilles; facteurs historiques et écologiques en association avec les stratégies biodémographiques. Stud. Neotrop. Fauna Environ. 1992, 27, 43–62. [Google Scholar] [CrossRef]
- Borges, A. Scorpionism and Dangerous Scorpions in Central America and the Caribbean Region. In Scorpion Venoms; Gopalakrishnakone, P., Possani, L.D., FSchwartz, E., Rodríguez de la Vega, R.C., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 215–244. [Google Scholar] [CrossRef]
- Chevalier, J.; Dewynter, M. Inventaire & Cartographie des Scorpions de la Martinique; Biotope: Mèze, France, 2020. [Google Scholar]
- Lourenço, W.R.; Goodman, S.M. Scorpion from the comoros archipelago: Description of a new species of Grosphus Simon (Scorpiones, Buthidae) from Mayotte (Maore). Bol. Soc. Entomol. Aragon 2009, 44, 35–38. [Google Scholar]
- Institut Louis Malardé. Fiche Thématique Scorpions. Lab Rech En Entomol 2019. Available online: https://www.ilm.pf/wp-content/uploads/2019/11/Fiche-thematique-scorpions-1.pdf (accessed on 6 April 2021).
- Lourenço, W.R. A new species of Tityus, C.L. Koch, 1836 (Scorpiones: Buthidae) from the island of Martinique, Lesser Antilles. Arthropoda Sel. 2013, 22, 227–231. [Google Scholar] [CrossRef]
- Lourenço, W.R. Première observation de Tityus metuendus Pocock, 1897 (Scorpiones, Buthidae) pour la Guyane française. Rev. Arachnol. 2019, 2, 52–56. [Google Scholar]
- Ganteaume, F.; Imbert, C. Le point en 2013 sur les envenimations liées aux arthropodes en Guyane française. Bull. Soc. Pathol. Exot. 2014, 107, 31–38. [Google Scholar] [CrossRef]
- Lourenço, W.R. The scorpions from the Mitaraka Massif in French Guiana (Scorpiones: Buthidae, Chactidae). Zoosystema 2018, 40, 367. [Google Scholar] [CrossRef]
- Touroult, J.; Poirier, E.; Moulin, N.; Deknuydt, F.; Dumbardon-Martial, E.; Ramage, T.; Daniel, R. Inventaire Entomologique des ZNIEFF de Martinique. Mission 2016 Pour le Compte de la Deal Martinique; Société Entomologique Antilles Guyane: Remire-Monjoly, French, 2017. [Google Scholar] [CrossRef]
- Ythier, E. On the genus Oiclus Simon, 1880 (Scorpiones: Diplocentridae) in Guadeloupe islands, with description of three new species. Arachnida 2019, 22, 17–49. [Google Scholar]
- Teruel, R.; Chazal, L.; Centro Oriental de Ecosistemas y Biodiversidad. A new species of the genus Oiclus Simon, 1880 (Scorpiones: Scorpionidae: Diplocentrinae) from Guadeloupe, Lesser Antilles. Euscorpius 2010, 2010, 1–9. [Google Scholar] [CrossRef]
- Colombo, M. New data on distribution and ecology of seven species of Euscorpius Thorell, 1876 (Scorpiones: Euscorpiidae). Euscorpius 2006, 2006, 1–40. [Google Scholar] [CrossRef]
- De Haro, L. Intoxication par les venins. La Revue Prat. 2000, 50, 401–406. [Google Scholar]
- De Haro, L.; Jouglard, J.; David, J.M. Scorpion bites in southern France: Experience at the poison-control centre of Marseilles. Presse Med. 1996, 25, 600. [Google Scholar]
- Teruel, R.; Questel, K. A new Lesser Antillean scorpion of the genus Didymocentrus Kraepelin, 1905 (Scorpiones: Diplocentridae) Euscorpius Occasional Publications in Scorpiology. Euscorpius 2020, 313, 1–15. [Google Scholar]
- de Haro, L. Animaux venimeux terrestres. EMC—Pathol. Prof. Environ. 2009, 4, 1–17. [Google Scholar] [CrossRef]
- Monod, L. Taxonomic emendations in the genus Liocheles Sundevall, 1833 (Scorpiones, Liochelidae). Rev. Suisse Zool. 2011, 118, 723–758. [Google Scholar]
- Kovařík, F. A review of the genus Isometrus Ehrenberg, 1828 (Scorpiones: Buthidae) with descriptions of four new species from Asia and Australia. Euscorpius 2003, 2003, 1–19. [Google Scholar] [CrossRef]
- Kraepelin, K. Die Skorpione und Pedipalpen von Neu-Caledonien und den benachbarten Inselgruppen. Nova Caledonia. Forschungen in Neu-Caledonien und auf den Loyalty-Inseln. Recherches scientifiques en Nouvelle-Calédonie et aux iles Loyalty. A. Zoologie. Available online: https://www.biodiversitylibrary.org/bibliography/6790 (accessed on 1 October 2022).
