Botulinum Neurotoxins beyond Neurons: Interplay with Glial Cells
Abstract
1. Introduction
2. Search Strategy and Inclusion Criteria
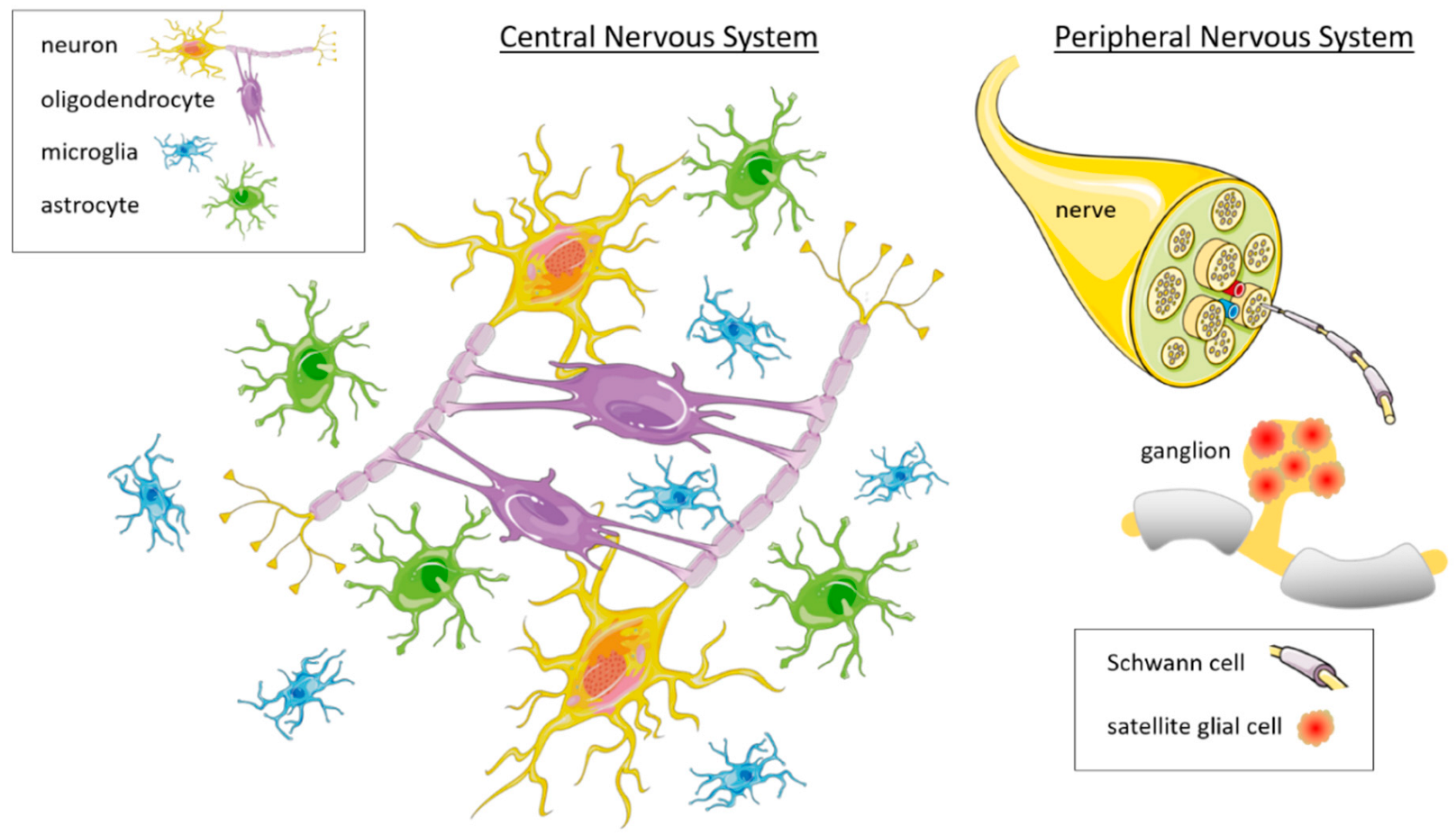
3. Glia
4. Interactions between BoNTs, Microglia, and Astrocytes
5. Interaction between BoNTs and Myelin Forming Cells
6. Interactions between BoNTs and Other Glial Cells
7. Summary
8. Concluding Remarks and Future Perspectives
- Are all the effects of peripheral BoNTs on central spinal glial cells solely due to direct interactions that occur after the retrograde transport of BoNTs?
