Time-Lagged Correlation Analysis of Shellfish Toxicity Reveals Predictive Links to Adjacent Areas, Species, and Environmental Conditions
Abstract
1. Introduction
2. Materials and Methods
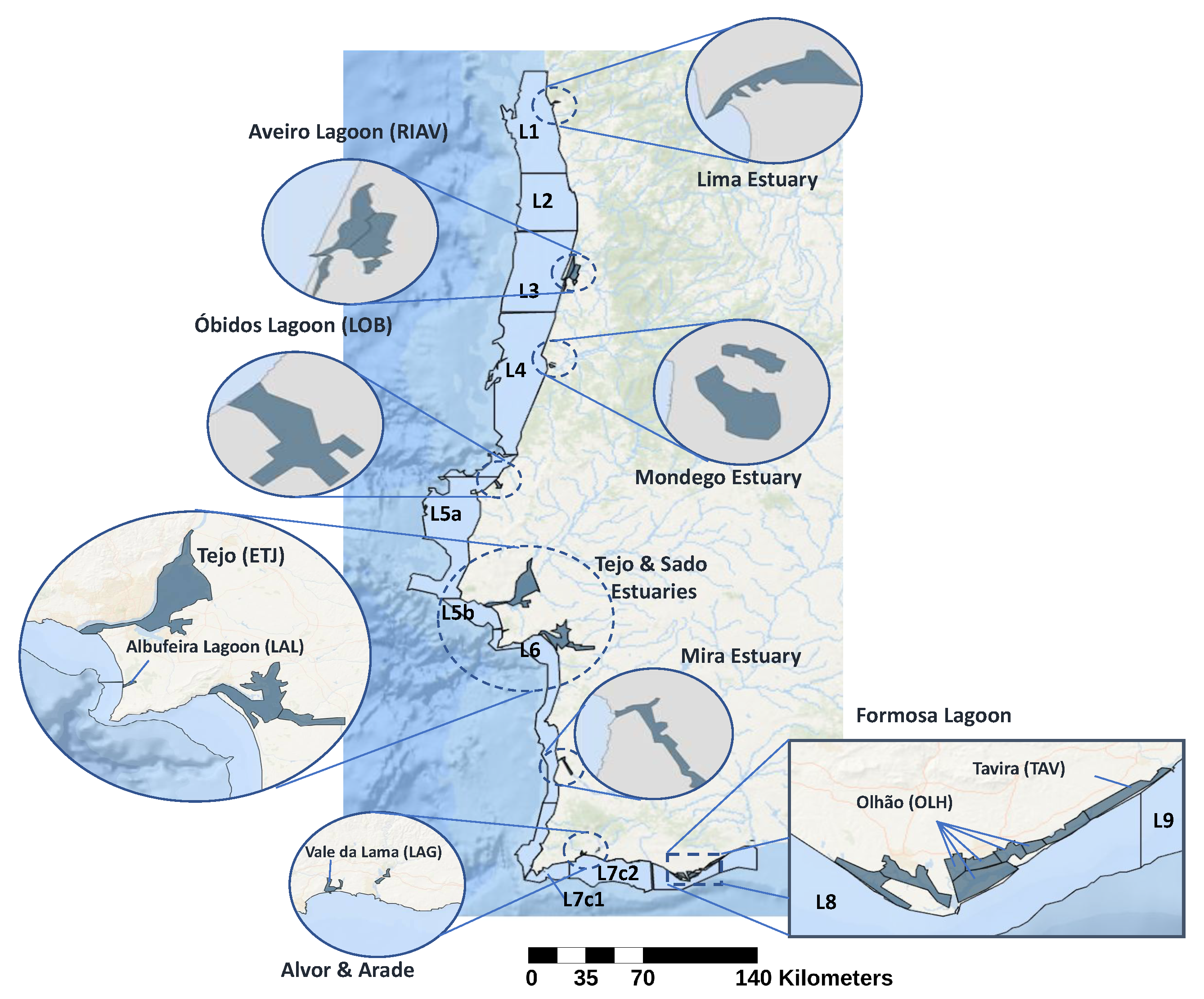
2.1. Data Sources