- Fet, V. (Ed.) Catalog of the Scorpions of the World (1758–1998); The New York Entomological Society: New York, NY, USA, 2000. [Google Scholar]
- Lourenço, W.R. The genus Microananteris Lourenço, 2003 in French Guiana (Scorpiones: Buthidae). Zoosystema 2021, 43, 377–386. [Google Scholar] [CrossRef]
- Ythier, E. The genus Buthus Leach, 1815 (Scorpiones: Buthidae) in France with description of a new species from the Eastern Pyrenees. Faunitaxys 2021, 10, 1–13. [Google Scholar]
- Kallel, H.; Mayence, C.; Guegueniat, P.; Hommel, D. Acute Necrotizing Pancreatitis after Tityus obscurus Scorpion Envenomation in French Guiana. J. Clin. Toxicol. 2016, 6, 1000328. [Google Scholar] [CrossRef]
- Vaucel, J.; Mutricy, R.; Hoarau, M.; Pujo, J.-M.; Elenga, N.; Labadie, M.; Kallel, H. Pediatric scorpionism in northern Amazonia: A 16-year study on epidemiological, environmental and clinical aspects. J. Venom. Anim. Toxins Trop. Dis. 2020, 26, e202000038. [Google Scholar] [CrossRef]
- Hommel, D.; Hulin, A.; Lourenço, W.R. Accident scorpionique létal par Tityus cambridgei Pocock: À propos d’un cas en Guyane Française. Concours Méd. Paris 2000, 122, 481–484. [Google Scholar]
- Vaucel, J.; Labadie, M.; Hoarau, M.; Kallel, H. Pediatric scorpionism in French Guiana: Epidemiological and clinical study—Preliminary result. Toxicol. Anal. Clin. 2019, 31, S29. [Google Scholar] [CrossRef]
- Schmitt, C.; Torrents, R.; Simon, N.; de Haro, L. First Described Envenomation by Centruroides pococki Scorpion in the French Caribbean Island Guadeloupe. Wilderness Environ. Med. 2017, 28, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Torrents, R.; Petit, M.; Simon, N.; De Haro, L. Piqure de scorpion Centruroides pococki en Guadeloupe. Toxicol. Anal. Clin. 2017, 29, S75. [Google Scholar] [CrossRef]
- Domangé, B.; Torrents, R.; Schmitt, C.; Boulamery, A.; Von Fabek, K.; Glaizal, M.; Simon, N.; De Haro, L. Présence confirmée et innocuité du scorpion Isometrus maculatus en polynésie française suite à une envenimation colligée pour la première fois en Océanie. Toxicol. Anal. Clin. 2018, 30, 177. [Google Scholar] [CrossRef]
- Bloch, J.; Sinno-Tellier, S.; Greillet, C.; Puskarczyk, E.; Manel, J. La toxicovigilance: Comment et est-ce que ça marche? Toxicol. Anal. Clin. 2019, 31, S24. [Google Scholar] [CrossRef]
- Vaucel, J.-A.; Gil-Jardine, C.; Labadie, M.; Larréché, S.; Paradis, C.; Nardon, A.; Courtois, A.; Langrand, J.; Kallel, H.; the French PCC Research Group. Comment on epidemiology of scorpionism in France: Nationwide scorpion exposure. Description of Buthus pyrenaeus envenoming. Clin. Toxicol. 2022, 60, 890–891. [Google Scholar] [CrossRef]
- Ythier, E.; Léo, L. Le genre Buthus Leach, 1815 (Scorpiones: Buthidae) en France avec la description d’une nouvelle espèce du Massif de la Sainte-BaumeThe genus Buthus Leach, 1815 (Scorpiones: Buthidae) in France with description of a new species from the Sainte-Baume Massif. Faunitaxys 2022, 10, 1–13. [Google Scholar] [CrossRef]
- Aboumaâd, B.; Lahssaini, M.; Tiger, A.; Benhassain, S.M. Clinical comparison of scorpion envenomation by Androctonus mauritanicus and Buthus occitanus in children. Toxicon 2014, 90, 337–343. [Google Scholar] [CrossRef]
- Achour, S.; Iken, I.; Hoummani, H.; Harandou, M.; Hida, M. Profil épidémiologique des envenimations scorpioniques chez l’enfant: CHU Hassan II de Fès. Toxicol. Anal. Clin. 2018, 30, 164. [Google Scholar] [CrossRef]
- Martin-Eauclaire, M.-F.; Bosmans, F.; Céard, B.; Diochot, S.; Bougis, P.E. A first exploration of the venom of the Buthus occitanus scorpion found in southern France. Toxicon 2014, 79, 55–63. [Google Scholar] [CrossRef][Green Version]
- Khattabi, A.; Soulaymani-Bencheikh, R.; Achour, S.; Salmi, L.-R.; Scorpion Consensus Expert Group. Classification of clinical consequences of scorpion stings: Consensus development. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, W.R.; Chevalier, J. A further new species of the genus Microananteris Lourenço, 2003, from French Guiana (Scorpiones, Buthidae). Bull. Soc. Entomol. Fr. 2022, 127, 91–99. [Google Scholar] [CrossRef]
