Nicotinic Acetylcholine Receptors Are Novel Targets of APETx-like Toxins from the Sea Anemone Heteractis magnifica
Abstract
1. Introduction
2. Results
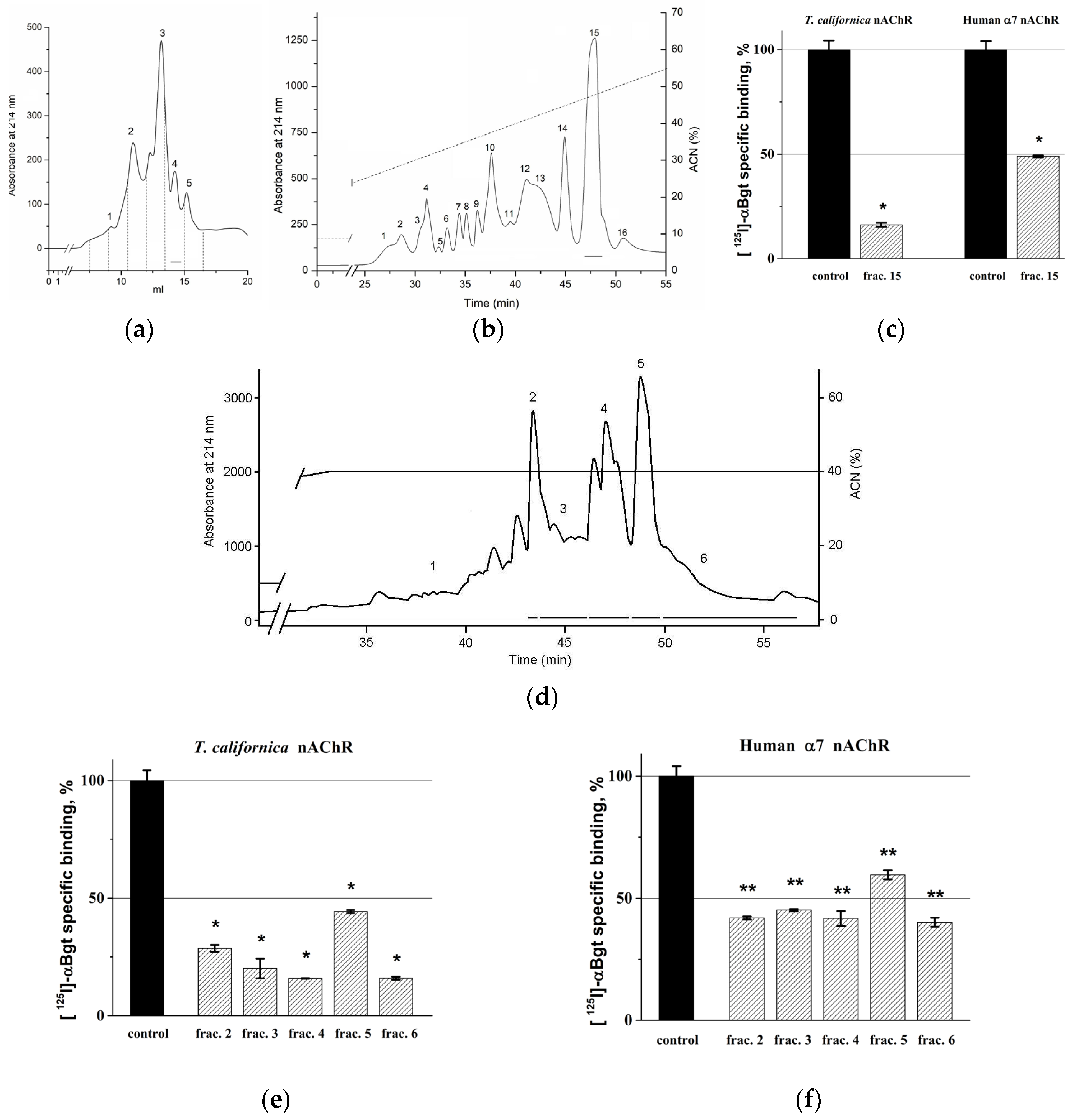
2.1. Activity-Guided Isolation of Toxins from H. magnifica
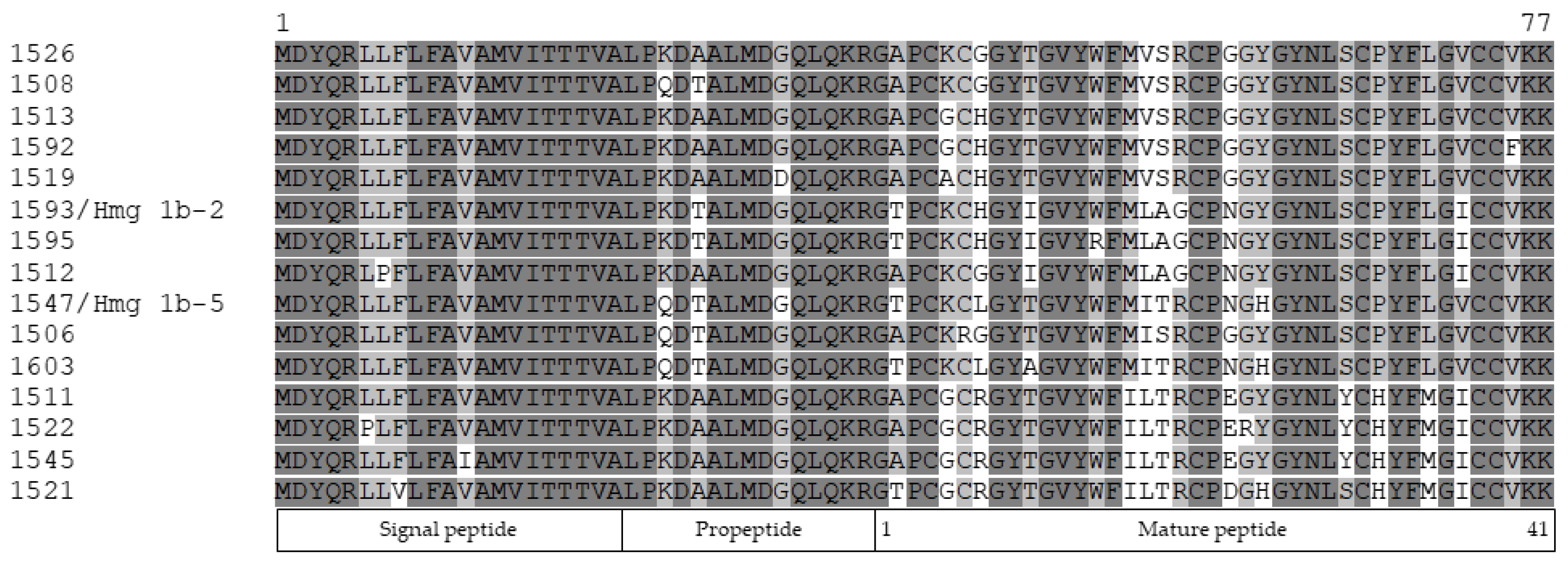
2.2. Peptide Sequences Determination
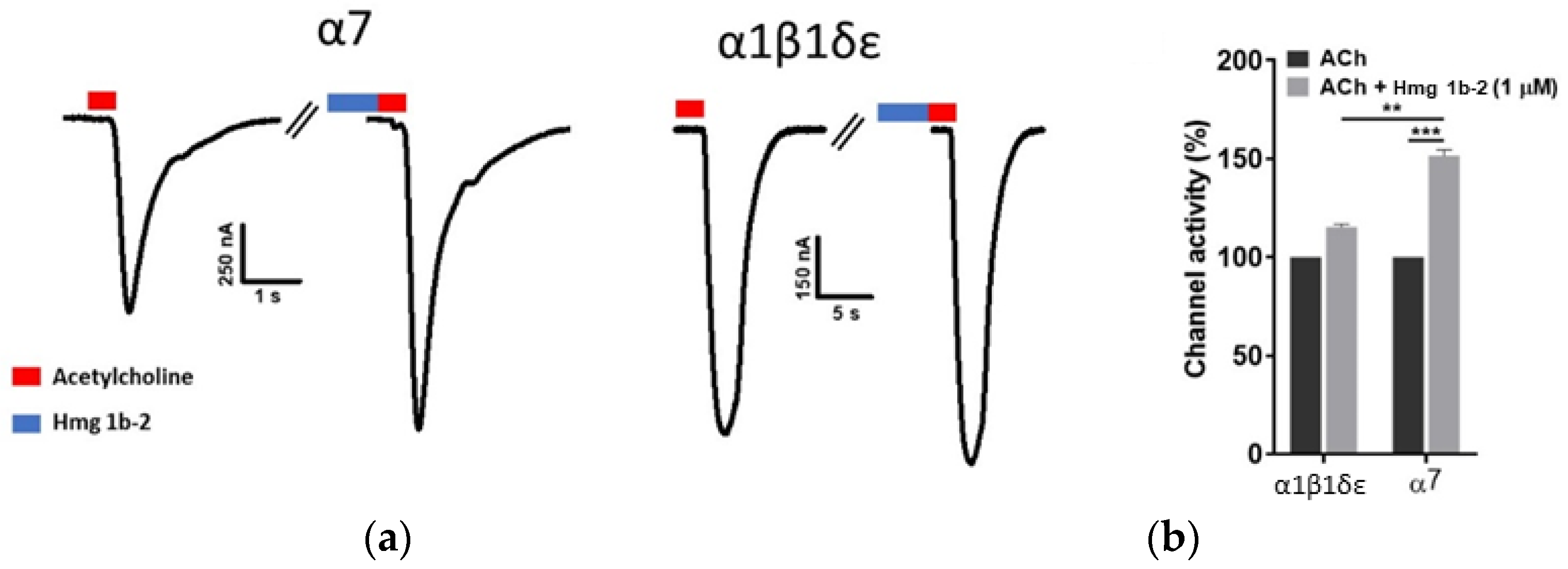
2.3. Electrophysiological Effects of Hmg 1b-2 on nAChRs
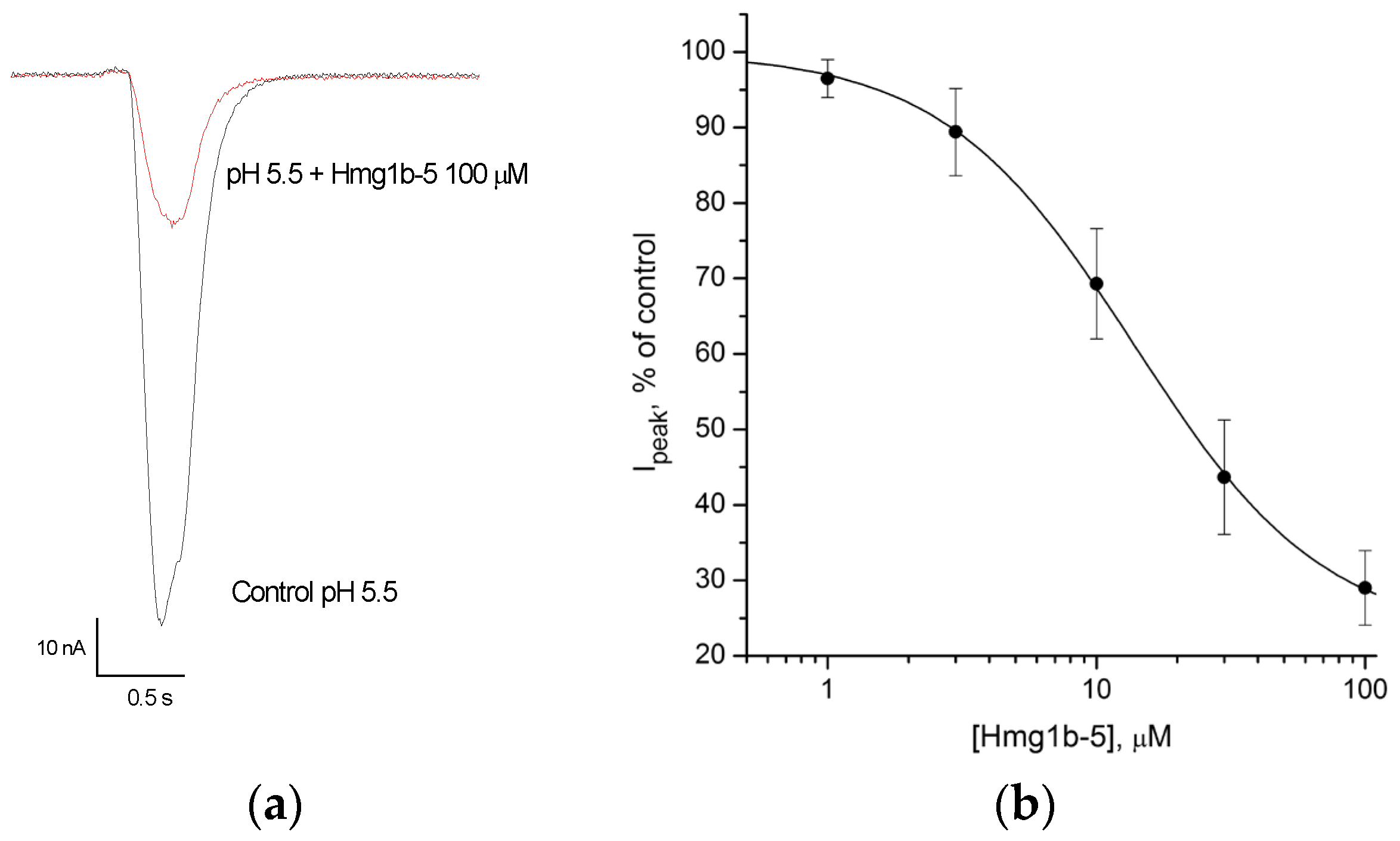
2.4. Electrophysiological Effects of Hmg 1b-2 Metox and Hmg 1b-5 on ASIC Channels
2.5. Sequence Identification and Analysis of H. magnifica APETx-like Toxins Diversity
2.6. Molecular Modeling of APETx-like Toxins
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Extraction and Chromatographic Procedure
5.2. Reduction and Alkylation of Disulfide Bridges
5.3. Cyanogen Bromide Cleavage of Alkylated Peptides
5.4. Mass Spectrometric Analysis
5.5. Tandem Mass Spectrometry (MS/MS), Sequence Determination and Analysis
5.6. cDNA and Gene Sequences Determination, Phylogenetic Analysis
5.7. Radioligand Competition Assay
5.8. Expression of nAChRs in Xenopus laevis Oocytes and Electrophysiological Recordings
5.9. Expression of ASIC Channels in Xenopus laevis Oocytes and Electrophysiological Recordings
5.10. Homology Modeling
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hone, A.J.; McIntosh, J.M. Nicotinic acetylcholine receptors in neuropathic and inflammatory pain. FEBS Lett. 2018, 592, 1045–1062. [Google Scholar] [CrossRef]
- Kudryavtsev, D.; Shelukhina, I.; Vulfius, C.; Makarieva, T.; Stonik, V.; Zhmak, M.; Ivanov, I.; Kasheverov, I.; Utkin, Y.; Tsetlin, V. Natural compounds interacting with nicotinic acetylcholine receptors: From low-molecular weight ones to peptides and proteins. Toxins 2015, 7, 1683–1701. [Google Scholar] [CrossRef]
- Dutertre, S.; Nicke, A.; Tsetlin, V.I. Nicotinic acetylcholine receptor inhibitors derived from snake and snail venoms. Neuropharmacology 2017, 127, 196–223. [Google Scholar] [CrossRef]
- Howard, R.J. Elephants in the dark: Insights and incongruities in pentameric ligand-gated ion channel models. J. Mol. Biol. 2021, 433, 167128. [Google Scholar] [CrossRef]
- Changeux, J.-P. Discovery of the first neurotransmitter receptor: The acetylcholine nicotinic receptor. Biomolecules 2020, 10, 547. [Google Scholar] [CrossRef]