- How should this retrograde transport be controlled so that it does not cause adverse effects after the BoNTs are injected?
- Which receptors on the membrane of the glial cell allow BoNTs to enter the glial cell and exert their proteolytic effect?
- Is the mechanism of action of BoNTs inside the glial cells, as has been widely demonstrated at the neuronal level? Are there specific internal glial cell targets involved?
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montecucco, C.; Rasotto, M.B. On botulinum neurotoxin variability. mBio 2015, 6, e02131. [Google Scholar] [CrossRef]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef]
- Peck, M.W.; Smith, T.J.; Anniballi, F.; Austin, J.W.; Bano, L.; Bradshaw, M.; Cuervo, P.; Cheng, L.W.; Derman, Y.; Dorner, B.G.; et al. Historical perspectives and guidelines for botulinum neurotoxin subtype nomenclature. Toxins 2017, 9, 38. [Google Scholar] [CrossRef]
- Steward, L.; Brin, M.F.; Brideau-Andersen, A. Novel Native and Engineered Botulinum Neurotoxins. In Botulinum Toxin Therapy; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2021; Volume 263, pp. 63–89. [Google Scholar]
- Rossetto, O.; Pirazzini, M.; Fabris, F.; Montecucco, C. Botulinum Neurotoxins: Mechanism of Action. Handb. Exp. Pharm. 2021, 263, 35–47. [Google Scholar]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology and Toxicology. Pharm. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef]
- Pavone, F.; Luvisetto, S. Botulinum Neurotoxin for Pain Management: Insights from Animal Models. Toxins 2010, 2, 2890–2913. [Google Scholar] [CrossRef]
- Yan, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways and the Major Nuerotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar]
- Park, J.; Park, H.J. Botulinum Toxin for the Treatement of Neuropathic Pain. Toxins 2017, 9, 260. [Google Scholar] [CrossRef]
- Lackovic, Z. Botulinum Toxin and Pain. Handb. Exp. Pharm. 2021, 263, 251–264. [Google Scholar]
- Attal, N. Pharmacological treatments of neuropathic pain: The latest recommendations. Rev. Neurol. 2019, 175, 46–50. [Google Scholar] [CrossRef]
- Jeftinija, S.D.; Jeftinija, K.V.; Stefanovic, G. Cultured astrocytes express proteins involved in vesicular glutamate release. Brain Res. 1997, 750, 41–47. [Google Scholar] [CrossRef]
- Verderio, C.; Coco, S.; Rossetto, O.; Montecucco, C.; Matteoli, M. Internalization and proteolytic action of botulinum toxins in CNS neurons and astrocytes. J. Neurochem. 1999, 73, 372–379. [Google Scholar] [CrossRef]
- Araque, A.; Li, N.; Doyle, R.T.; Haydon, P.G. SNARE protein-dependent glutamate release from astrocytes. J. Neurosci. 2000, 20, 666–673. [Google Scholar] [CrossRef]
- Yaguchi, T.; Nishizaki, T. Extracellular high K+ stimulates glutamate release from astrocytes by activating voltage-dependent calcium channels. J. Cell Physiol. 2010, 225, 512–518. [Google Scholar] [CrossRef]
- Abdipranoto, A.; Liu, G.J.; Werry, E.L.; Bennett, M.R. Mechanisms of secretion of ATP from cortical astrocytes triggered by uridine triphosphate. Neuroreport 2003, 14, 2177–2178. [Google Scholar] [CrossRef]
- Liu, G.J.; Werry, E.L.; Bennett, M.R. Secretion of ATP from Schwann cells in response to uridine triphosphate. Eur. J. Neurosci. 2005, 21, 151–160. [Google Scholar] [CrossRef]