- Vaucel, J.-A.; Larréché, S.; Paradis, C.; Labadie, M.; Courtois, A.; Grenet, G.; Langrand, J.; Tournoud, C.; Nisse, P.; Gallart, J.-C.; et al. Relationship Between Scorpion Stings Events and Environmental Conditions in Mainland France. J. Med. Entomol. 2021, 58, 2146–2153. [Google Scholar] [CrossRef]
- Vaucel, J.-A.; Paradis, C.; Courtois, A.; Bragança, C.; Moltini, A.; Labadie, M.; Kallel, H. Les Scorpions en France Métropolitaine: Clés de Détermination et Répartition Géographique. Toxicol. Anal. Clin. 2021, 33, S48–S49. [Google Scholar] [CrossRef]
- Kovarik, F. Review of European scorpions, with a key to species. Serket 1999, 6, 38–44. [Google Scholar]
- Lourenço, W.R. The coevolution between telson morphology and venom glands in scorpions (Arachnida). J. Venom. Anim. Toxins Trop. Dis. 2020, 26, e20200128. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Cao, Z.; Li, W.; Wu, Y. Diverse Structural Features of Potassium Channels Characterized by Scorpion Toxins as Molecular Probes. Molecules 2019, 24, 2045. [Google Scholar] [CrossRef]
- de Oliveira-Mendes, B.B.R.; Miranda, S.E.M.; Sales-Medina, D.F.; de Freitas Magalhães, B.; Kalapothakis, Y.; de Souza, R.P.; Cardoso, V.N.; de Barros, A.L.B.; Guerra-Duarte, C.; Kalapothakis, E.; et al. Inhibition of Tityus serrulatus venom hyaluronidase affects venom biodistribution. PLoS Negl. Trop. Dis. 2019, 13, e0007048. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Knerr, J.M.; Argemi, L.; Bordon, K.C.F.; Pucca, M.B.; Cerni, F.A.; Arantes, E.C.; Çalışkan, F.; Laustsen, A.H. Scorpion Venom: Detriments and Benefits. Biomedicines 2020, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, R.C.R.; Vidal, N.; Possani, L.D. Scorpion Peptides. Handb. Biol. Act. Pept. 2013, 423–429. [Google Scholar] [CrossRef]
- Girish, K.S.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef] [PubMed]
- Ramos, O.H.P.; Selistre-de-Araujo, H.S. Snake venom metalloproteases—Structure and function of catalytic and disintegrin domains. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 142, 328–346. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Hernández, V.; Jiménez-Vargas, J.M.; Gurrola, G.B.; Valdivia, H.H.; Possani, L.D. Scorpion venom components that affect ion-channels function. Toxicon 2013, 76, 328–342. [Google Scholar] [CrossRef]
- Santibáñez-López, C.E.; Possani, L.D. Overview of the Knottin scorpion toxin-like peptides in scorpion venoms: Insights on their classification and evolution. Toxicon 2015, 107, 317–326. [Google Scholar] [CrossRef]
- Clairfeuille, T.; Cloake, A.; Infield, D.T.; Llongueras, J.P.; Arthur, C.P.; Li, Z.R.; Jian, Y.; Martin-Eauclaire, M.-F.; Bougis, P.E.; Ciferri, C.; et al. Structural basis of α-scorpion toxin action on Nav channels. Science 2019, 363, eaav8573. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Vargas, J.A.; Mourão, C.B.F.; Quintero-Hernández, V.; Possani, L.D.; Schwartz, E.F. Identification and phylogenetic analysis of Tityus pachyurus and Tityus obscurus novel putative Na+-channel scorpion toxins. PLoS ONE 2012, 7, e30478. [Google Scholar] [CrossRef]
- Rodríguez de la Vega, R.C.; Possani, L.D. Overview of scorpion toxins specific for Na+ channels and related peptides: Biodiversity, structure–function relationships and evolution. Toxicon 2005, 46, 831–844. [Google Scholar] [CrossRef]
- Yang, F.; Wang, D.; Tong, Y.; Qin, C.; Yang, L.; Yu, F.; Huang, X.; Liu, S.; Cao, Z.; Guo, L.; et al. Thermostable potassium channel-inhibiting neurotoxins in processed scorpion medicinal material revealed by proteomic analysis: Implications of its pharmaceutical basis in traditional Chinese medicine. J. Proteom. 2019, 206, 103435. [Google Scholar] [CrossRef]
- Martin-Eauclaire, M.-F.; Bougis, P.E. Potassium Channels Blockers from the Venom of Androctonus mauretanicus mauretanicus. J. Toxicol. 2012, 2012, 103608. [Google Scholar] [CrossRef]
