Aflatoxin Contamination of Maize, Groundnut, and Sorghum Grown in Burkina Faso, Mali, and Niger and Aflatoxin Exposure Assessment
Abstract
1. Introduction
2. Materials and Methods
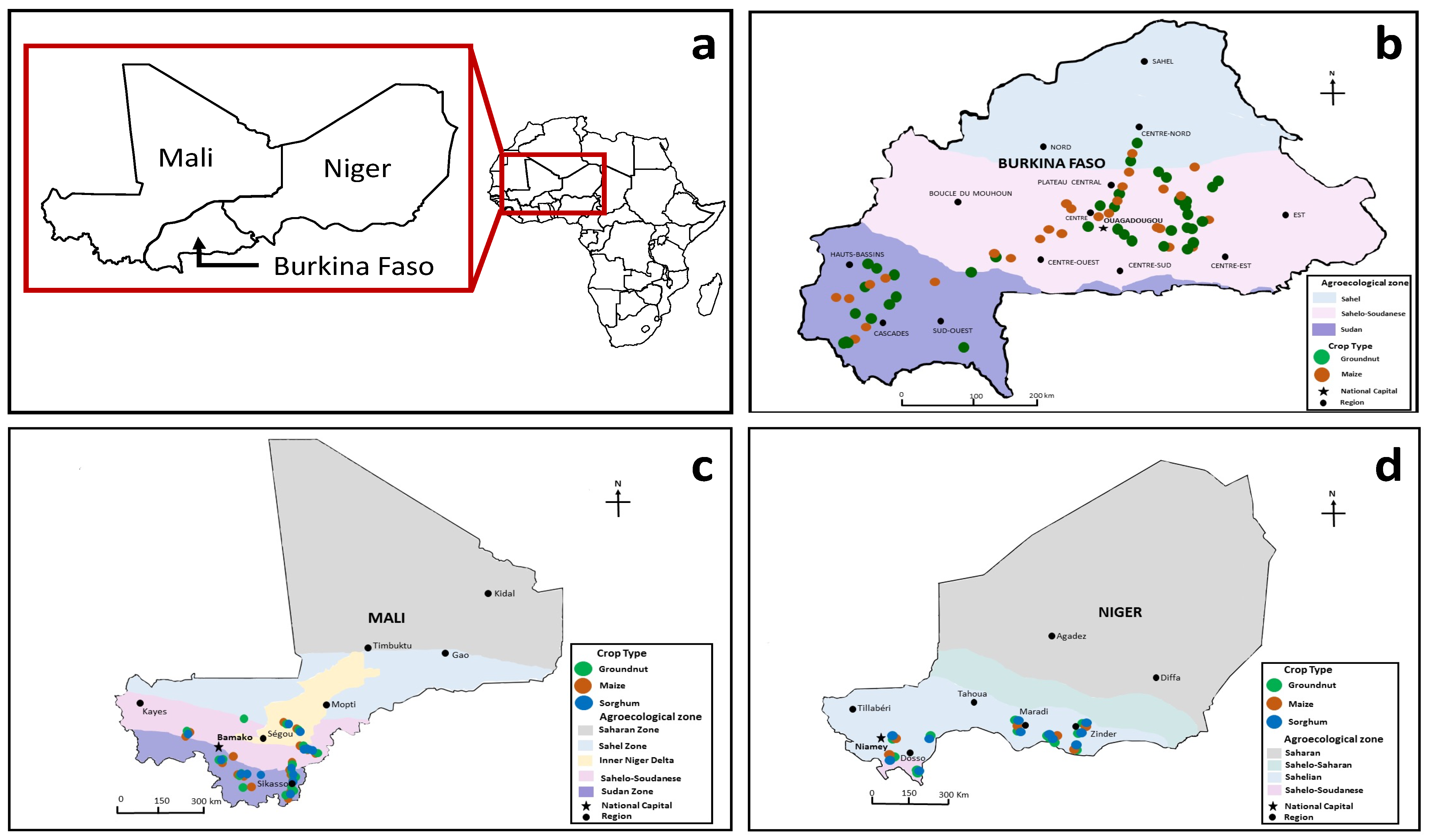
2.1. Sample Collection
2.2. Sample Processing
2.3. Aflatoxin Quantification
2.4. Data Analysis
2.5. Assessment of Exposure
3. Results
4. Burkina Faso
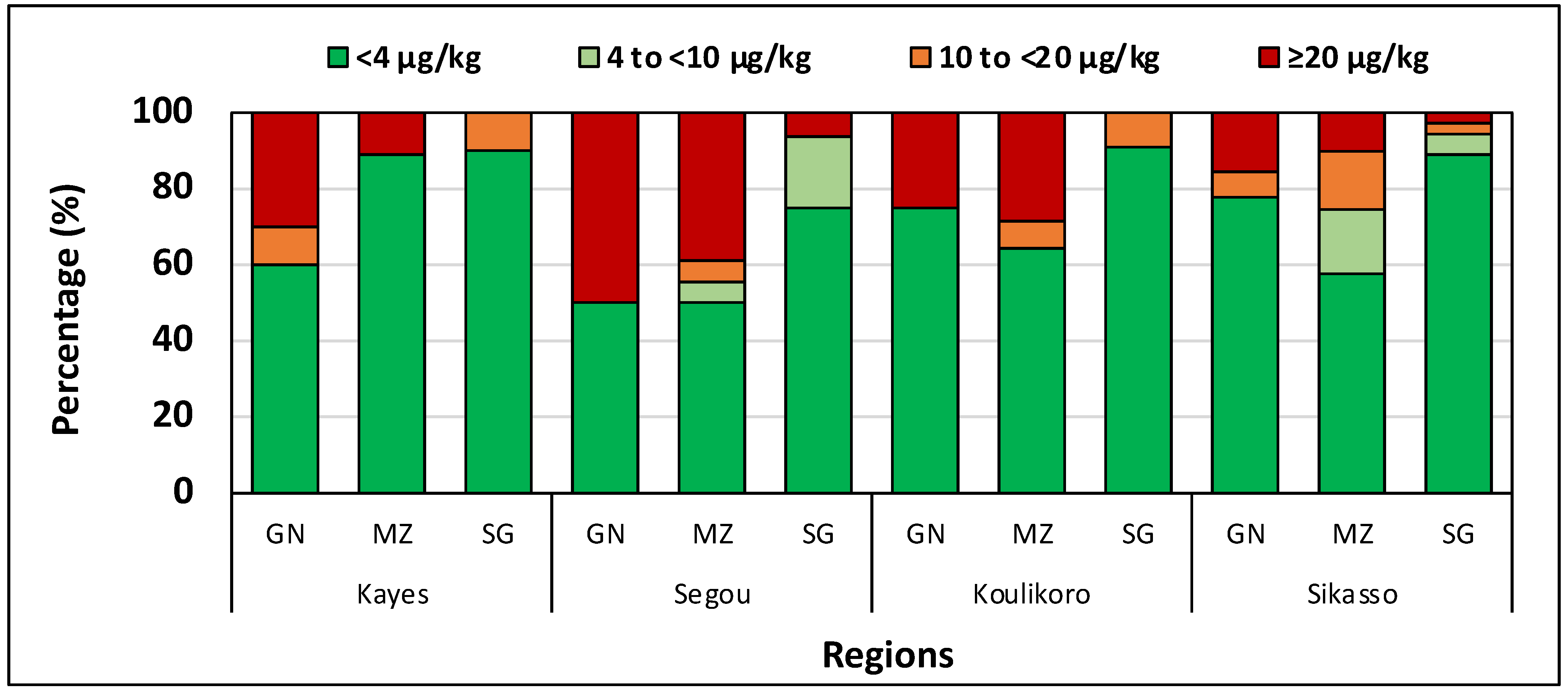
5. Mali
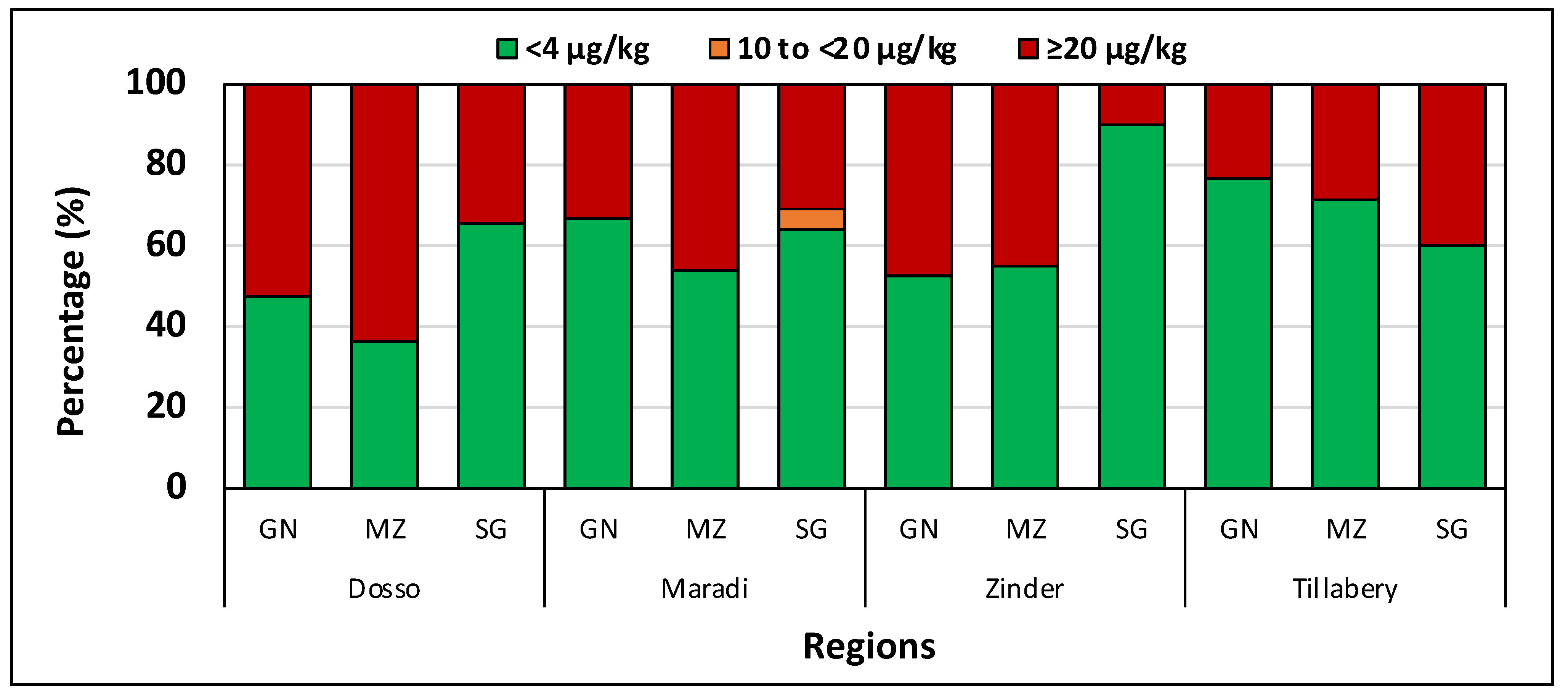
6. Niger
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Statement of Compliance
References
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Hong, S.-B.; Nováková, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklenář, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Gieseker, K.E. Centers