- Lebedev, D.S.; Kryukova, E.V.; Ivanov, I.A.; Egorova, N.S.; Timofeev, N.D.; Spirova, E.N.; Tufanova, E.Y.; Siniavin, A.E.; Kudryavtsev, D.S.; Kasheverov, I.E.; et al. Oligoarginine peptides, a new family of nicotinic acetylcholine receptor inhibitors. Mol. Pharmacol. 2019, 96, 664–673. [Google Scholar] [CrossRef]
- Zhang, B.; Ren, M.; Yang, F.; Li, R.; Yu, L.; Luo, A.; Zhangsun, D.; Luo, S.; Dong, S. Oligo-basic amino acids, potential nicotinic acetylcholine receptor inhibitors. Biomed. Pharmacother. 2022, 152, 113215. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Vulfius, C.A.; Spirova, E.N.; Serebryakova, M.V.; Shelukhina, I.V.; Kudryavtsev, D.S.; Kryukova, E.V.; Starkov, V.G.; Kopylova, N.V.; Zhmak, M.N.; Ivanov, I.A.; et al. Peptides from puff adder Bitis arietans venom, novel inhibitors of nicotinic acetylcholine receptors. Toxicon 2016, 121, 70–76. [Google Scholar] [CrossRef]
- Abraham, N.; Lewis, R. Neuronal nicotinic acetylcholine receptor modulators from cone snails. Mar. Drugs 2018, 16, 208. [Google Scholar] [CrossRef]
- Kasheverov, I.E.; Oparin, P.B.; Zhmak, M.N.; Egorova, N.S.; Ivanov, I.A.; Gigolaev, A.M.; Nekrasova, O.V.; Serebryakova, M.V.; Kudryavtsev, D.S.; Prokopev, N.A.; et al. Scorpion toxins interact with nicotinic acetylcholine receptors. FEBS Lett. 2019, 593, 2779–2789. [Google Scholar] [CrossRef]
- Miwa, J.M.; Anderson, K.R.; Hoffman, K.M. Lynx prototoxins: Roles of endogenous mammalian neurotoxin-like proteins in modulating nicotinic acetylcholine receptor function to influence complex biological processes. Front. Pharmacol. 2019, 10, 343. [Google Scholar] [CrossRef]
- Utkin, Y.N. Three-finger toxins, a deadly weapon of elapid venom–milestones of discovery. Toxicon 2013, 62, 50–55. [Google Scholar] [CrossRef]
- Duque, H.M.; Dias, S.C.; Franco, O.L. Structural and functional analyses of cone snail toxins. Mar. Drugs 2019, 17, 370. [Google Scholar] [CrossRef]
- Garateix, A.; Castellanos, M.; Hernández, J.L.; Más, R.; Menéndez, R.; Romero, L.; Chávez, M. Effects of a high molecular weight toxin from the sea anemone Condylactis gigantea on cholinergic responses. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1992, 103, 403–409. [Google Scholar] [CrossRef]
- Kalina, R.; Gladkikh, I.; Dmitrenok, P.; Chernikov, O.; Koshelev, S.; Kvetkina, A.; Kozlov, S.; Kozlovskaya, E.; Monastyrnaya, M. New APETx-like peptides from sea anemone Heteractis crispa modulate ASIC1a channels. Peptides 2018, 104, 41–49. [Google Scholar] [CrossRef]
- Kalina, R.S.; Koshelev, S.G.; Zelepuga, E.A.; Kim, N.Y.; Kozlov, S.A.; Kozlovskaya, E.P.; Monastyrnaya, M.M.; Gladkikh, I.N. APETx-like peptides from the sea anemone Heteractis crispa, diverse in their effect on ASIC1a and ASIC3 ion channels. Toxins 2020, 12, 266. [Google Scholar] [CrossRef]