- Tytgat, J.; Chandy, K.G.; Garcia, M.L.; Gutman, G.A.; Martin-Eauclaire, M.-F.; van der Walt, J.J.; Possani, L.D. A unified nomenclature for short-chain peptides isolated from scorpion venoms: α-KTx molecular subfamilies. Trends Pharmacol. Sci. 1999, 20, 444–447. [Google Scholar] [CrossRef]
- Cremonez, C.; Maiti, M.; Peigneur, S.; Cassoli, J.; Dutra, A.; Waelkens, E.; Lescrinier, E.; Herdewijn, P.; De Lima, M.E.; Pimenta, A.M.C.; et al. Structural and Functional Elucidation of Peptide Ts11 Shows Evidence of a Novel Subfamily of Scorpion Venom Toxins. Toxins 2016, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenkov, A.I.; Krylov, N.A.; Chugunov, A.O.; Grishin, E.V.; Vassilevski, A.A. Kalium: A database of potassium channel toxins from scorpion venom. Database 2016, 2016, baw056. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Gurrola, G.B.; Zhang, J.; Valdivia, C.R.; SanMartin, M.; Zamudio, F.Z.; Zhang, L.; Possani, L.D.; Valdivia, H.H. Structure–function relationships of peptides forming the calcin family of ryanodine receptor ligands. J. Gen. Physiol. 2016, 147, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Nencioni, A.L.A.; Neto, E.B.; de Freitas, L.A.; Dorce, V.A.C. Effects of Brazilian scorpion venoms on the central nervous system. J. Venom. Anim. Toxins Trop. Dis. 2018, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, A.K.; Caricati, C.P.; Lima, M.L.S.R.; Dos Santos, M.C.; Kipnis, T.L.; Eickstedt, V.R.D.; Knysak, I.; Da Silva, M.H.; Higashi, H.G.; Da Silva, W.D. Antigenic cross-reactivity among the venoms from several species of Brazilian scorpions. Toxicon 1994, 32, 989–998. [Google Scholar] [CrossRef]
- Batista, C.V.F.; Gómez-Lagunas, F.; Lucas, S.; Possani, L.D. Tc1, from Tityus cambridgei, is the first member of a new subfamily of scorpion toxin that blocks K + -channels. FEBS Lett. 2000, 486, 117–120. [Google Scholar] [CrossRef]
- Borja-Oliveira, C.R.; Pertinhez, T.A.; Rodrigues-Simioni, L.; Spisni, A. Positive inotropic effects of Tityus cambridgei and T. serrulatus scorpion venoms on skeletal muscle. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2009, 149, 404–408. [Google Scholar] [CrossRef]
- de Paula Santos-da-Silva, A.; Candido, D.M.; Nencioni, A.L.A.; Kimura, L.F.; Prezotto-Neto, J.P.; Barbaro, K.C.; Chalkidis, H.M.; Dorce, V.A.C. Some pharmacological effects of Tityus obscurus venom in rats and mice. Toxicon 2017, 126, 51–58. [Google Scholar] [CrossRef]
- Batista, C.V.F.; Zamudio, F.Z.; Lucas, S.; Fox, J.W.; Frau, A.; Prestipino, G.; Possani, L.D. Scorpion toxins from Tityus cambridgei that affect Na+-channels. Toxicon 2002, 40, 557–562. [Google Scholar] [CrossRef]
- Tibery, D.V.; Campos, L.A.; Mourão, C.B.F.; Peigneur, S.; Carvalho, A.C.E.; Tytgat, J.; Schwartz, E.F. Electrophysiological characterization of Tityus obscurus β toxin 1 (To1) on Na+-channel isoforms. Biochim. Biophys. Acta BBA Biomembr. 2019, 1861, 142–150. [Google Scholar] [CrossRef]
- Batista, C.V.F.; Martins, J.G.; Restano-Cassulini, R.; Coronas, F.I.V.; Zamudio, F.Z.; Procópio, R.; Possani, L. Venom characterization of the Amazonian scorpion Tityus metuendus. Toxicon 2018, 143, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Kitanaka, A.; Yakio, M.; Yamazaki, Y.; Nakagawa, Y.; Miyagawa, H. Complete de novo sequencing of antimicrobial peptides in the venom of the scorpion Isometrus maculatus. Toxicon 2017, 139, 1–12. [Google Scholar] [CrossRef] [PubMed]
- So, W.L.; Leung, T.C.N.; Nong, W.; Bendena, W.G.; Ngai, S.M.; Hui, J.H.L. Transcriptomic and proteomic analyses of venom glands from scorpions Liocheles australasiae, Mesobuthus martensii, and Scorpio maurus palmatus. Peptides 2021, 146, 170643. [Google Scholar] [CrossRef] [PubMed]
- Corona, M.; Gurrola, G.B.; Merino, E.; Cassulini, R.R.; Valdez-Cruz, N.A.; García, B.; Ramírez-Domínguez, M.E.; Coronas, F.I.; Zamudio, F.Z.; Wanke, E.; et al. A large number of novel Ergtoxin-like genes and ERG K+-channels blocking peptides from scorpions of the genus Centruroides. FEBS Lett. 2002, 532, 21–26. [Google Scholar] [CrossRef]