for Disease Control and Prevention. Outbreak of Aflatoxin Poisoning-Eastern and Central Provinces, Kenya, January-July 2004. Morb. Mortal. Wkly. Rep. 2004, 53, 790–793. [Google Scholar]
- Kamala, A.; Shirima, C.; Jani, B.; Bakari, M.; Sillo, H.; Rusibamayila, N.; Saeger, S.D.; Kimanya, M.; Gong, Y.Y.; Simba, A.; et al. Outbreak of an acute aflatoxicosis in Tanzania during 2016. World Mycotoxin J. 2018, 11, 311–320. [Google Scholar] [CrossRef]
- Williams, J.H.; Grubb, J.A.; Davis, J.W.; Wang, J.-S.; Jolly, P.E.; Ankrah, N.-A.; Ellis, W.O.; Afriyie-Gyawu, E.; Johnson, N.M.; Robinson, A.G.; et al. HIV and hepatocellular and esophageal carcinomas related to consumption of mycotoxin-prone foods in sub-Saharan Africa. Am. J. Clin. Nutr. 2010, 92, 154–160. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Aflatoxins. 100F. 2012. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100F-23.pdf (accessed on 1 July 2022).
- Kuiper-Goodman, T. Mycotoxins: Risk assessment and legislation. Toxicol. Lett. 1995, 82/83, 853–859. [Google Scholar] [CrossRef]
- Brera, C.; Debegnach, F.; Gregori, E.; Colicchia, S.; Soricelli, S.; Miano, B.; Magri, M.C.; De Santis, B. Dietary assessment of European population to mycotoxins: A review. In Environmental Mycology in Public Health; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 16; pp. 223–259. [Google Scholar] [CrossRef]
- WHO. Aflatoxins. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 1 July 2022).