- Heusser, S.A.; Pless, S.A. Acid-sensing ion channels as potential therapeutic targets. Trends Pharmacol. Sci. 2021, 42, 1035–1050. [Google Scholar] [CrossRef]
- Ortega-Ramírez, A.; Vega, R.; Soto, E. Acid-sensing ion channels as potential therapeutic targets in neurodegeneration and neuroinflammation. Mediat. Inflamm. 2017, 2017, 3728096. [Google Scholar] [CrossRef]
- Uchitel, O.D.; González Inchauspe, C.; Weissmann, C. Synaptic signals mediated by protons and acid-sensing ion channels. Synapse 2019, 73, e22120. [Google Scholar] [CrossRef]
- Sintsova, O.; Gladkikh, I.; Chausova, V.; Monastyrnaya, M.; Anastyuk, S.; Chernikov, O.; Yurchenko, E.; Aminin, D.; Isaeva, M.; Leychenko, E.; et al. Peptide fingerprinting of the sea anemone Heteractis magnifica mucus revealed neurotoxins, Kunitz-type proteinase inhibitors and a new β-defensin α-amylase inhibitor. J. Proteom. 2017, 173, 12–21. [Google Scholar] [CrossRef]
- Kozlov, S.A.; Osmakov, D.I.; Andreev, Y.A.; Koshelev, S.G.; Gladkikh, I.N.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Grishin, E.V. A sea anemone polypeptide toxin inhibiting the ASIC3 acid-sensitive channel. Russ. J. Bioorganic Chem. 2012, 38, 578–583. [Google Scholar] [CrossRef]
- Lu, G.; Moriyama, E.N. Vector NTI, a balanced all-in-one sequence analysis suite. Brief. Bioinform. 2004, 5, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Isaeva, M.P.; Chausova, V.E.; Zelepuga, E.A.; Guzev, K.V.; Tabakmakher, V.M.; Monastyrnaya, M.M.; Kozlovskaya, E.P. A new multigene superfamily of Kunitz-type protease inhibitors from sea anemone Heteractis crispa. Peptides 2012, 34, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, S.; Grishin, E. Convenient nomenclature of cysteine-rich polypeptide toxins from sea anemones. Peptides 2012, 33, 240–244. [Google Scholar] [CrossRef]
- Chagot, B.; Escoubas, P.; Diochot, S.; Bernard, C.; Lazdunski, M.; Darbon, H. Solution structure of APETx2, a specific peptide inhibitor of ASIC3 proton-gated channels. Protein Sci. 2005, 14, 2003–2010. [Google Scholar] [CrossRef]
- Báez, A.; Salceda, E.; Fló, M.; Graña, M.; Fernández, C.; Vega, R.; Soto, E. α-Dendrotoxin inhibits the ASIC current in dorsal root ganglion neurons from rat. Neurosci. Lett. 2015, 606, 42–47. [Google Scholar] [CrossRef]