2.2. Preprocessing and Data Preparation
2.3. Seasonality-Trend Analysis
2.4. Correlation Analysis
3. Results
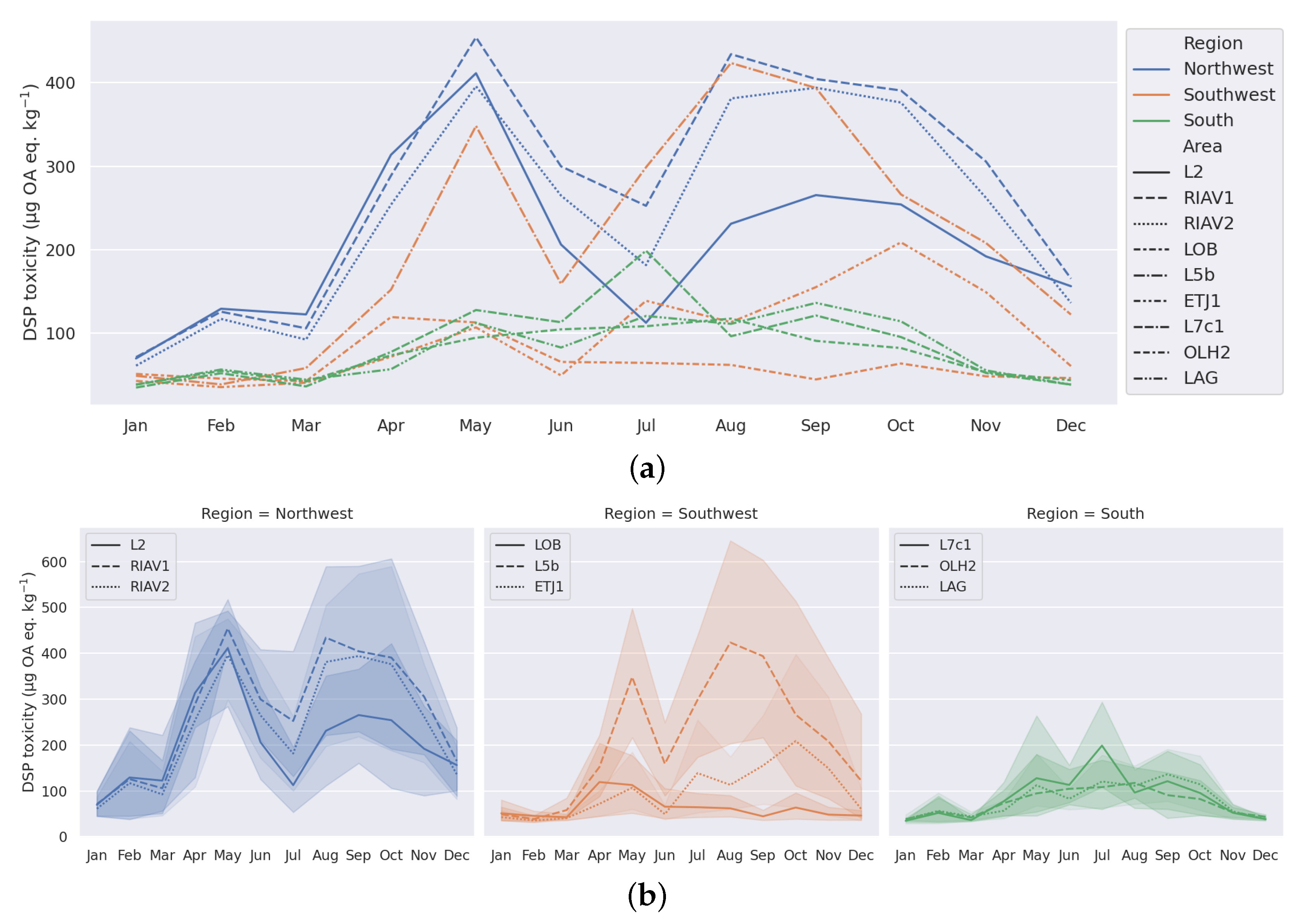
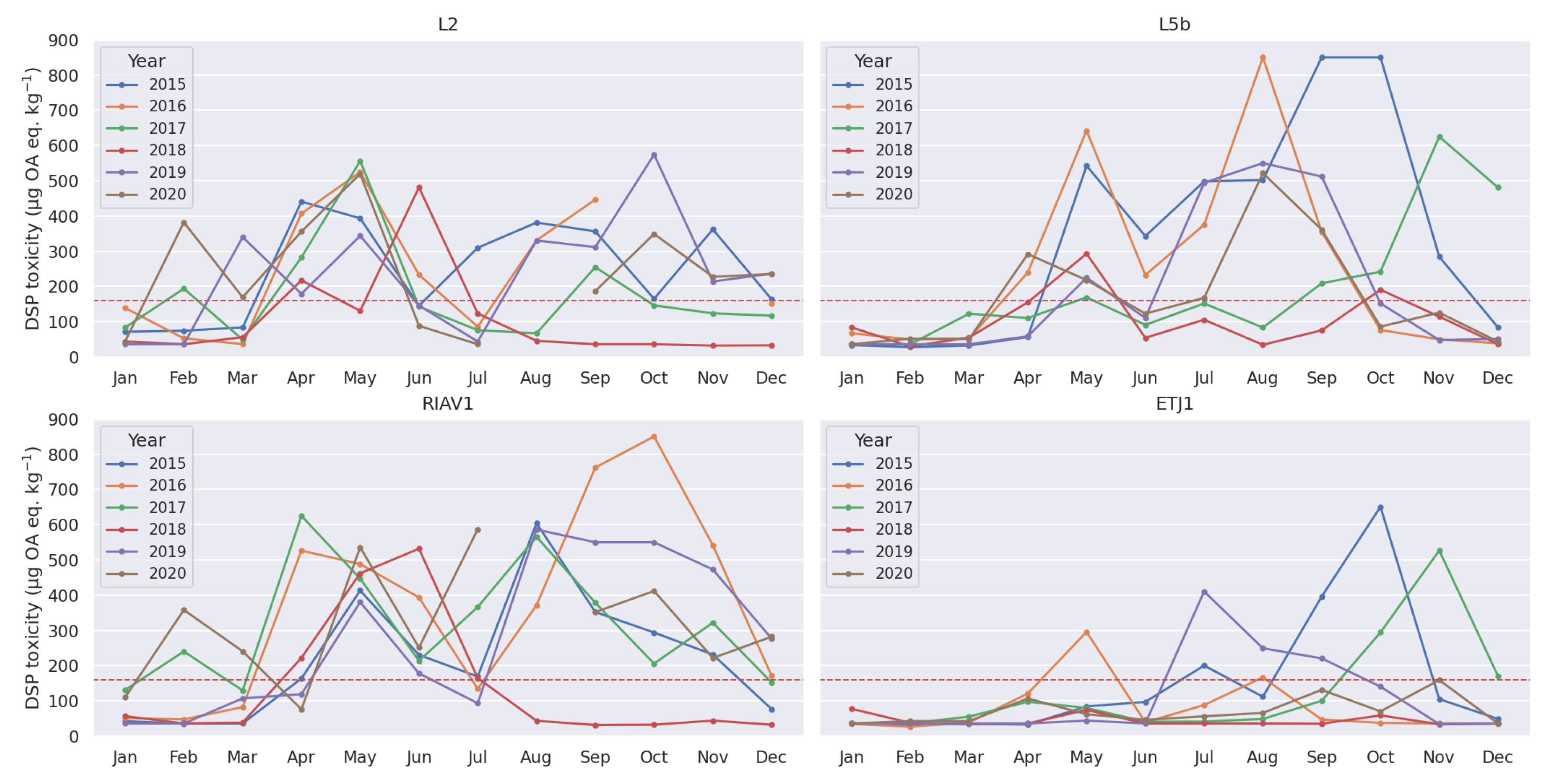
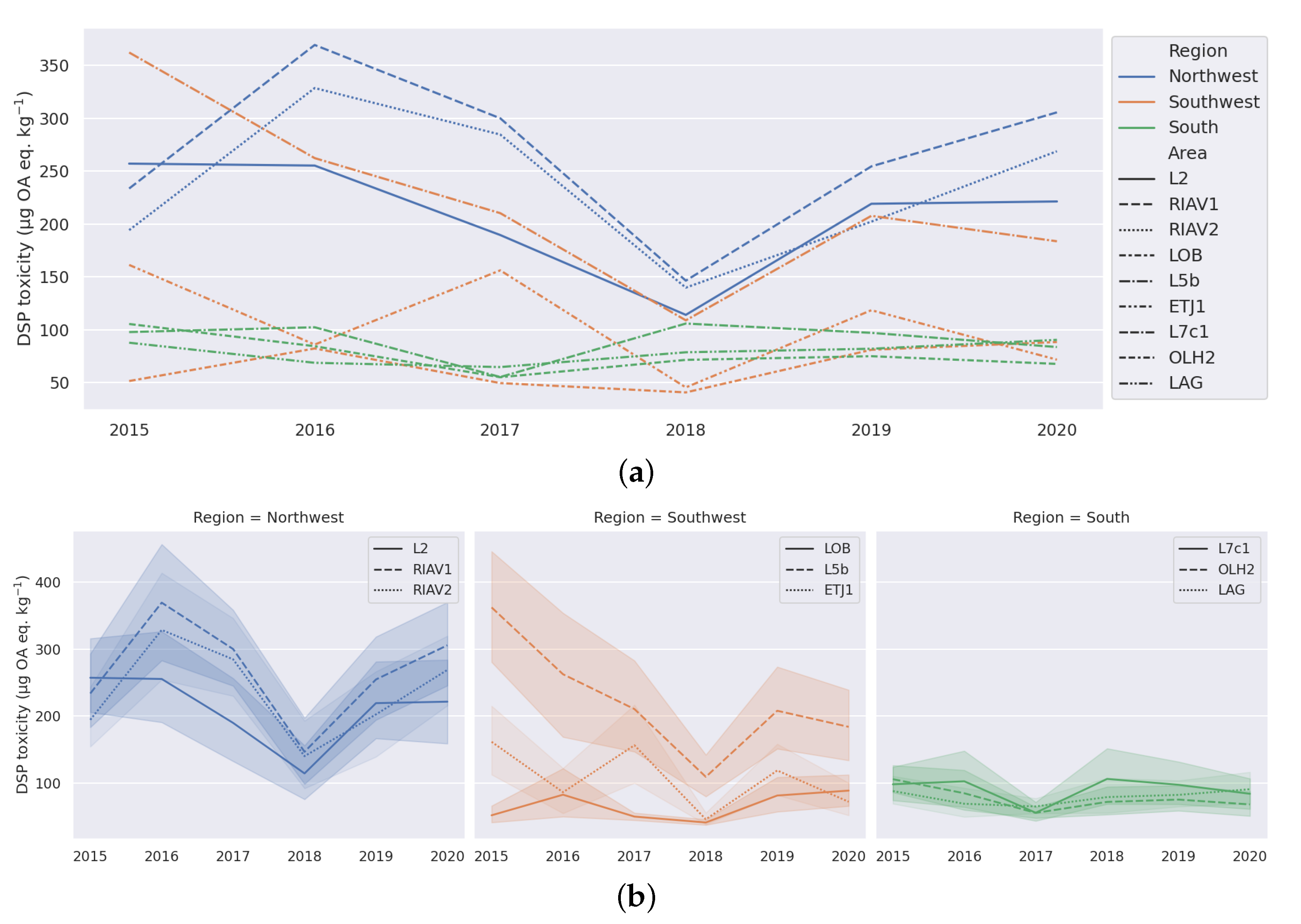
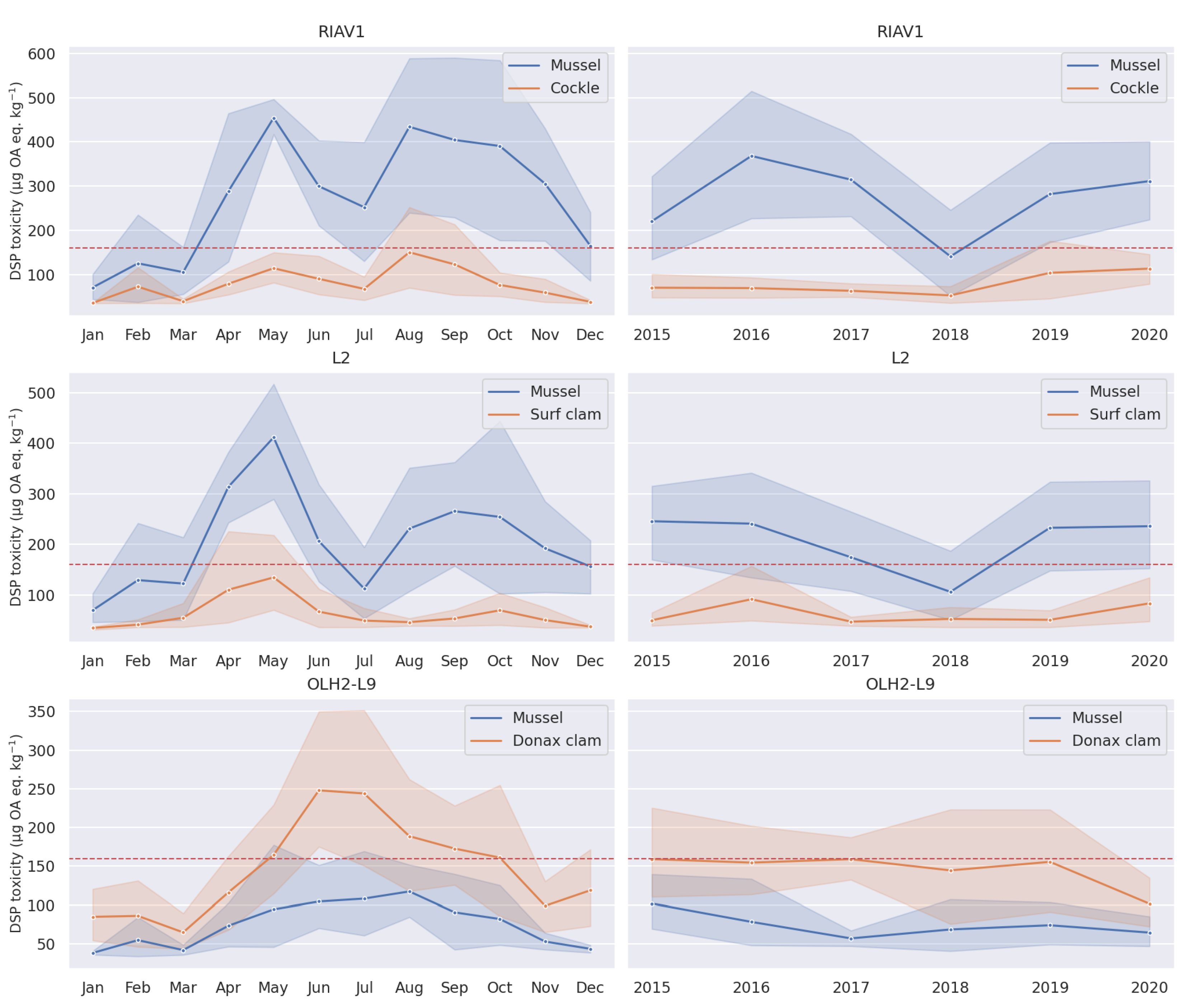