- Marinelli, S.; Vacca, V.; Ricordy, R.; Uggenti, C.; Tata, A.M.; Luvisetto, S.; Pavone, F. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS ONE 2012, 7, e47977. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- Barateiro, A.; Brites, D.; Fernandes, A. Oligodendrocyte Development and Myelination in Neurodevelopment: Molecular Mechanisms in Health and Disease. Curr. Pharm. Des. 2016, 22, 656–679. [Google Scholar] [CrossRef]
- Zhou, B.; Zhu, Z.; Ransom, B.R.; Tong, X. Oligodendrocyte lineage cells and depression. Mol. Psychiatry 2021, 26, 103–117. [Google Scholar] [CrossRef]
- Yamazaki, Y. Oligodendrocyte Physiology Modulating Axonal Excitability and Nerve Conduction. Adv. Exp. Med. Biol. 2019, 1190, 123–144. [Google Scholar]
- Del Bigio, M.R. Ependymal cells: Biology and pathology. Acta Neuropathol. 2010, 119, 55–73. [Google Scholar] [CrossRef]
- Moreno-Manzano, V. Ependymal cells in the spinal cord as neuronal progenitors. Curr. Opin. Pharm. 2020, 50, 82–87. [Google Scholar] [CrossRef]
- Seo, J.S.; Mantas, J.; Svenningsson, P.; Greengard, P. Ependymal cells-CSF flow regulates stress-induced depression. Mol. Psychiatry 2021, 26, 7308–7315. [Google Scholar] [CrossRef]
- Barry, D.S.; Pakan, J.M.; McDermott, K.W. Radial glial cells: Key organisers in CNS development. Int. J. Biochem. Cell Biol. 2014, 46, 76–79. [Google Scholar] [CrossRef]
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef]
- Sofronien, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Araque, A. Astrocytes process synaptic information. Neuron Glia Biol. 2008, 4, 3–10. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Salzer, J.L. Schwann cell meylination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020529. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef] [PubMed]
- Ohara, P.T.; Vit, J.P.; Bhargava, A.; Romero, M.; Sundberg, C.; Charles, A.C.; Jasmin, L. Gliopathic pain: When satellite glial cells go bad. Neuroscientist 2009, 15, 450–463. [Google Scholar] [CrossRef]
- Hanani, M.; Verkhrastky, A. Satellite Glial Cells and Astrocytes, a Comparative Review. Neurochem. Res. 2021, 46, 2525–2537. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M. How Is Peripheral Injury Signaled to Satellite Glial Cells in Sensory Ganglia? Cells 2022, 11, 512. [Google Scholar] [CrossRef]
- Huang, L.Y.; Gu, Y.; Chen, Y. Communication between neuronal somata and satellite glial cells in sensory ganglia. Glia 2013, 61, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Cortes, F.; Turco, F.; Linan-Rico, A.; Soghomonyan, S.; Whitaker, E.; Wehner, S.; Cuomo, R.; Christofi, F.L. Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22, 433–449. [Google Scholar] [CrossRef]
- Seguella, L.; Gulbransen, B.D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 571–587. [Google Scholar] [CrossRef]
- Sharkey, K.A. Emerging roles for enteric glia in gastrointestinal disorders. J. Clin. Investig. 2015, 125, 918–925. [Google Scholar] [CrossRef]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Prinz, M. Microglia Heterogeneity in the Single-Cell Era. Cell Rep. 2020, 30, 1271–1281. [Google Scholar] [CrossRef]