- Richter, S.; Wenzel, A.; Stein, M.; Gabdoulline, R.R.; Wade, R.C. WebPIPSA: A web server for the comparison of protein interaction properties. Nucleic Acids Res. 2008, 36, W276–W280. [Google Scholar] [CrossRef] [PubMed]
- Dineley, K.T.; Pandya, A.A.; Yakel, J.L. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci. 2015, 36, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Philip, N.S.; Carpenter, L.L.; Tyrka, A.R.; Price, L.H. The nicotinic acetylcholine receptor as a target for antidepressant drug development. Sci. World J. 2012, 2012, 104105. [Google Scholar] [CrossRef]
- Santos-Torres, J.; Ślimak, M.A.; Auer, S.; Ibañez-Tallon, I. Cross-reactivity of acid-sensing ion channel and Na+-H+ exchanger antagonists with nicotinic acetylcholine receptors. J. Physiol. 2011, 589, 5109–5123. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.-T.; Tu, P.-H.; Chen, C.-J.; Lin, Y.-W.; Hsieh, S.-T.; Chen, C.-C. Role of acid-sensing ion channel 3 in sub-acute-phase inflammation. Mol. Pain 2009, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro-Junior, E.L.; Kalina, R.; Gladkikh, I.; Leychenko, E.; Tytgat, J.; Peigneur, S. A Tale of toxin promiscuity: The versatile pharmacological effects of Hcr 1b-2 sea anemone peptide on voltage-gated ion channels. Mar. Drugs 2022, 20, 147. [Google Scholar] [CrossRef]
- Sintsova, O.V.; Kalina, R.S.; Gladkikh, I.N.; Palikova, Y.A.; Palikov, V.A.; Borozdina, N.A.; Klimovich, A.A.; Menshov, A.S.; Dyachenko, I.A.; Leychenko, E.V. Anxiolytic effect of peptides from the sea anemone Heteractis crispa, modulators of TRPV1 And ASIC channels. Dokl. Biochem. Biophys. 2022, 505, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, M.; Dabrowski, M.; Gurley, D.A.; Larsson, O.; Johnson, E.C.; Fredholm, B.B.; Eriksson, L.I. Activation and inhibition of human muscular and neuronal nicotinic acetylcholine receptors by succinylcholine. Anesthesiology 2006, 104, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Bekbossynova, A.; Zharylgap, A.; Filchakova, O. Venom-derived neurotoxins targeting nicotinic acetylcholine receptors. Molecules 2021, 26, 3373. [Google Scholar] [CrossRef]
- Williams, D.K.; Wang, J.; Papke, R.L. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochem. Pharmacol. 2011, 82, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Miwa, J.M.; Ibaňez-Tallon, I.; Crabtree, G.W.; Sánchez, R.; Šali, A.; Role, L.W.; Heintz, N. Lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron 1999, 23, 105–114. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Khasanov, T.A.; Andreev, Y.A.; Lyukmanova, E.N.; Kozlov, S.A. Animal, herb, and microbial toxins for structural and pharmacological study of acid-sensing ion channels. Front. Pharmacol. 2020, 11, 991. [Google Scholar] [CrossRef]