3.1. Exploratory Analysis
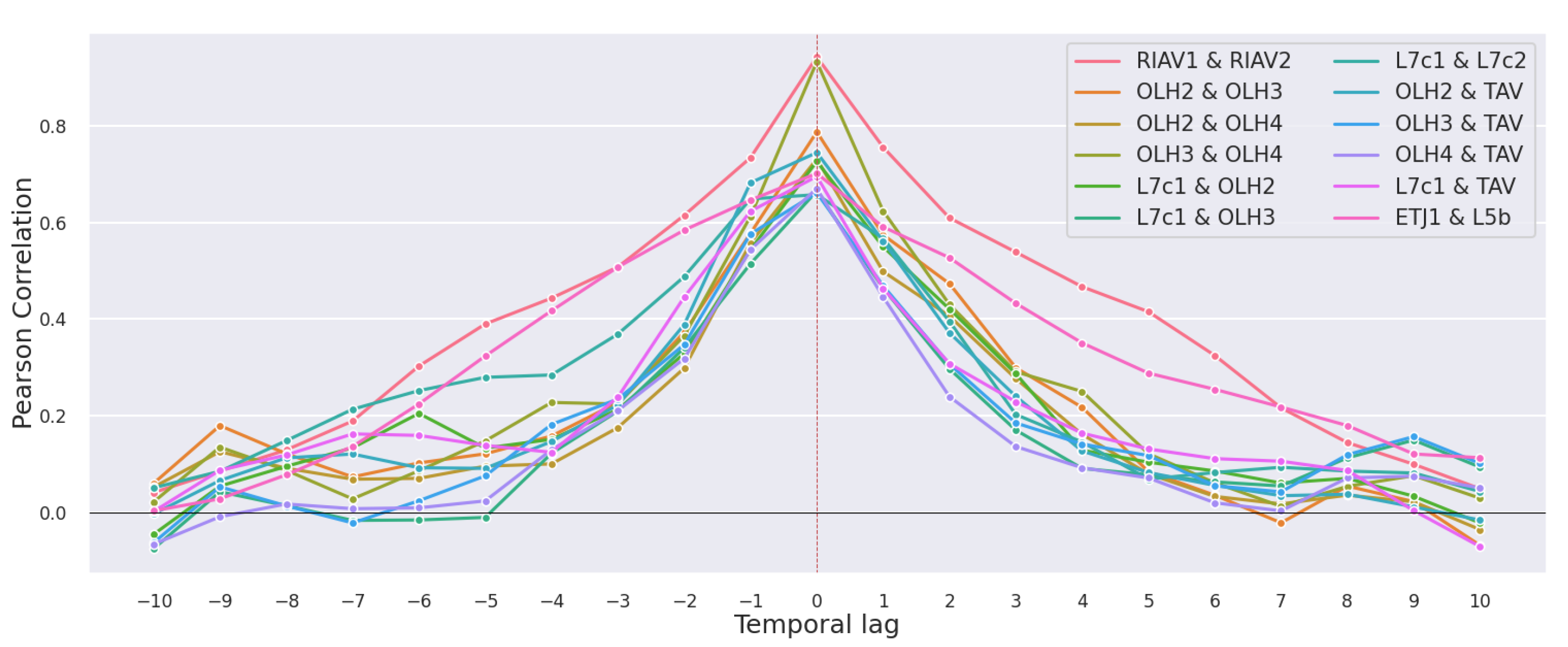
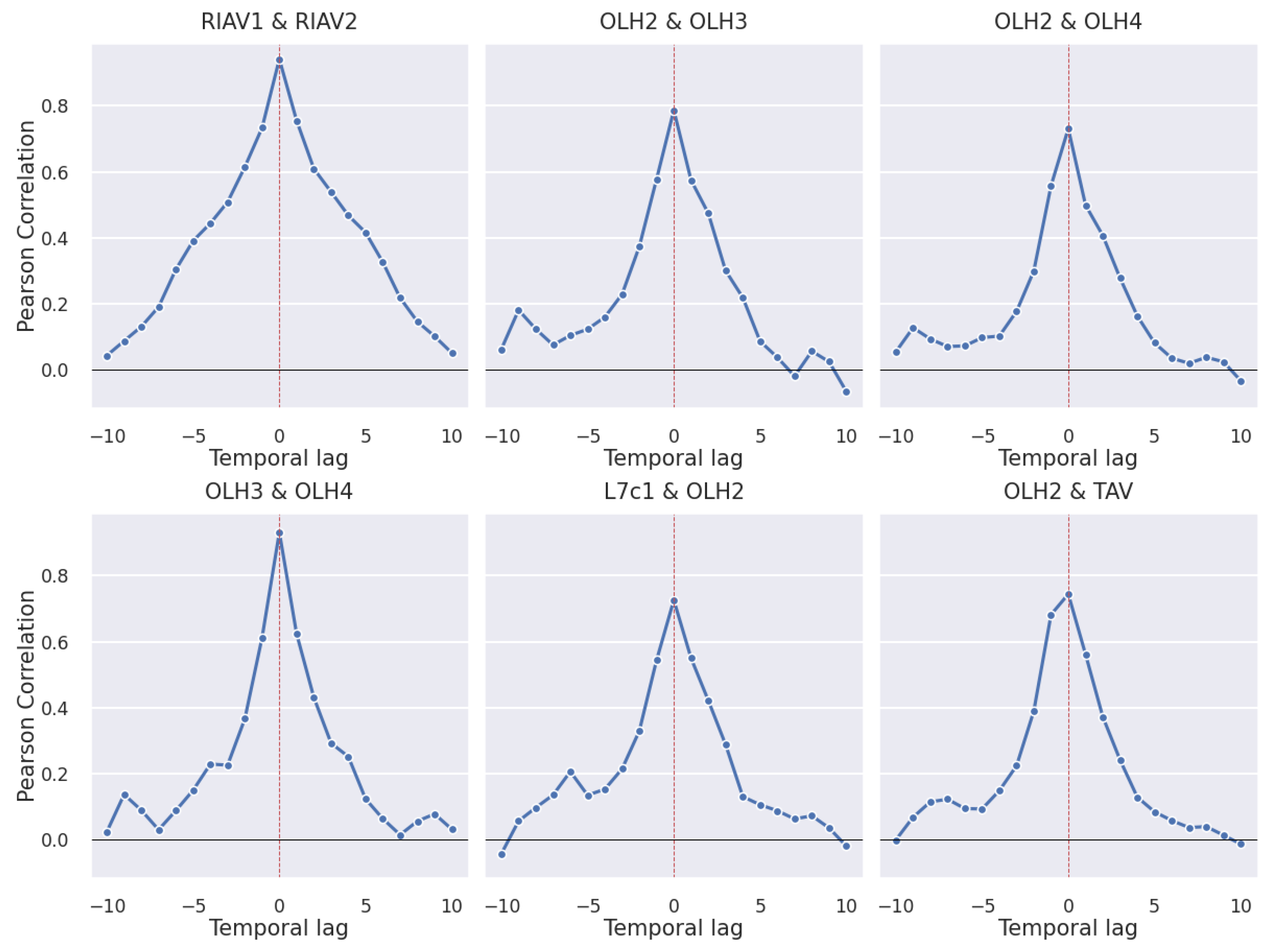
3.2. Correlations
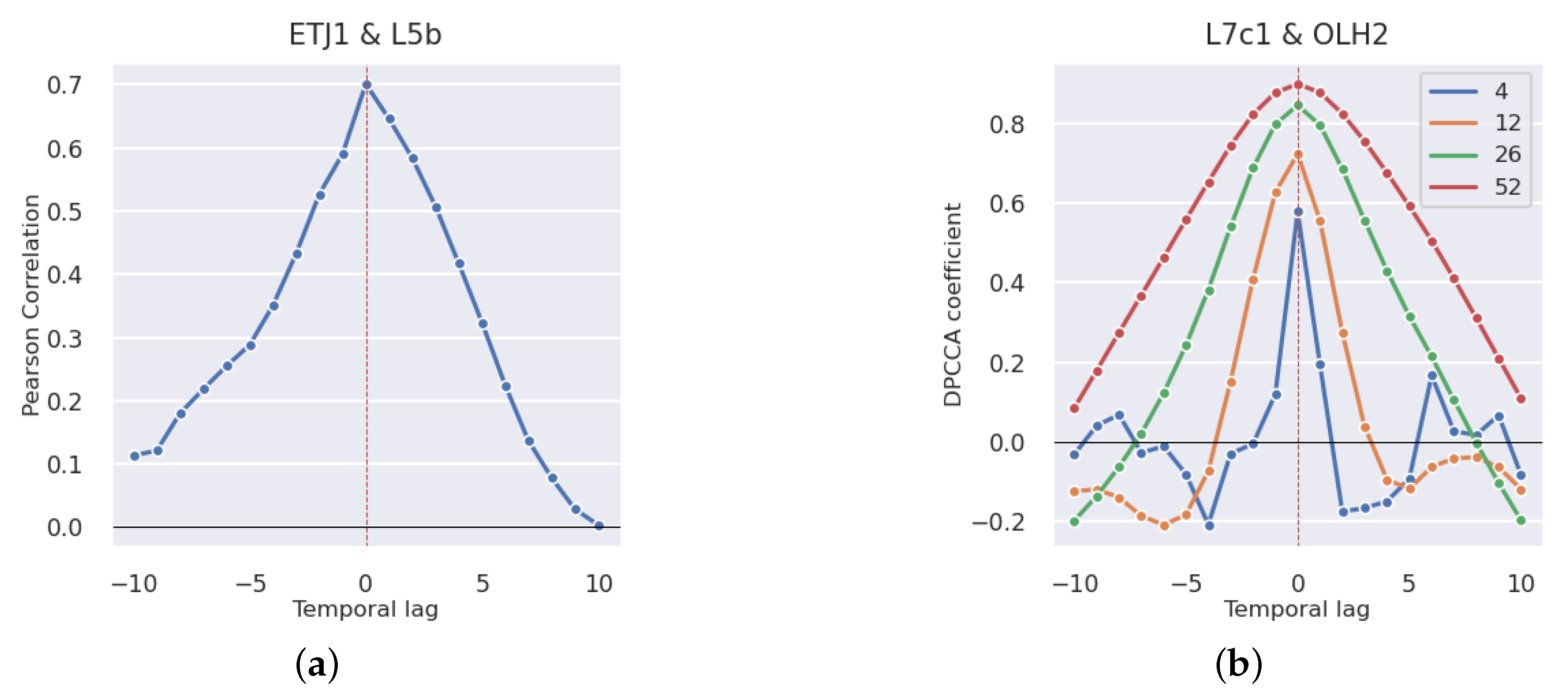
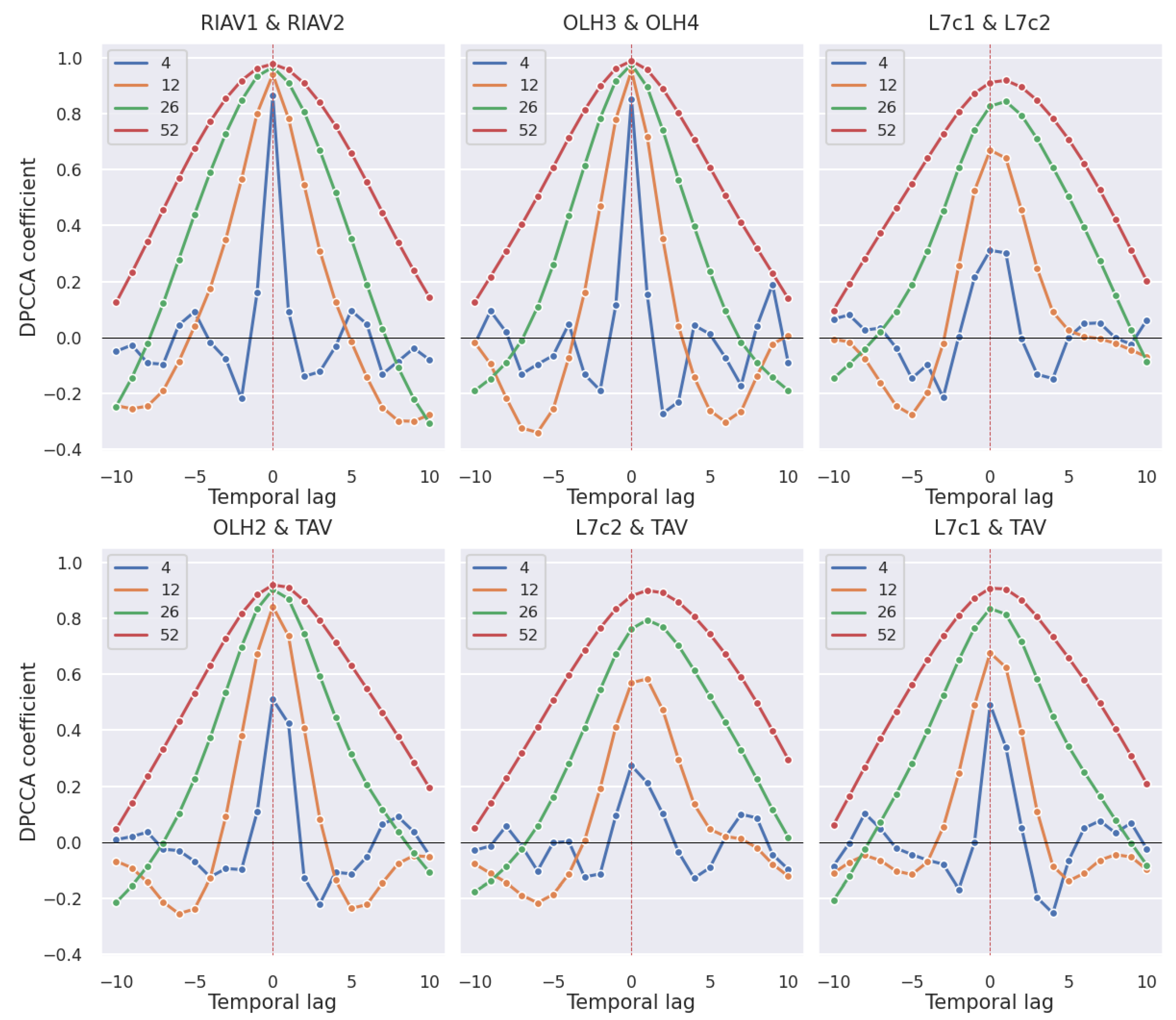
3.2.1. Production Areas
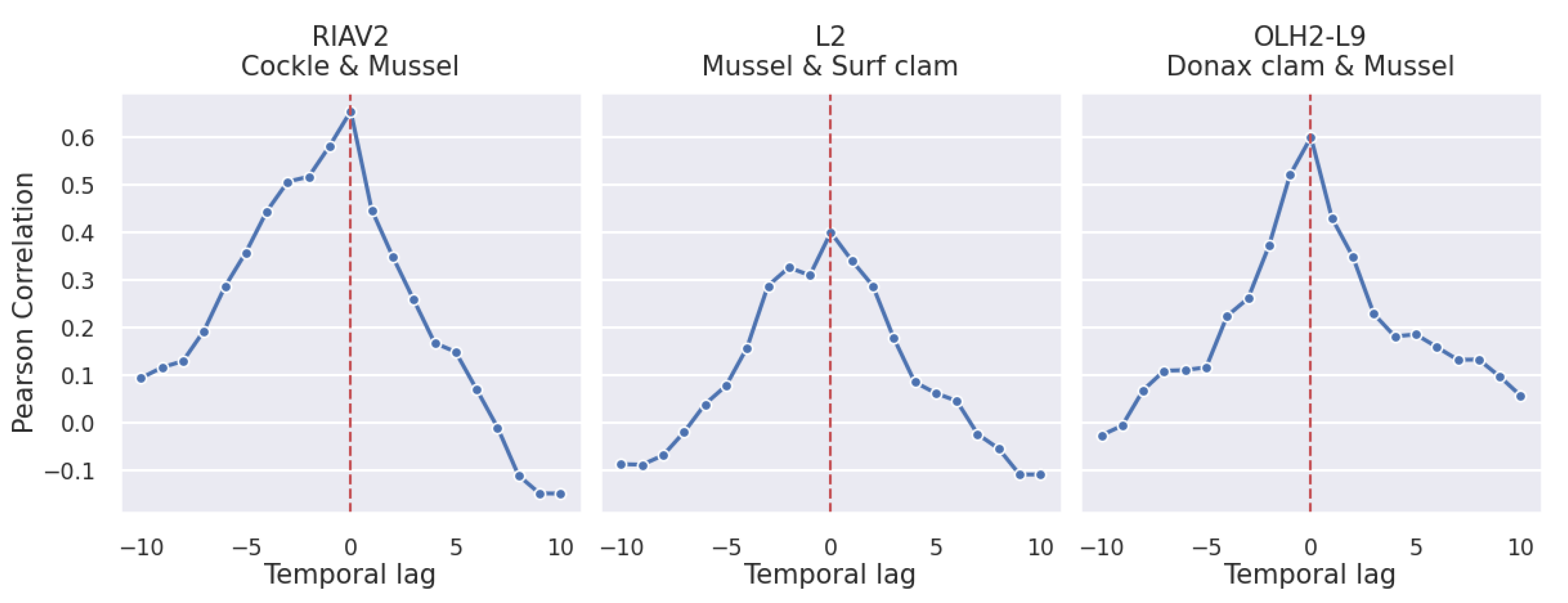