- Valdez-Velázquez, L.L.; Cid-Uribe, J.; Romero-Gutierrez, M.T.; Olamendi-Portugal, T.; Jimenez-Vargas, J.M.; Possani, L.D. Transcriptomic and proteomic analyses of the venom and venom glands of Centruroides hirsutipalpus, a dangerous scorpion from Mexico. Toxicon 2020, 179, 21–32. [Google Scholar] [CrossRef]
- Carcamo-Noriega, E.N.; Possani, L.D.; Ortiz, E. Venom content and toxicity regeneration after venom gland depletion by electrostimulation in the scorpion Centruroides limpidus. Toxicon 2019, 157, 87–92. [Google Scholar] [CrossRef]
- Ward, M.J.; Ellsworth, S.A.; Rokyta, D.R. Venom-gland transcriptomics and venom proteomics of the Hentz striped scorpion (Centruroides hentzi; Buthidae) reveal high toxin diversity in a harmless member of a lethal family. Toxicon 2018, 142, 14–29. [Google Scholar] [CrossRef]
- Díaz, C.; Rivera, J.; Lomonte, B.; Bonilla, F.; Diego-García, E.; Camacho, E.; Tytgat, J.; Sasa, M. Venom characterization of the bark scorpion Centruroides edwardsii (Gervais 1843): Composition, biochemical activities and in vivo toxicity for potential prey. Toxicon 2019, 171, 7–19. [Google Scholar] [CrossRef]
- Gómez-Ramírez, I.V.; Riaño-Umbarila, L.; Olamendi-Portugal, T.; Restano-Cassulini, R.; Possani, L.D.; Becerril, B. Biochemical, electrophysiological and immunological characterization of the venom from Centruroides baergi, a new scorpion species of medical importance in Mexico. Toxicon 2020, 184, 10–18. [Google Scholar] [CrossRef]
- Abd El-Aziz, T.M.; Xiao, Y.; Kline, J.; Gridley, H.; Heaston, A.; Linse, K.D.; Ward, M.J.; Rokyta, D.R.; Stockand, J.D.; Cummins, T.R.; et al. Identification and Characterization of Novel Proteins from Arizona Bark Scorpion Venom That Inhibit Nav1.8, a Voltage-Gated Sodium Channel Regulator of Pain Signaling. Toxins 2021, 13, 501. [Google Scholar] [CrossRef]
- Estrada-Gómez, S.; Vargas Muñoz, L.J.; Saldarriaga-Córdoba, M.; Quintana Castillo, J.C. Venom from Opisthacanthus elatus scorpion of Colombia, could be more hemolytic and less neurotoxic than thought. Acta Trop. 2016, 153, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.F.; Bartok, A.; Schwartz, C.A.; Papp, F.; Gómez-Lagunas, F.; Panyi, G.; Possani, L.D. OcyKTx2, a new K+-channel toxin characterized from the venom of the scorpion Opisthacanthus cayaporum. Peptides 2013, 46, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Azofeifa, D.; Sasa, M.; Lomonte, B.; Diego-García, E.; Ortiz, N.; Bonilla, F.; Murillo, R.; Tytgat, J.; Díaz, C. Biochemical characterization of the venom of Central American scorpion Didymocentrus krausi Francke, 1978 (Diplocentridae) and its toxic effects in vivo and in vitro. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 217, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.R.J.; McIntyre, L.; Northfield, T.D.; Daly, N.L.; Wilson, D.T. Small Molecules in the Venom of the Scorpion Hormurus waigiensis. Biomedicines 2020, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Housley, D.M.; Pinyon, J.L.; von Jonquieres, G.; Perera, C.J.; Smout, M.; Liddell, M.J.; Jennings, E.A.; Wilson, D.; Housley, G.D. Australian Scorpion Hormurus waigiensis Venom Fractions Show Broad Bioactivity Through Modulation of Bio-Impedance and Cytosolic Calcium. Biomolecules 2020, 10, 617. [Google Scholar] [CrossRef]
- Abroug, F.; Ouanes-Besbes, L.; Tilouche, N.; Elatrous, S. Scorpion envenomation: State of the art. Intensive Care Med. 2020, 46, 401–410. [Google Scholar] [CrossRef]
- Goyffon, M.; Tournier, J.-N. Scorpions: A Presentation. Toxins 2014, 6, 2137–2148. [Google Scholar] [CrossRef]
- Bouaziz, M.; Bahloul, M.; Kallel, H.; Samet, M.; Ksibi, H.; Dammak, H.; Ben Ahmed, M.N.; Chtara, K.; Chelly, H.; Ben Hamida, C.; et al. Epidemiological, clinical characteristics and outcome of severe scorpion envenomation in South Tunisia: Multivariate analysis of 951 cases. Toxicon 2008, 52, 918–926. [Google Scholar] [CrossRef]
- Güllü, U.U.; İpek, S.; Dalkıran, T.; Dinçer, S.; Yurttutan, S.; Aynacı, E. The Role of ProBNP on Prognosis in Scorpion Stings. Wilderness Environ. Med. 2021, 32, 137–142. [Google Scholar] [CrossRef]