- Cristofori-Armstrong, B.; Rash, L.D. Acid-sensing ion channel (ASIC) structure and function: Insights from spider, snake and sea anemone venoms. Neuropharmacology 2017, 127, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.; Cristofori-armstrong, B.; Rash, L.D.; Román-gonzález, S.A.; Arreguín, R.; Lewis, R.J.; Alewood, P.F.; Vetter, I. Novel conorfamides from Conus austini venom modulate both nicotinic acetylcholine receptors and acid-sensing ion channels. Biochem. Pharmacol. 2019, 164, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, K. Novel peptide toxins recently isolated from sea anemones. Toxicon 2009, 54, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Madio, B.; King, G.F.; Undheim, E.A.B. Sea anemone toxins: A structural overview. Mar. Drugs 2019, 17, 325. [Google Scholar] [CrossRef]
- Diochot, S. APETx1, a new toxin from the sea anemone Anthopleura elegantissima, blocks voltage-gated human ether-a-go-go-related gene potassium channels. Mol. Pharmacol. 2003, 64, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.Y.M. Modulation of Kv3 subfamily potassium currents by the sea anemone toxin BDS: Significance for CNS and biophysical studies. J. Neurosci. 2005, 25, 8735–8745. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; Beress, L.; Moller, C.; Mari, F.; Forssmann, W.-G.; Tytgat, J. A natural point mutation changes both target selectivity and mechanism of action of sea anemone toxins. FASEB J. 2012, 26, 5141–5151. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jo, S.; Bean, B.P. Modulation of neuronal sodium channels by the sea anemone peptide BDS-I. J. Neurophysiol. 2012, 107, 3155–3167. [Google Scholar] [CrossRef]
- Diochot, S.; Baron, A.; Rash, L.D.; Deval, E.; Escoubas, P.; Scarzello, S.; Salinas, M.; Lazdunski, M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004, 23, 1516–1525. [Google Scholar] [CrossRef]
- Jensen, J.E.; Cristofori-Armstrong, B.; Anangi, R.; Rosengren, K.J.; Lau, C.H.Y.; Mobli, M.; Brust, A.; Alewood, P.F.; King, G.F.; Rash, L.D. Understanding the molecular basis of toxin promiscuity: The analgesic sea anemone peptide apetx2 interacts with acid-sensing ion channel 3 and hERG channels via overlapping pharmacophores. J. Med. Chem. 2014, 57, 9195–9203. [Google Scholar] [CrossRef]