- Cserép, C.; Pósfai, B.; Dénes, Á. Shaping Neuronal Fate: Functional Heterogeneity of Direct Microglia-Neuron Interactions. Neuron 2021, 109, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Ferretti, M.T. Function and Dysfunction of Microglia during Brain Development: Consequences for Synapses and Neural Circuits. Front. Synaptic Neurosci. 2017, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.R.; Milligan, E.D.; Maier, S.F. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001, 24, 450–455. [Google Scholar] [CrossRef]
- Austin, P.J.; Moalem-Taylor, G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 2010, 229, 26–50. [Google Scholar] [CrossRef]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef]
- Tsuda, M. Microglia in the spinal cord and neuropathic pain. J. Diabetes Investig. 2016, 7, 17–26. [Google Scholar] [CrossRef]
- Sommer, C.; Schäfers, M. Mechanisms of neuropathic pain: The role of cytokines. Drug Discov. Today Dis. Mech. 2004, 1, 441–448. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Kishioka, S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr. Opin. Pharmacol. 2012, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, E.; Piotrowska, A.; Popiolek-Barczyk, K.; Mika, J. Botulinum Toxin Type A-A Modulator of Spinal Neuron-Glia Interactions under Neuropathic Pain Conditions. Toxins 2018, 10, 145. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; Ninomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke. Int. J. Mol. Sci. 2017, 18, 2135. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Wang, H.; Wu, L.; Tian, S.; Wang, X.; Zheng, H.; Wu, W. Botulinum toxin type A promotes M2 polarization and suppresses chronic constriction injury-induced neuropathic pain through the P2X7 receptor. Cell Biosci. 2020, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, S.; Wang, H.; Liu, P.; Zheng, H.; Wu, L.; Liu, Q.; Wu, W. Botulinum toxin type A alleviates neuropathic pain and suppresses inflammatory cytokines release from microglia by targeting TLR2/MyD88 and SNAP23. Cell Biosci. 2020, 10, 141. [Google Scholar] [CrossRef]
- Chen, W.J.; Niu, J.Q.; Chen, Y.T.; Deng, W.J.; Xu, Y.Y.; Liu, J.; Luo, W.F.; Liu, T. Unilateral facial injectionn of Botulinum neurotoxin A attenuates bilateral trigeminal neuropathic pain and anxiety-like behaviors through inhibition of TLR2-mediated neuroinflammation in mice. J. Headache Pain 2021, 22, 38. [Google Scholar] [CrossRef]
- Piotrowska, A.; Popiolek-Barczyk, K.; Pavone, F.; Mika, J. Comparison of the Expression Changes after Botulinum Toxin Type A and Minocycline Administration in Lipopolysaccharide-Stimulated Rat Microglial and Astroglial Cultures. Front. Cell. Infect. Microbiol. 2017, 7, 141. [Google Scholar] [CrossRef]
- Hepp, R.; Perraut, M.; Chasserot-Golaz, S.; Galli, T.; Aunis, D.; Langley, K.; Grant, N.J. Cultured glial cells express the SNAP-25 analogue SNAP-23. Glia 1999, 27, 181–187. [Google Scholar] [CrossRef]
- Vaidyanathan, V.V.; Yoshino, K.; Jahnz, M.; Dörries, C.; Bade, S.; Nauenburg, S.; Niemann, H.; Binz, T. Proteolysis of SNAP-25 isoforms by botulinum neurotoxin types A, C, and E: Domains and amino acid residues controlling the formation of enzyme-substrate complexes and cleavage. J. Neurochem. 1999, 72, 327–337. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, J.; Sun, L.; Wang, Y.; Meng, H. Combination of Botulinum Toxin and Minocycline Ameliorates Neuropathic Pain through Antioxidant Stress and Anti-inflammation via Promoting SIRT1 Pathway. Front. Pharm. 2021, 11, 602417. [Google Scholar] [CrossRef]