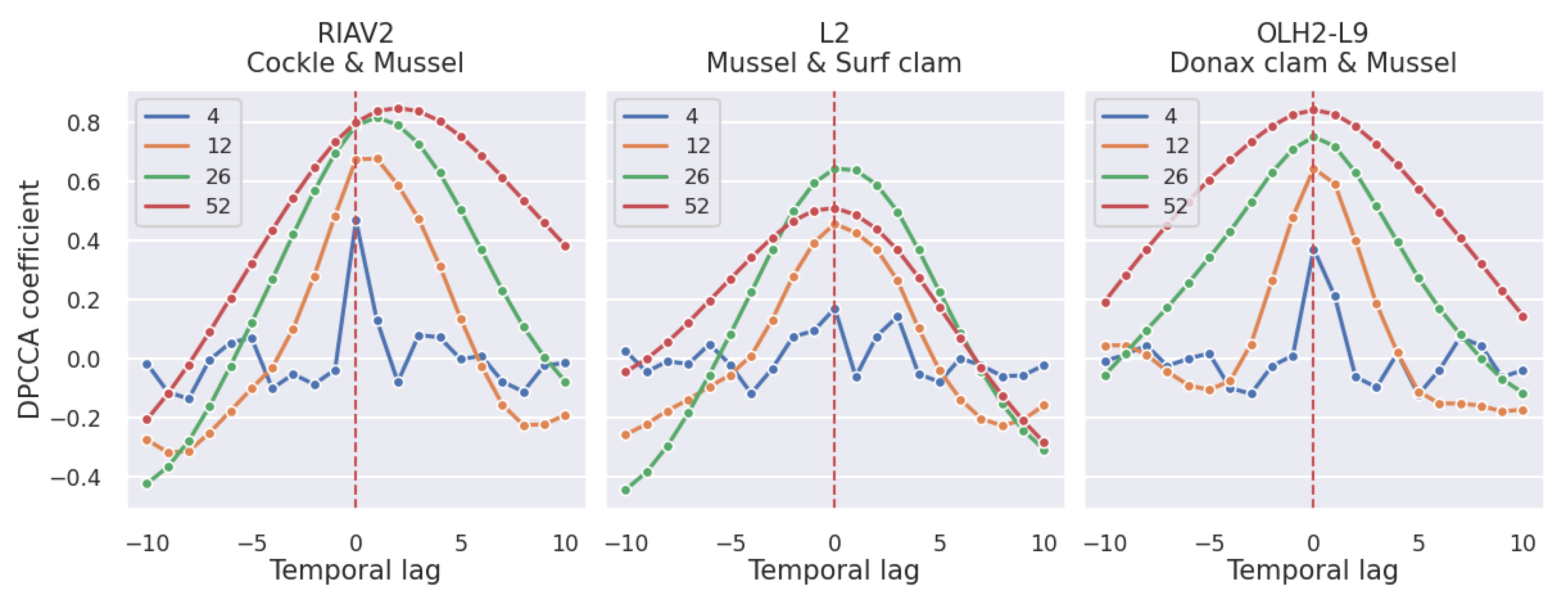
3.2.2. Shellfish Species
3.2.3. Toxic Phytoplankton Abundances
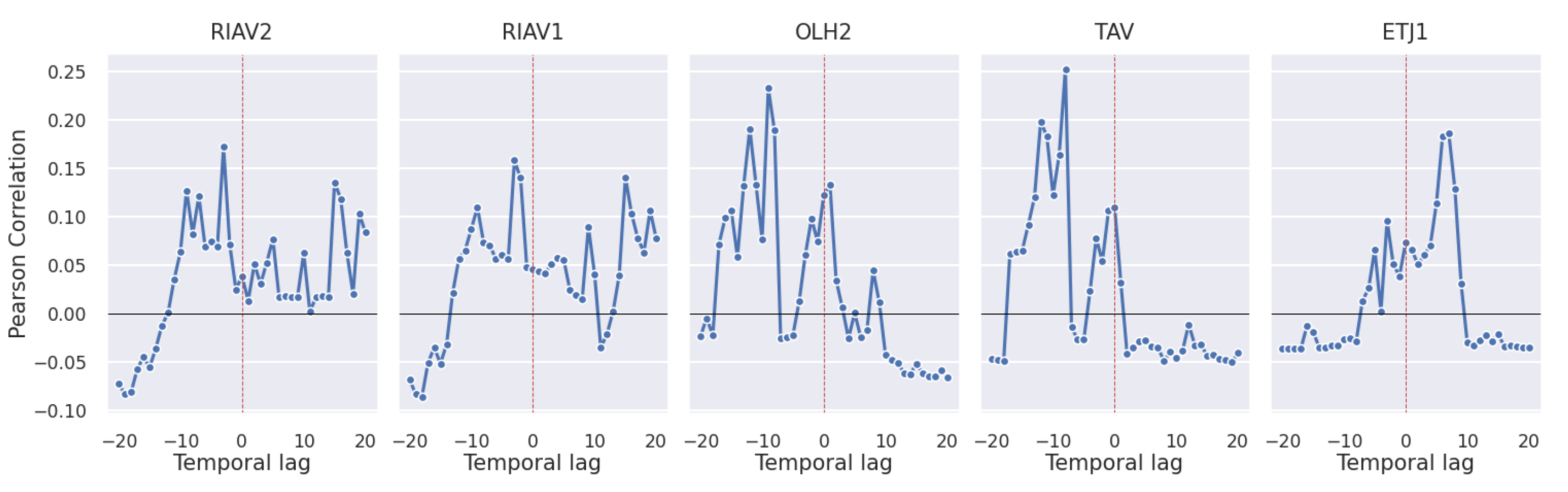
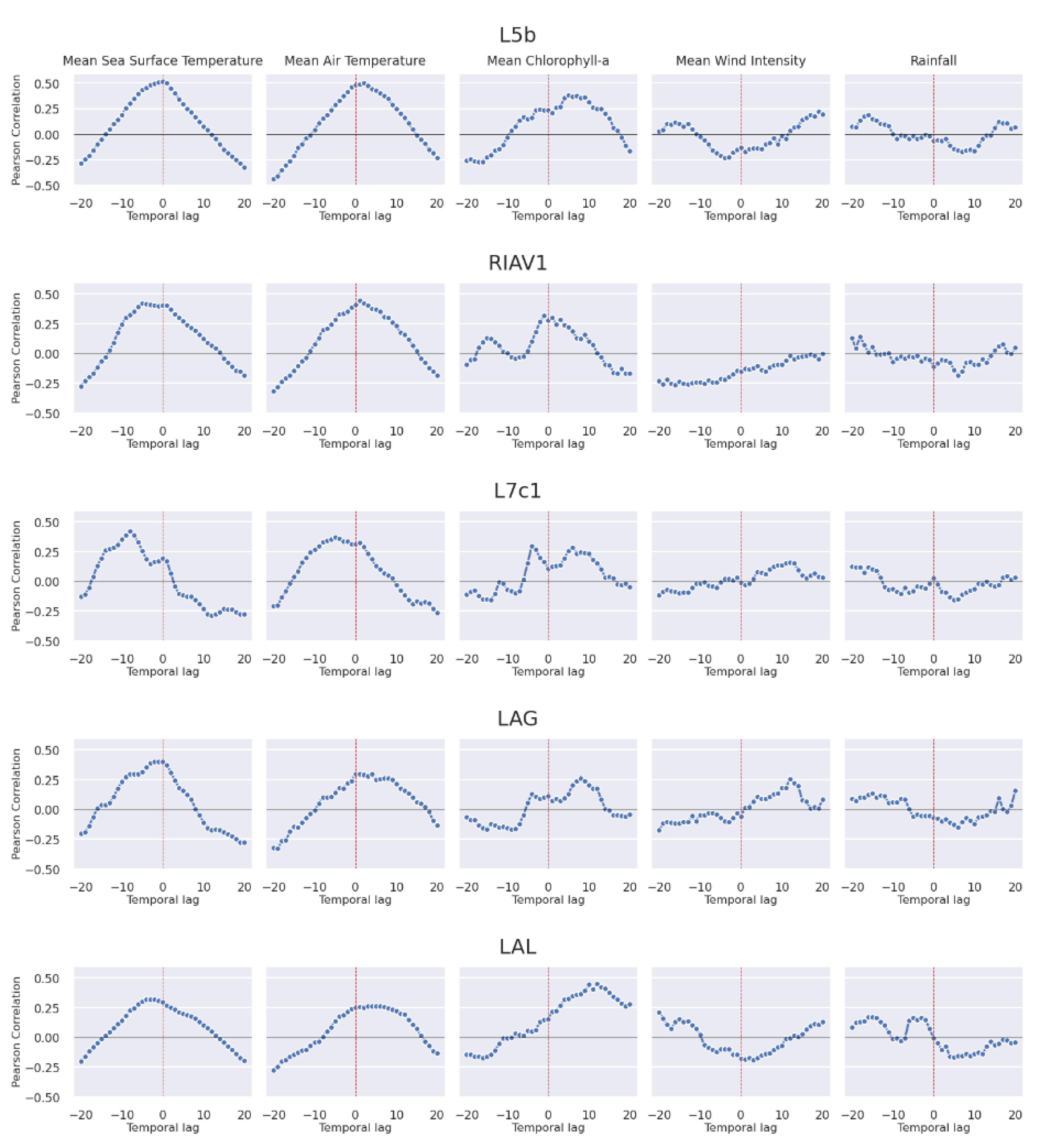
3.2.4. Oceanographic and Meteorological Conditions
4. Discussion