- Moreels, L.; Peigneur, S.; Galan, D.T.; De Pauw, E.; Béress, L.; Waelkens, E.; Pardo, L.A.; Quinton, L.C.; Tytgat, J. APETx4, a novel sea anemone toxin and a modulator of the cancer-relevant potassium channel KV10.1. Mar. Drugs 2017, 15, 287. [Google Scholar] [CrossRef]
- Kvetkina, A.; Leychenko, E.; Chausova, V.; Zelepuga, E.; Chernysheva, N.; Guzev, K.; Pislyagin, E.; Yurchenko, E.; Menchinskaya, E.; Aminin, D.; et al. A new multigene HCIQ subfamily from the sea anemone Heteractis crispa encodes Kunitz-peptides exhibiting neuroprotective activity against 6-hydroxydopamine. Sci. Rep. 2020, 10, 4205. [Google Scholar] [CrossRef] [PubMed]
- Leychenko, E.; Isaeva, M.; Tkacheva, E.; Zelepuga, E.; Kvetkina, A.; Guzev, K.; Monastyrnaya, M.; Kozlovskaya, E. Multigene family of pore-forming toxins from sea anemone Heteractis crispa. Mar. Drugs 2018, 16, 183. [Google Scholar] [CrossRef] [PubMed]
- Leichenko, E.V.; Monastirnaya, M.M.; Zelepuga, E.A.; Tkacheva, E.S.; Isaeva, M.P.; Likhatskaya, G.N.; Anastyuk, S.D.; Kozlovskaya, E.P. Hct-a is a new actinoporin family from the Heteractis crispa sea anemone. Acta Nat. 2014, 6, 89–98. [Google Scholar] [CrossRef]
- Nicosia, A.; Mikov, A.; Cammarata, M.; Colombo, P.; Andreev, Y.; Kozlov, S.; Cuttitta, A. The Anemonia viridis venom: Coupling biochemical purification and RNA-Seq for translational research. Mar. Drugs 2018, 16, 407. [Google Scholar] [CrossRef]
- MacRander, J.; Broe, M.; Daly, M. Multi-copy venom genes hidden in de novo transcriptome assemblies, a cautionary tale with the snakelocks sea anemone Anemonia sulcata (Pennant, 1977). Toxicon 2015, 108, 184–188. [Google Scholar] [CrossRef]
- Babenko, V.V.; Mikov, A.N.; Manuvera, V.A.; Anikanov, N.A.; Kovalchuk, S.I.; Andreev, Y.A.; Logashina, Y.A.; Kornilov, D.A.; Manolov, A.I.; Sanamyan, N.P.; et al. Identification of unusual peptides with new Cys frameworks in the venom of the cold-water sea anemone Cnidopus Japonicus. Sci. Rep. 2017, 7, 14534. [Google Scholar] [CrossRef]
- Macrander, J.; Brugler, M.R.; Daly, M. A RNA-seq approach to identify putative toxins from acrorhagi in aggressive and non-aggressive Anthopleura elegantissima polyps. BMC Genom. 2015, 16, 221. [Google Scholar] [CrossRef]
- Rodríguez, A.A.; Cassoli, J.S.; Sa, F.; Dong, Z.Q.; De Freitas, J.C.; Pimenta, A.M.C.; De Lima, M.E.; Konno, K.; Lee, S.M.Y.; Garateix, A.; et al. Peptide fingerprinting of the neurotoxic fractions isolated from the secretions of sea anemones Stichodactyla helianthus and Bunodosoma granulifera. New members of the APETx-like family identified by a 454 pyrosequencing approach. Peptides 2012, 34, 26–38. [Google Scholar] [CrossRef]