- Feng, X.; Xiong, D.; Li, J.; Xiao, L.; Xie, W.; Qiu, Y. Direct Inhibition of Microglia Activation by Pretreatment with Botulinum Neurotoxin A for the Prevention of Neuropathic Pain. Front. Neurosci. 2021, 15, 760403. [Google Scholar] [CrossRef]
- Vacca, V.; Marinelli, S.; Luvisetto, S.; Pavone, F. Botulinum toxin A increases analgesic effects of morphine, counters development of morphine tolerance and modulates glia activation and μ opioid receptor expression in neuropathic mice. Brain Behav. Immun. 2013, 32, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Vacca, V.; Madaro, L.; De Angelis, F.; Proietti, D.; Cobianchi, S.; Orsini, T.; Puri, P.L.; Luvisetto, S.; Pavone, F.; Marinelli, S. Revealing the therapeutic potential of Botulinum neurotoxin type A in counteracting paralysis and neuropathic pain in spinally injured mice. Toxins 2020, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, A.; Marinelli, S.; De Angelis, F.; Vacca, V.; Luvisetto, S.; Pavone, F. Botulinum Toxin B Affects Neuropathic Pain but Not Functional Recovery after Peripheral Nerve Injury in a Mouse Model. Toxins 2018, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Gao, C.; Wang, L.; Chu, X.; Shi, Q.; Yang, H.; Li, T. Botulinum toxin type A ameliorates adjuvant-arthritis pain by inhibiting microglial activation-mediated neuroinflammation and intracellular molecular signaling. Toxicon 2020, 178, 33–40. [Google Scholar] [CrossRef]
- Munoz-Lora, V.R.M.; Abdalla, H.B.; Del Bel Cury, A.A.; Clemente-Napimoga, J.T. Modulatory effect of botulinum toxin type A on the microglia P2X7/CatS/FKN activated-pathway in antigen-induced arthritis of the temporomandibular joint of rats. Toxicon 2020, 187, 116–121. [Google Scholar] [CrossRef]
- Munoz-Lora, V.R.M.; Dugonjic Okrosa, A.; Matak, I.; Del Bel Cury, A.A.; Kalinichev, M.; Lackovic, Z. Antinociceptive Actions of Botulinum Toxin A1 on Immunogenic Hypersensitivity in Temporomandibular Joint of Rats. Toxins 2022, 14, 161. [Google Scholar] [CrossRef]
- Luvisetto, S. Botulinum Neurotoxins in Central Nervous System: An Overview from Animal Models to Human Therapy. Toxins 2021, 13, 751. [Google Scholar] [CrossRef]
- Ferrari, E.; Gu, C.; Niranjan, D.; Restani, L.; Rasetti-Escargueil, C.; Obara, I.; Geranton, S.M.; Arsenault, J.; Goetze, T.A.; Harper, C.B.; et al. Synthetic Self-Assembling Clostridial Chimera for Modulation of Sensory Functions. Bioconjug. Chem. 2013, 24, 1750–1759. [Google Scholar] [CrossRef]
- Webb, R.P. Engineering of Botulinum Neurotoxins for Biomedical Applications. Toxins 2018, 10, 231. [Google Scholar] [CrossRef]
- Fonfria, E.; Elliott, M.; Beard, M.; Chaddock, J.A.; Krupp, J. Engineering Botulinum Toxins to Improve and Expand Targeting and SNARE Cleavage Activity. Toxins 2018, 10, 278. [Google Scholar] [CrossRef]
- Li, N.; Leung, G.K.K. Oligodendrocyte precursor cells in spinal cord injury: A review and update. BioMed Res. Int. 2015, 2015, 235195. [Google Scholar] [CrossRef] [PubMed]
- Bartholdi, D.; Schwab, M.E. Oligodendroglial reaction following spinal cord injury in rat: Transient upregulation of MBP mRNA. Glia 1998, 23, 278–284. [Google Scholar] [CrossRef]