- Osnaya-Romero, N.; Acosta-Saavedra, L.C.; Goytia-Acevedo, R.; Lares-Asseff, I.; Basurto-Celaya, G.; Perez-Guille, G.; Possani, L.; Calderón-Aranda, E. Serum level of scorpion toxins, electrolytes and electrocardiogram alterations in Mexican children envenomed by scorpion sting. Toxicon 2016, 122, 103–108. [Google Scholar] [CrossRef]
- Bompelli, N.; Reddy, C.R.; Deshpande, A. Scorpion bite-induced unilateral pulmonary oedema. BMJ Case Rep. 2018, 2018, bcr2018224476. [Google Scholar] [CrossRef] [PubMed]
- Razi, E.; Malekanrad, E. Asymmetric pulmonary edema after scorpion sting: A case report. Rev. Inst. Med. Trop. Sao Paulo 2008, 50, 347–350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Isbister, G.K.; Bawaskar, H.S. Scorpion Envenomation. N. Engl. J. Med. 2014, 371, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H. Neurological effects of venomous bites and stings. Handb. Clin. Neurol. 2013, 114, 349–368. [Google Scholar] [CrossRef]
- Torrez, P.P.Q.; Quiroga, M.M.M.; Abati, P.A.M.; Mascheretti, M.; Costa, W.S.; Campos, L.P.; França, F.O. Acute cerebellar dysfunction with neuromuscular manifestations after scorpionism presumably caused by Tityus obscurus in Santarém, Pará/Brazil. Toxicon Off. J. Int. Soc. Toxinol. 2015, 96, 68–73. [Google Scholar] [CrossRef]
- Udayakumar, N.; Rajendiran, C.; Srinivasan, A. Cerebrovascular manifestations in scorpion sting:A case series. Indian J. Med. Sci. 2006, 60, 241. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Sarkar, S.; Paswan, A. Cerebrovascular manifestations and alteration of coagulation profile in scorpion sting: A case series. Indian J. Crit. Care Med. 2008, 12, 15–17. [Google Scholar] [CrossRef][Green Version]
- Bahloul, M.; Chaari, A.; Khlaf-Bouaziz, N.; Hergafi, L.; Ksibi, H.; Kallel, H.; Chaari, A.; Chelly, H.; Ben Hamida, C.; Rekik, N.; et al. Gastrointestinal manifestations in severe scorpion envenomation. Gastroentérol. Clin. Biol. 2005, 29, 1001–1005. [Google Scholar] [CrossRef]
- Chtara, K.; Bahloul, M.; Turki, O.; Baccouche, N.; Regaieg, K.; Ben Hamida, C.; Chelly, H.; Chabchoub, I.; Chaari, A.; Bouaziz, M. Incidence and impact outcome of hyperglycaemia in severe scorpion envenomed children requiring intensive care admission. Intensive Care Med. 2015, 41, 1871–1872. [Google Scholar] [CrossRef]
- Rodrigo, C.; Gnanathasan, A. Management of scorpion envenoming: A systematic review and meta-analysis of controlled clinical trials. Syst. Rev. 2017, 6, 74. [Google Scholar] [CrossRef]
- Sifi, A.; Adi-Bessalem, S.; Laraba-Djebari, F. Role of angiotensin II and angiotensin type-1 receptor in scorpion venom-induced cardiac and aortic tissue inflammation. Exp. Mol. Pathol. 2017, 102, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Bahloul, M.; Chaari, A.; Ammar, R.; Allala, R.; Dammak, H.; Turki, O.; Chelly, H.; Ben Hamida, C.; Bouaziz, M. Severe scorpion envenomation among children: Does hydrocortisone improve outcome? A case-control study. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Malaque, C.M.S.A.; de Bragança, A.C.; Sanches, T.R.; Volpini, R.A.; Shimizu, M.H.; Hiyane, M.I.; Câmara, N.O.S.; Seguro, A.C.; Andrade, L. The role of dexamethasone in scorpion venom-induced deregulation of sodium and water transport in rat lungs. Intensive Care Med. Exp. 2015, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Alberts, M.B.; Shalit, M.; LoGalbo, F. Suction for venomous snakebite: A study of “mock venom” extraction in a human model. Ann. Emerg. Med. 2004, 43, 181–186. [Google Scholar] [CrossRef]
- de Oliveira, U.C.; Nishiyama, M.Y.; dos Santos, M.B.V.; de Paula Santos-da-Silva, A.; de Menezes Chalkidis, H.; Souza-Imberg, A.; Candido, D.M.; Yamanouye, N.; Dorce, V.A.C.; Junqueira-De-Azevedo, I.D.L.M. Proteomic endorsed transcriptomic profiles of venom glands from Tityus obscurus and T. serrulatus scorpions. PLoS ONE 2018, 13, e0193739. [Google Scholar] [CrossRef] [PubMed]