- Madio, B.; Undheim, E.A.B.; King, G.F. Revisiting venom of the sea anemone Stichodactyla haddoni: Omics techniques reveal the complete toxin arsenal of a well-studied sea anemone genus. J. Proteom. 2017, 166, 83–92. [Google Scholar] [CrossRef]
- Cassoli, J.S.; Verano-Braga, T.; Oliveira, J.S.; Montandon, G.G.; Cologna, C.T.; Peigneur, S.; de Castro Pimenta, A.M.; Kjeldsen, F.; Roepstorff, P.; Tytgat, J.; et al. The proteomic profile of Stichodactyla duerdeni secretion reveals the presence of a novel O-linked glycopeptide. J. Proteom. 2013, 87, 89–102. [Google Scholar] [CrossRef]
- Kryukova, E.; Ivanov, I.; Lebedev, D.; Spirova, E.; Egorova, N.; Zouridakis, M.; Kasheverov, I.; Tzartos, S.; Tsetlin, V. Orthosteric and/or allosteric binding of α-conotoxins to nicotinic acetylcholine receptors and their models. Mar. Drugs 2018, 16, 460. [Google Scholar] [CrossRef] [PubMed]
- Osipov, A.V.; Kasheverov, I.E.; Makarova, Y.V.; Starkov, V.G.; Vorontsova, O.V.; Ziganshin, R.K.; Andreeva, T.V.; Serebryakova, M.V.; Benoit, A.; Hogg, R.C.; et al. Naturally occurring disulfide-bound dimers of three-fingered toxins. J. Biol. Chem. 2008, 283, 14571–14580. [Google Scholar] [CrossRef] [PubMed]
- Sencic, L.; Macek, P. New method for isolation of venom from the sea anemone Actinia cari. Purification and characterization of cytolytic toxins. Comp. Biochem. Physiol. B 1990, 97, 687–693. [Google Scholar] [CrossRef]
- Sokotun, I.N.; Il’ina, A.P.; Monastyrnaya, M.M.; Leychenko, E.V.; Es’kov, A.A.; Anastuk, S.D.; Kozlovskaya, E.P. Proteinase inhibitors from the tropical sea anemone Radianthus macrodactylus: Isolation and characteristic. Biochemistry 2007, 72, 301–306. [Google Scholar] [CrossRef]
- Sokotun, I.N.; Leĭchenko, E.V.; Vakorina, T.I.; Es’kov, A.A.; Il’ina, A.P.; Monastyrnaia, M.M.; Kozlovskaia, E.P. A serine protease inhibitor from the anemone Radianthus macrodactylus: Isolation and physicochemical characteristics. Bioorg. Khim. 2007, 33, 448–455. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Kvetkina, A.; Kostina, E.; Gladkikh, I.; Chausova, V.; Yurchenko, E.; Bakunina, I.; Pivkin, M.; Anastyuk, S.; Popov, R.; Monastyrnaya, M.; et al. Deep-sea anemones are prospective source of new antimicrobial and cytotoxic compounds. Mar. Drugs 2021, 19, 654. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Pinheiro-Junior, E.L.; Boldrini-França, J.; Takeda, A.A.S.; Costa, T.R.; Peigneur, S.; Cardoso, I.A.; de Oliveira, I.S.; Sampaio, S.V.; de Mattos Fontes, M.R.; Tytgat, J.; et al. Towards toxin PEGylation: The example of rCollinein-1, a snake venom thrombin-like enzyme, as a PEGylated biopharmaceutical prototype. Int. J. Biol. Macromol. 2021, 190, 564–573. [Google Scholar] [CrossRef]