- Marinelli, S.; Luvisetto, S.; Cobianchi, S.; Makuch, W.; Obara, I.; Mezzaroma, E.; Caruso, M.; Straface, E.; Przewlocka, B.; Pavone, F. Botulinum neurotoxin type A counteracts neuropathic pain and facilitates fucntional recovery after peripheral nerve injury in animal models. Neuroscience 2010, 171, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef]
- Barden, J.A.; Cottee, L.J.; Bennett, M.R. Vesicle-associated proteins and P2X receptor clusters at single sympathetic varicosities in mouse vas deferens. J. Neurocytol. 1999, 28, 469–480. [Google Scholar] [CrossRef]
- Loreti, S.; Ricordy, R.; De Stefano, M.E.; Augusto-Tocco, G.; Tata, M.A. Acetylcholine inhibits cell cycle progression in rat Schwann cells by activation of the M2 receptor subtype. Neuron Glia Biol. 2007, 3, 269–279. [Google Scholar] [CrossRef]
- Uggenti, C.; De Stefano, M.E.; Costantino, M.; Loreti, S.; Pisano, A.; Avallone, B.; Talora, C.; Magnaghi, V.; Tata, A.M. M2 muscarinic receptor activation regulates Schwann cell differentiation and myelin organization. Dev. Neurobiol. 2014, 74, 676–691. [Google Scholar] [CrossRef]
- Cobianchi, S.; Jaramillo, J.; Luvisetto, S.; Pavone, F.; Navarro, X. Botulinum neurotoxin A promotes functional recovery after peripheral nerve injury by increasing regeneration of myelinated fibers. Neuroscience 2017, 359, 82–91. [Google Scholar] [CrossRef]
- Seo, M.; Lim, D.; Kim, S.; Kim, T.; Kwon, B.S.; Nam, K. Effect of Botulinum Injection and Extracorporeal Shock Wave Therapy on Nerve Regeneration in Rats with Experimentally Induced Sciatic Nerve Injury. Toxins 2021, 13, 879. [Google Scholar] [CrossRef]
- da Silva, L.B.; Poulsen, J.N.; Arendt-Nielsen, L.; Gazerani, P. Botulinum neurotoxin type A modulates vesicular release of glutamate from satellite glial cells. J. Cell Mol. Med. 2015, 19, 1900–1909. [Google Scholar] [CrossRef]
- Gazerani, P. Satellite Glial Cells in Pain Research: A Targeted Viewpoint of Potential and Future Directions. Front. Pain Res. 2021, 2, 646068. [Google Scholar] [CrossRef] [PubMed]
- Edvinson, J.; Warfvinge, K.; Edvinsson, L. Modulation of inflammatory mediators in the trigeminal ganglion by botulinum neurotoxin type A: An organ culture study. J. Headache Pain 2015, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, A.; Frederiksen, S.D.; Blixt, F.W.; Warfvinge, K.; Edvinsson, L. Expression of messenger molecules and receptors in rat and human sphenopalatine ganglion indicating therapeutic targets. J. Headache Pain 2016, 17, 78. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luvisetto, S. Botulinum Neurotoxins beyond Neurons: Interplay with Glial Cells. Toxins 2022, 14, 704. https://doi.org/10.3390/toxins14100704
Luvisetto S. Botulinum Neurotoxins beyond Neurons: Interplay with Glial Cells. Toxins. 2022; 14(10):704. https://doi.org/10.3390/toxins14100704
Chicago/Turabian StyleLuvisetto, Siro. 2022. "Botulinum Neurotoxins beyond Neurons: Interplay with Glial Cells" Toxins 14, no. 10: 704. https://doi.org/10.3390/toxins14100704
APA StyleLuvisetto, S. (2022). Botulinum Neurotoxins beyond Neurons: Interplay with Glial Cells. Toxins, 14(10), 704. https://doi.org/10.3390/toxins14100704