- JECFA. Safety Evaluation of Certain Contaminants in Food: Prepared by the Eighty-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization and Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2018; (WHO Food Additives Series, No. 74; FAO JECFA Monographs 19 bis); License: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Ismail, A.; Naeem, I.; Gong, Y.Y.; Routledge, M.N.; Akhtar, S.; Riaz, M.; Ramalho, L.N.Z.; de Oliveira, C.A.F.; Ismail, Z. Early life exposure to dietary aflatoxins, health impact and control perspectives: A review. Trends Food Sci. Technol. 2021, 112, 212–224. [Google Scholar] [CrossRef]
- Jin, S.; Yang, H.; Jiao, Y.; Pang, Q.; Wang, Y.; Wang, M.; Shan, A.; Feng, X. Dietary curcumin alleviated acute ileum damage of ducks (Anas platyrhynchos) induced by AFB1 through regulating Nrf2-ARE and NF-κB signaling pathways. Foods 2021, 10, 1370. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.G.; Avramescu, S.; Marin, D.E.; Țăranu, I.; Georgescu, S.E.; Dinischiotu, A. The reduction of the combined effects of aflatoxin and ochratoxin A in piglet livers and kidneys by dietary antioxidants. Toxins 2021, 13, 648. [Google Scholar] [CrossRef] [PubMed]
- Aikore, M.O.S.; Ortega-Beltran, A.; Eruvbetine, D.; Atehnkeng, J.; Falade, T.D.O.; Cotty, P.J.; Bandyopadhyay, R. Performance of broilers fed with maize colonized by either toxigenic or atoxigenic strains of Aspergillus flavus with and without an aflatoxin-sequestering agent. Toxins 2019, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Guclu, H. Aflatoxin regulations in a network of global maize trade. PLoS ONE 2012, 7, e45151. [Google Scholar] [CrossRef]
- Matumba, L.; Van Poucke, C.; Njumbe Ediage, E.; De Saeger, S. Keeping mycotoxins away from the food: Does the existence of regulations have any impact in Africa? Crit. Rev. Food Sci. Nutr. 2017, 57, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Waliyar, F.; Umeh, V.C.; Traore, A.; Osiru, M.; Ntare, B.R.; Diarra, B.; Kodio, O.; Kumar, K.V.K.; Sudini, H. Prevalence and distribution of aflatoxin contamination in groundnut (Arachis hypogaea L.) in Mali, West Africa. Crop Prot. 2015, 70, 1–7. [Google Scholar] [CrossRef]
- Afolabi, C.G.; Ezekiel, C.N.; Kehinde, I.A.; Olaolu, A.W.; Ogunsanya, O.M. Contamination of groundnut in south-western Nigeria by aflatoxigenic fungi and aflatoxins in relation to processing. J. Phytopathol. 2015, 163, 279–286. [Google Scholar] [CrossRef]
- Sirma, A.J.; Senerwa, D.M.; Grace, D.; Makita, K.; Mtimet, N.; Kang’ethe, E.K.; Lindahl, J.F. Aflatoxin B1 occurrence in millet, sorghum and maize from four agro-ecological zones in Kenya. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 10991–11003. [Google Scholar] [CrossRef]
- Warth, B.; Parich, A.; Atehnkeng, J.; Bandyopadhyay, R.; Schuhmacher, R.; Sulyok, M.; Krska, R. Quantitation of mycotoxins in food and feed from Burkina Faso and Mozambique using a modern LC-MS/MS multitoxin method. J. Agric. Food Chem. 2012, 60, 9352–9363. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Kumar, M.; Leslie, J. Relative severity of aflatoxin contamination of cereal crops in West Africa. Food Addit. Contam. 2007, 24, 1109–1114. [Google Scholar] [CrossRef]
- Solidarités International (2020) ‘The Sahel in the Midst of Climate Change’, Relief Web, 17 March. Available online: https://reliefweb.int/report/chad/sahel-midst-climate-change (accessed on 1 July 2022).
- Logrieco, A.; Battilani, P.; Leggieri, M.C.; Jiang, Y.; Haesaert, G.; Lanubile, A.; Mahuku, G.; Mesterházy, A.; Ortega-Beltran, A.; Pasti, M.; et al. Perspectives on global mycotoxin issues and management from the Mycokey Maize Working Group. Plant Dis. 2021, 105, 525–537. [Google Scholar] [CrossRef]
- Cotty, P.J. Virulence and cultural characteristics of two Aspergillus flavus strains pathogenic on cotton. Phytopathology 1989, 79, 808–814. [Google Scholar] [CrossRef]
- Chang, P.K.; Horn, B.W.; Dorner, J.W. Sequence breakpoints in the aflatoxin biosynthesis gene cluster and flanking regions in nonaflatoxigenic Aspergillus flavus isolates. Fungal Genet. Biol. 2005, 42, 914–923. [Google Scholar] [CrossRef]
- Moral, J.; Garcia-Lopez, M.T.; Camiletti, B.X.; Jaime, R.; Michailides, T.J.; Bandyopadhyay, R.; Ortega-Beltran, A. Present status and perspective on the future use of aflatoxin biocontrol products. Agronomy 2020, 10, 491. [Google Scholar] [CrossRef]
- Dorner, J.W.; Cole, R.J. Variability among peanut subsamples prepared for aflatoxin analysis with four mills. J. AOAC Int. 1993, 76, 983–987. [Google Scholar] [CrossRef]