- Dubinnyi, M.A.; Osmakov, D.I.; Koshelev, S.G.; Kozlov, S.A.; Andreev, Y.A.; Zakaryan, N.A.; Dyachenko, I.A.; Bondarenko, D.A.; Arseniev, A.S.; Grishin, E.V. Lignan from thyme possesses inhibitory effect on ASIC3 channel current. J. Biol. Chem. 2012, 287, 32993–33000. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Šali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.; Witham, S.; Sarkar, S.; Zhang, J.; Li, L.; Li, C.; Alexov, E. DelPhi web server v2: Incorporating atomic-style geometrical figures into the computational protocol. Bioinformatics 2012, 28, 1655–1657. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalina, R.S.; Kasheverov, I.E.; Koshelev, S.G.; Sintsova, O.V.; Peigneur, S.; Pinheiro-Junior, E.L.; Popov, R.S.; Chausova, V.E.; Monastyrnaya, M.M.; Dmitrenok, P.S.; et al. Nicotinic Acetylcholine Receptors Are Novel Targets of APETx-like Toxins from the Sea Anemone Heteractis magnifica. Toxins 2022, 14, 697. https://doi.org/10.3390/toxins14100697
Kalina RS, Kasheverov IE, Koshelev SG, Sintsova OV, Peigneur S, Pinheiro-Junior EL, Popov RS, Chausova VE, Monastyrnaya MM, Dmitrenok PS, et al. Nicotinic Acetylcholine Receptors Are Novel Targets of APETx-like Toxins from the Sea Anemone Heteractis magnifica. Toxins. 2022; 14(10):697. https://doi.org/10.3390/toxins14100697
Chicago/Turabian StyleKalina, Rimma S., Igor E. Kasheverov, Sergey G. Koshelev, Oksana V. Sintsova, Steve Peigneur, Ernesto Lopes Pinheiro-Junior, Roman S. Popov, Victoria E. Chausova, Margarita M. Monastyrnaya, Pavel S. Dmitrenok, and et al. 2022. "Nicotinic Acetylcholine Receptors Are Novel Targets of APETx-like Toxins from the Sea Anemone Heteractis magnifica" Toxins 14, no. 10: 697. https://doi.org/10.3390/toxins14100697
APA StyleKalina, R. S., Kasheverov, I. E., Koshelev, S. G., Sintsova, O. V., Peigneur, S., Pinheiro-Junior, E. L., Popov, R. S., Chausova, V. E., Monastyrnaya, M. M., Dmitrenok, P. S., Isaeva, M. P., Tytgat, J., Kozlov, S. A., Kozlovskaya, E. P., Leychenko, E. V., & Gladkikh, I. N. (2022). Nicotinic Acetylcholine Receptors Are Novel Targets of APETx-like Toxins from the Sea Anemone Heteractis magnifica. Toxins, 14(10), 697. https://doi.org/10.3390/toxins14100697