- Abroug, F.; Ouanes-Besbes, L.; Ouanes, I.; Dachraoui, F.; Hassen, M.F.; Haguiga, H.; Elatrous, S.; Brun-Buisson, C. Meta-analysis of controlled studies on immunotherapy in severe scorpion envenomation. Emerg. Med. J. 2011, 28, 963–969. [Google Scholar] [CrossRef]
- Medhioub Kaaniche, F.; Allala, R.; Baccouch, N.; Boujelbène, M.; Nasri, A. Réaction d’hypersensibilité au sérum anti-scorpionique: À propos d’un cas et revue de littérature. Toxicol. Anal. Clin. 2019, 31, 116–118. [Google Scholar] [CrossRef]
- Descotes, J. Immunothérapie spécifique des intoxications: Passé, présent, avenir. Toxicol. Anal. Clin. 2015, 27, 123. [Google Scholar] [CrossRef]
- Gupta, B.D.; Parakh, M.; Purohit, A. Management of Scorpion Sting: Prazosin or Dobutamine. J. Trop. Pediatr. 2010, 56, 115–118. [Google Scholar] [CrossRef]
- Gupta, V. Prazosin: A Pharmacological Antidote for Scorpion Envenomation. J. Trop. Pediatr. 2005, 52, 150–151. [Google Scholar] [CrossRef]
- Bouaziz, M.; Ben Hamida, C.; Chelly, H.; Bahloul, M.; Kallel, H. Dobutamine in the treatment of severe scorpion envenoming. Toxicon 2020, 182, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Abroug, F.; Ouanes-Besbes, L.; Elatrous, S. Should dobutamine be used in severe scorpion envenomation. Clin. Toxicol. 2015, 53, 584. [Google Scholar] [CrossRef] [PubMed]
- Bahloul, M.; Chaari, A.; Dammak, H.; Samet, M.; Chtara, K.; Chelly, H.; Ben Hamida, C.; Kallel, H.; Bouaziz, M. Pulmonary edema following scorpion envenomation: Mechanisms, clinical manifestations, diagnosis and treatment. Int. J. Cardiol. 2013, 162, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Najafian, M.; Ghorbani, A.; Zargar, M.; Baradaran, M.; Baradaran, N. Scorpion stings in pregnancy: An analysis of outcomes in 66 envenomed pregnant patients in Iran. J. Venom. Anim. Toxins Trop. Dis. 2020, 26, e20190039. [Google Scholar] [CrossRef]
- Ates, S.; Karahan, M.A.; Altay, N.; Akelci, K.; Ikiz, N.; Guzel, B.; Ozer, M.W.; Yilmaz, H.D. Approach to scorpion stings in pregnancy: A retrospective case series and literature review. Taiwan J. Obstet. Gynecol. 2018, 57, 692–695. [Google Scholar] [CrossRef]
- Ben Nasr, H.; Hammami, T.S.; Sahnoun, Z.; Rebai, T.; Bouaziz, M.; Kassis, M.; Zeghal, K.M. Scorpion envenomation symptoms in pregnant women. J. Venom. Anim. Toxins Trop. Dis. 2007, 13, 94–102. [Google Scholar] [CrossRef]
- Kaplanoglu, M.; Helvaci, M.R. Scorpion stings in pregnant women: An analysis of 11 cases and review of literature. Clin. Exp. Obstet. Gynecol. 2015, 42, 228–230. [Google Scholar] [CrossRef]
- Zengin, S.; Al, B.; Oktay, M.M.; Kilic, H. Scorpion sting: Eclampsia. Case Rep. 2012, 2012, bcr1220115401. [Google Scholar] [CrossRef]
- do Nascimento Martins, A.; Nencioni, A.L.A.; Dorce, A.L.C.; Paulo, M.E.F.V.; Frare, E.O.; Dorce, V.A.C. Effect of maternal exposure to Tityus bahiensis scorpion venom during lactation on the offspring of rats. Reprod. Toxicol. 2016, 59, 147–158. [Google Scholar] [CrossRef]
| Class I: Local Symptoms | Class II: Minor Symptoms | Class III: Severe Symptoms | |
|---|---|---|---|
| Local symptoms | General symptoms | Pulmonary symptoms | Neurological failure |
| Bullous eruption | Hyperthermia | Stridor | Glasgow Score ≤ 6 (spontaneous) |
| Burning sensation | Hypothermia | Wheezing | Paralysis |
| Ecchymosis | Sweating | Abdominal and urogenital symptoms | |
| Erythema | Thirst | Diarrhea | Cardiogenic failure |
| Hyperesthesia | Pallor | Gastrointestinal hemorrhage | Bradycardia |
| Itching | Neurological symptoms | Hematuria | Cardiovascular collapse |
| Necrosis | Agitation/Restlessness/Excitement | Abdominal distension | Hypotension |
| Local paresthesia | Anisocoria | Nausea | Ventricular arrhythmia |
| Pain | Ataxia | Pancreatitis | |
| Purpura/Petechia | Confusion | Priapism | Respiratory failure |
| Swelling | Convulsion | Urinary retention | Cyanosis |
| Tingling | Dystonia | Vomiting | Dyspnea |
| Encephalopathy | ENT symptoms | Pulmonary edema | |
| Fasciculation | Salivation | ||
| Headache | Lacrimation | ||
| Miosis | Odynophagia | ||
| Mydriasis | Rhinorrhea | ||
| Nystagmus | Dry mouth | ||