- Atehnkeng, J.; Ojiambo, P.S.; Ikotun, T.; Sikora, R.A.; Cotty, P.J.; Bandyopadhyay, R. Evaluation of atoxigenic isolates of Aspergillus flavus as potential biocontrol agents for aflatoxin in maize. Food Addit. Contam.-Part A 2008, 25, 1264–1271. [Google Scholar] [CrossRef]
- Probst, C.; Cotty, P.J. Relationships between in vivo and in vitro aflatoxin production: Reliable prediction of fungal ability to contaminate maize with aflatoxins. Fungal Biol. 2012, 116, 503–510. [Google Scholar] [CrossRef]
- Yeh, F.S.; Yu, M.C.; Mo, C.C.; Luo, S.; Tong, M.J.; Henderson, B.E. Hepatitis B virus, aflatoxins, and hepatocellular carcinoma in southern Guangxi, China. Cancer Res. 1989, 49, 2506–2509. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef]
- Adetunji, M.C.; Olusegun, O.A.; Ezekiel, C.N. Risk assessment of mycotoxins in stored maize grains consumed by infants and young children in Nigeria. Children 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Akello, J.; Ortega-Beltran, A.; Katati, B.; Atehnkeng, J.; Augusto, J.; Mwila, C.M.; Mahuku, G.; Chikoye, D.; Bandyopadhyay, R. Prevalence of aflatoxin- and fumonisin-producing fungi associated with cereal crops grown in Zimbabwe and their associated risks in a climate change scenario. Foods 2021, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M. Major cereal grains production and use around the world. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; Awika, J.M., Piironen, V., Bean, S., Eds.; American Chemical Society: Washington, DC, USA, 2011; pp. 1–13. [Google Scholar]
- Smale, M.; Kergna, A.; Diakité, L. An Economic Assessment of Sorghum Improvement in Mali. Impact Assessment Report No. 2. Patancheru 502 324; International Crops Research Institute for the Semi-Arid Tropics: Telangana, India, 2016; p. 52. [Google Scholar]
- Ndjeunga, J.; Nelson, C.H. Prospects for a pearl millet and sorghum food processing industry in west Africa semi-arid tropics. In Towards Sustainable Sorghum Production, Utilization, and Commercialization in West and Central Africa. Proceedings of a Technical Workshop of the West and Central Africa Sorghum Research Network; ICRISAT: Lomé, Togo, 1999; Available online: http://oar.icrisat.org/4891/ (accessed on 1 July 2022).
- Traore, T.M.; Fields, D. Households demand for staple cereal commodities in Burkina Faso. In Proceedings of the 2016 Annual Meeting of the Southern Agricultural Economics Association, San Antonio, TX, USA, 6–9 February 2016; pp. 1–34. [Google Scholar]
- Dettwyler, K.A. Nutritional status of adults in rural Mali. Am. J. Phys. Anthropol. 1992, 88, 309–321. [Google Scholar] [CrossRef]
- Breakwell, L.; Tevi-Benissan, C.; Childs, L.; Mihigo, R.; Tohme, R. The status of hepatitis B control in the African region. Pan Afr. Med. J. 2017, 27, 17. [Google Scholar] [CrossRef] [PubMed]
- Lingani, M.; Akita, T.; Ouoba, S.; Sanou, A.M.; Sugiyama, A.; Tarnagda, Z.; Ohisa, M.; Tinto, H.; Mishiro, S.; Tanaka, J. High prevalence of hepatitis B infections in Burkina Faso (1996–2017): A systematic review with meta-analysis of epidemiological studies. BMC Public Health 2018, 18, 551. [Google Scholar] [CrossRef]
- Matumba, L.; Sulyok, M.; Njoroge, S.; Njumbe Ediage, E.; Van Poucke, C.; De Saeger, S.; Krska, R. Uncommon occurrence ratios of aflatoxin B1, B2, G1, and G2 in maize and groundnuts from Malawi. Mycotoxin Res. 2014, 31, 57–62. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived products. EFSA J. 2007, 446, 1–127. [Google Scholar]
- Soler, T.; Hoogenboom, G.; Olatinwo, R.; Diarra, B.; Waliyar, F.; Traore, S. Peanut contamination by Aspergillus flavus and aflatoxin B1 in granaries of villages and markets of Mali, West Africa. J. Food Agric. Environ. 2010, 8, 195–203. Available online: http://oar.icrisat.org/id/eprint/7541 (accessed on 10 April 2022).
- Ritchie, H.; Roser, M. Diet Compositions. Available online: https://ourworldindata.org/diet-compositions (accessed on 20 April 2022).
- Wu, F.; Mitchell, N.J.; Male, D.; Kensler, T.W. Reduced foodborne toxin exposure is a benefit of improving dietary diversity. Soc. Toxicol. 2014, 141, 329–334. [Google Scholar] [CrossRef]
- Ware, L.; Durand, N.; Nikiema, P.A.; Alter, P.; Fontana, A.; Montet, D.; Barro, N. Occurrence of mycotoxins in commercial infant formulas locally produced in Ouagadougou (Burkina Faso). Food Control 2017, 73, 518–523. [Google Scholar] [CrossRef]
- Sousa, F.M.; Nombre, A.; Miningou, A.; Traore, S.A.; Lindahl, J.F.; Ayantunde, A.A.; Sanchez, J.; Alonso, S. Aflatoxin M1-related health risk for milk consumers in dairy farms in rural and peri-urban areas of Burkina Faso. Agriculture, Nutrition and Health Academy Week. 2021. Available online: https://cgspace.cgiar.org/bitstream/handle/10568/114174/Burkina%20faso%20aflatoxin%20risk.pdf?sequence=1 (accessed on 1 July 2022).
- Amadou, H.; Dossa, L.H.; Lompo, D.J.P.; Abdulkadir, A.; Schlecht, E. A comparison between urban livestock production strategies in Burkina Faso, Mali and Nigeria in West Africa. Trop. Anim. Health Prod. 2012, 44, 1631–1642. [Google Scholar] [CrossRef]
- Ingenbleek, L.; Sulyok, M.; Adegboye, A.; Hossou, S.E.; Koné, A.Z.; Oyedele, A.D.; Kisito, C.S.; Koreissi Dembélé, Y.; Eyangoh, S.; Verger, P.; et al. Regional sub-Saharan Africa total diet study in Benin, Cameroon, Mali and Nigeria reveals the presence of 164 mycotoxins and other secondary metabolites in foods. Toxins 2019, 11, 54. [Google Scholar] [CrossRef]
- Bakoye, O.N.; Baoua, I.B.; Seyni, H.; Amadou, L.; Murdock, L.L.; Baributsa, D. Quality of maize for sale in markets in Benin and Niger. J. Stored Prod. Res. 2017, 71, 99–105. [Google Scholar] [CrossRef]
- Craufurd, P.Q.; Prasad, P.V.V.; Waliyar, F.; Taheri, A. Drought, pod yield, pre-harvest Aspergillus infection and aflatoxin contamination on peanut in Niger. Field Crops Res. 2006, 98, 20–29. [Google Scholar] [CrossRef]
- Wu, F.; Khlangwiset, P. Health economic impacts and cost-effectiveness of aflatoxin-reduction strategies in Africa: Case studies in biocontrol and post-harvest interventions. Food Addit. Contam.-Part A. 2010, 27, 496–509. [Google Scholar] [CrossRef]
- Wu, F. Global impacts of aflatoxin in maize: Trade and human health. World Mycotoxin J. 2015, 8, 137–142. [Google Scholar] [CrossRef]
- US FDA. Section 683.100. Action Levels for Aflatoxins in Animal Feed. Complaince Policy Guide. 2019. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-683100-action-levels-aflatoxins-animal-feeds (accessed on 20 April 2022).
- Anitha, S.; Muzanila, Y.; Tsusaka, T.W.; Kachulu, L.; Kumwenda, N.; Musoke, M.; Swai, E.; Shija, J.; Siambi, M.; Monyo, E.S.; et al. Reducing child undernutrition through dietary diversification, reduced aflatoxin exposure, and improved hygiene practices: The immediate impacts in central Tanzania. Ecol. Food Nutr. 2019, 59, 243–262. [Google Scholar] [CrossRef]
- Khlangwiset, P.; Shephard, G.S.; Wu, F. Aflatoxins and growth impairment: A review. Crit. Rev. Toxicol. 2011, 41, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.M.; Abdelgadir, A.M.; Moss, M.O. Exposure of infants to aflatoxin M1 from mothers’ breast milk in Abu Dhabi, UAE. Food Addit. Contam. 1995, 12, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Shirima, C.P.; Kimanya, M.E.; Kinabo, J.L.; Routledge, M.N.; Srey, C.; Wild, C.P.; Gong, Y.Y. Dietary exposure to aflatoxin and fumonisin among Tanzanian children as determined using biomarkers of exposure. Mol. Nutr. Food Res. 2013, 57, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Partanen, H.A.; El-Nezami, H.S.; Leppänen, J.M.; Myllynen, P.K.; Woodhouse, H.J.; Vähäkangas, K.H. Aflatoxin B1 transfer and metabolism in human placenta. Toxicol. Sci. 2010, 113, 216–225. [Google Scholar] [CrossRef]
- Hsieh, L.; Hsieh, T. Detection of aflatoxin B1-DNA adducts in human placenta and cord blood. Cancer Res. 1993, 53, 1278–1280. [Google Scholar]
- Huang, W.; Liu, M.; Xiao, B.; Zhang, J.; Song, M.; Li, Y.; Cao, Z. Aflatoxin B1 disrupts blood-testis barrier integrity by reducing junction protein and promoting apoptosis in mice testes. Food Chem. Toxicol. 2021, 148, 111972. [Google Scholar] [CrossRef]