| General paresthesia | Muscular symptoms | ||
| Prostration | Arthralgia | ||
| Ptosis | Local muscular cramps | ||
| Somnolence/Lethargy/Drowsiness | Myoclonia | ||
| Cardiovascular symptoms | |||
| Hypertension | |||
| Tachycardia | |||
| Clinical Presentation | Management | Monitoring Time/Location |
|---|---|---|
| Asymptomatic Patient | 4 h/Emergency Department (ED) | |
| General | Washing and disinfection of the wound | |
| Measurement of vital parameters, capillary glycemia | ||
| Class I envenoming | 8 h/ED or hospitalization unit | |
| General | Same as “asymptomatic patient” | |
| Local pain | Local analgesia: topical anesthetic and external cooling | |
| Class II envenoming or presence of isolated hyperglycemia | Until symptoms improve (8 h minimum)/hospitalization unit | |
| General | Same as “asymptomatic patient” and “class I envenoming” Biology: blood count, blood ionogram (K, Na, Cl), Ca, ASAT, ALAT, lipase, urea, creatinine, CRP, troponin, BNP, lactate Frontal chest X-ray 12-lead electrocardiogram (ECG) | |
| Neuromuscular disorder | Diazepam IVL - Adult: 10 mg - Child: 0.2 mg/kg, not to exceed 10 mg | |
| Acute pancreatitis | Fasting 72 h Abdominal and pelvic CT scan injected at D3 | |
| Electrocardiographic abnormality | Continuous monitoring by cardio-tensional scope Repeat transthoracic echography (TTE) to assess left heart function | Same/Critical Care Unit |
| Acute lung edema | Continuous monitoring by cardio-tensional scope Furosemide IVSE - Adult: IVSE 250 to 1000 mg/24 h - Child: IVSE 0.5 to 1 mg/kg/24 h, not to exceed 20 mg Repeat TTE to assess left heart function | Same/Intensive care |
| Arterial hypertension | Prazosin per os: initial dose then 30 μg/kg/6 h as long as the ventricular ejection fraction is less than 60% - Adult: loading dose and maintenance dose 0.5 mg every 3 h - Child: loading and maintenance dose 30 μg/kg every 3 h Repeat TTE to assess left heart function | Same/Critical Care Unit |
| Class III envenoming | Until symptoms improve (8 h minimum)/Intensive care | |
| General | Same as “asymptomatic patient” and “class I and II envenoming” Biology: arterial blood gas 18-lead ECG Repeat TTE to assess left heart function Continuous monitoring by cardio-tensional scope Discuss oro-tracheal intubation to protect the airway | |
| Cardiogenic shock | Dobutamine 5–10 μg/kg/min (adult and child) Repeat TTE to assess left heart function | |
| Paralysis | Monitoring of respiratory and swallowing disorders | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaucel, J.-A.; Larréché, S.; Paradis, C.; Courtois, A.; Pujo, J.-M.; Elenga, N.; Résière, D.; Caré, W.; de Haro, L.; Gallart, J.-C.; et al. French Scorpionism (Mainland and Oversea Territories): Narrative Review of Scorpion Species, Scorpion Venom, and Envenoming Management. Toxins 2022, 14, 719. https://doi.org/10.3390/toxins14100719
Vaucel J-A, Larréché S, Paradis C, Courtois A, Pujo J-M, Elenga N, Résière D, Caré W, de Haro L, Gallart J-C, et al. French Scorpionism (Mainland and Oversea Territories): Narrative Review of Scorpion Species, Scorpion Venom, and Envenoming Management. Toxins. 2022; 14(10):719. https://doi.org/10.3390/toxins14100719
Chicago/Turabian StyleVaucel, Jules-Antoine, Sébastien Larréché, Camille Paradis, Arnaud Courtois, Jean-Marc Pujo, Narcisse Elenga, Dabor Résière, Weniko Caré, Luc de Haro, Jean-Christophe Gallart, and et al. 2022. "French Scorpionism (Mainland and Oversea Territories): Narrative Review of Scorpion Species, Scorpion Venom, and Envenoming Management" Toxins 14, no. 10: 719. https://doi.org/10.3390/toxins14100719
APA StyleVaucel, J.-A., Larréché, S., Paradis, C., Courtois, A., Pujo, J.-M., Elenga, N., Résière, D., Caré, W., de Haro, L., Gallart, J.-C., Torrents, R., Schmitt, C., Chevalier, J., Labadie, M., Kallel, H., & French PCC Research Group. (2022). French Scorpionism (Mainland and Oversea Territories): Narrative Review of Scorpion Species, Scorpion Venom, and Envenoming Management. Toxins, 14(10), 719. https://doi.org/10.3390/toxins14100719