- Turna, N.S.; Wu, F. Risk assessment of aflatoxin-related liver cancer in Bangladesh. Food Addit. Contam. Part A 2019, 36, 320–326. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). WHO IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. World Health Organ 2002, 82, 1–590. [Google Scholar]
- Ndom, P. Cancer prevention in Africa: Liver cancer. ecancer 2019, 13, 950. [Google Scholar] [CrossRef]
- Maina, A.W.; Wagacha, J.M.; Mwaura, F.B.; Muthomi, J.W.; Woloshuk, C.P. Postharvest practices of maize farmers in Kaiti District, Kenya and the impact of hermetic storage on populations of Aspergillus spp. and aflatoxin contamination. J. Food Res. 2016, 5, 53–66. [Google Scholar] [CrossRef]
- Bradford, K.J.; Dahal, P.; Van Asbrouck, J.; Kunusoth, K.; Bello, P.; Thompson, J.; Wu, F. The dry chain: Reducing postharvest losses and improving food safety in humid climates. Trends Food Sci. Technol. 2018, 71, 84–93. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Atehnkeng, J.; Ortega-Beltran, A.; Akande, A.; Falade, T.D.O.; Cotty, P.J. “Ground-truthing” efficacy of biological control for aflatoxin mitigation in farmers’ fields in Nigeria: From field trials to commercial usage, a 10-year study. Front. Microbiol. 2019, 10, 2528. [Google Scholar] [CrossRef]
- Adhikari, B.N.; Bandyopadhyay, R.; Cotty, P.J. Degeneration of aflatoxin gene clusters in Aspergillus flavus from Africa and North America. AMB Express 2016, 6, 62. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Ortega-Beltran, A.; Akande, A.; Mutegi, C.; Atehnkeng, J.; Kaptoge, L.; Senghor, A.L.; Adhikari, B.N.; Cotty, P.J. Biological control of aflatoxins in Africa: Current status and potential challenges in the face of climate change. World Mycotoxin J. 2016, 9, 771–789. [Google Scholar] [CrossRef]
| Total Aflatoxin (µg/kg) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maize | Groundnut | |||||||||||
| AEZ | n | Min | Max | Mean | Median | Variance | n | Min | Max | Mean | Median | Variance |
| NGS | 21 | 3 | 140 | 20.1 Aa | 11.8 | 836 | 15 | ND | 53 | 11.2 Ab | 7.1 | 231 |
| SS | 34 | 2 | 517 | 54.3 Aa | 17.9 | 12,930 | 33 | ND | 926 | 47.7 Ab | 8.8 | 27,239 |
| SGS | 7 | ND | 12 | 7.7 | 8.2 | 15 | 5 | ND | 22 | 13.7 | 13.9 | 73 |
| AEZ | Provinces | PDI (ng/kg bw/day) | MOE | HCC Rates |
|---|---|---|---|---|
| NGS | Balé | 76 | 2.2 | 3.2 |
| Boulgou | 44 | 3.9 | 1.9 | |
| Houet | 143 | 1.2 | 6.1 | |
| Kénédougou | 79 | 2.2 | 3.4 | |
| Kouritenga | 58 | 2.9 | 2.5 | |
| SGS | Léraba | 34 | 5.0 | 1.5 |
| Comoé | 29 | 5.9 | 1.2 | |
| SS | Bazéga | 56 | 3.1 | 2.4 |
| Boulkiemdé | 84 | 2.0 | 3.6 | |
| Gnagna | 255 | 0.7 | 10.8 | |
| Gourma | 66 | 2.6 | 2.8 | |
| Kadiogo | 102 | 1.7 | 4.3 | |
| Komandjari | 672 | 0.3 | 28.6 | |
| Kourwéogo | 156 | 1.1 | 6.6 | |
| Oubritenga | 432 | 0.4 | 18.3 | |
| Sanmatenga | 59 | 2.9 | 2.5 |
| Region | Total Aflatoxin (µg/kg) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maize | Groundnut | Sorghum | ||||||||||||||||
| n | Min | Max | Mean | Median | Variance | n | Min | Max | Mean | Median | Variance | n | Min | Max | Mean | Median | Variance | |
| Kayes | 9 | ND | 1076 | 119.5 Aa | ND | 128,586 | 10 | ND | 939 | 115.4 Aa | ND | 86,552 | 10 | ND | 11 | 1.1 Ba | ND | 12.8 |
| Koulikoro | 14 | ND | 159 | 27.7 Aa | ND | 3190 | 12 | ND | 210 | 33.8 Aa | ND | 4530 | 11 | ND | 16 | 1.4 Ba | ND | 23.0 |
| Ségou | 18 | ND | 1849 | 156.3 Aa | 3.1 | 189,468 | 14 | ND | 1245 | 124.4 Aa | 27.3 | 106,065 | 16 | ND | 27 | 2.9 Ba | ND | 49.0 |
| Sikasso | 59 | ND | 188 | 12.6 Aa | ND | 1147 | 44 | ND | 1235 | 58.6 Aa | ND | 47,177 | 36 | ND | 35 | 1.5 Ba | ND | 36.0 |
| Maize | Sorghum | |||||
|---|---|---|---|---|---|---|
| Region | PDI (ng/kg bw/day) | MOE | HCC Rates | PDI (ng/kg bw/day) | MOE | HCC Rates |
| Kayes | 69 | 2.5 | 4.7 | 2 | 74.6 | 0.2 |
| Koulikoro | 19 | 9.2 | 1.3 | 3 | 58.5 | 0.2 |
| Ségou | 59 | 2.9 | 4.0 | 133 | 1.3 | 9.1 |
| Sikasso | 6 | 27.1 | 0.4 | 2 | 68.5 | 0.2 |
| Total Aflatoxin (µg/kg) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maize | Groundnut | Sorghum | ||||||||||||||||
| Region | n | Min | Max | Mean | Median | Variance | n | Min | Max | Mean | Median | Variance | n | Min | Max | Mean | Median | Variance |
| Dosso | 32 | ND | 5886 | 658.9 Aa | 209.6 | 1.5 × 106 | 40 | ND | 8593 | 627.5 Aab | 72.7 | 2.1 × 106 | 38 | ND | 1934 | 106.7 Aa | 6.4 | 106,663 |
| Zinder | 40 | ND | 3721 | 276.1 Ba | ND | 506,246 | 40 | ND | 7162 | 702.6 Aa | ND | 2.5 × 106 | 38 | ND | 1988 | 63.4 Bb | ND | 104,206 |
| Maradi | 37 | ND | 924 | 99.5 Ba | ND | 44,838 | 39 | ND | 5142 | 343.9 ABa | ND | 848,354 | 39 | ND | 354 | 35.3 Ab | ND | 6209 |
| Tillabéri | 14 | ND | 1368 | 210.6 Ba | ND | 241,488 | 30 | ND | 1531 | 89.9 Ba | ND | 93,912 | 30 | ND | 531 | 79.4 Aab | ND | 20,391 |
| Niamey | - | - | - | - | - | - | - | - | - | 4 | ND | 655 | 258.7 | ND | 57,534 | |||
| Region | Maize | Sorghum | ||||
|---|---|---|---|---|---|---|
| PDI (ng/kg bw/day) | MOE | HCC Rates | PDI (ng/kg bw/day) | MOE | HCC Rates | |
| Dosso | 2100 | 0.1 | 119.7 | 706 | 0.2 | 40.2 |
| Zinder | 899 | 0.2 | 51.2 | 534 | 0.3 | 30.4 |
| Maradi | 310 | 0.5 | 17.7 | 253 | 0.7 | 14.4 |
| Tillabéri | 729 | 0.2 | 41.6 | 659 | 0.3 | 37.6 |
| Niamey | - | - | - | 2221 | 0.1 | 126.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falade, T.D.O.; Neya, A.; Bonkoungou, S.; Dagno, K.; Basso, A.; Senghor, A.L.; Atehnkeng, J.; Ortega-Beltran, A.; Bandyopadhyay, R. Aflatoxin Contamination of Maize, Groundnut, and Sorghum Grown in Burkina Faso, Mali, and Niger and Aflatoxin Exposure Assessment. Toxins 2022, 14, 700. https://doi.org/10.3390/toxins14100700
Falade TDO, Neya A, Bonkoungou S, Dagno K, Basso A, Senghor AL, Atehnkeng J, Ortega-Beltran A, Bandyopadhyay R. Aflatoxin Contamination of Maize, Groundnut, and Sorghum Grown in Burkina Faso, Mali, and Niger and Aflatoxin Exposure Assessment. Toxins. 2022; 14(10):700. https://doi.org/10.3390/toxins14100700
Chicago/Turabian StyleFalade, Titilayo D. O., Adama Neya, Saïdou Bonkoungou, Karim Dagno, Adamou Basso, Amadou Lamine Senghor, Joseph Atehnkeng, Alejandro Ortega-Beltran, and Ranajit Bandyopadhyay. 2022. "Aflatoxin Contamination of Maize, Groundnut, and Sorghum Grown in Burkina Faso, Mali, and Niger and Aflatoxin Exposure Assessment" Toxins 14, no. 10: 700. https://doi.org/10.3390/toxins14100700
APA StyleFalade, T. D. O., Neya, A., Bonkoungou, S., Dagno, K., Basso, A., Senghor, A. L., Atehnkeng, J., Ortega-Beltran, A., & Bandyopadhyay, R. (2022). Aflatoxin Contamination of Maize, Groundnut, and Sorghum Grown in Burkina Faso, Mali, and Niger and Aflatoxin Exposure Assessment. Toxins, 14(10), 700. https://doi.org/10.3390/toxins14100700