4.1. Seasonality-Trend Analysis
4.2. Correlation Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tachibana, K.; Scheuer, P.J.; Tsukitani, Y.; Kikuchi, H.; Van Engen, D.; Clardy, J.; Gopichand, Y.; Schmitz, F.J. Okadaic acid, a cytotoxic polyether from two marine sponges of the genus Halichondria. J. Am. Chem. Soc. 1981, 103, 2469–2471. [Google Scholar] [CrossRef]
- Murata, M.; Shimatani, M.; Sugitani, H.; Oshima, Y.; Yasumoto, T. Isolation and Structural Elucidation of the Causative Toxin of the Diarrhetic Shellfish Poisoning. Nippon Suisan Gakkaishi 1982, 48, 549–552. [Google Scholar] [CrossRef]
- Yasumoto, T.; Oshima, Y.; Yamaguchi, M. Occurrence of new type of toxic shellfish in Japan and chemical properties of the toxin. Dev. Mar. Biol. 1979, 1, 395–398. [Google Scholar]
- Vale, P.; Antónia, M.; Sampayo, M. Esters of okadaic acid and dinophysistoxin-2 in Portuguese bivalves related to human poisonings. Toxicon 1999, 37, 1109–1121. [Google Scholar] [CrossRef]
- Council of the European Union. Council Directive 91/492/EEC of 15 July 1991 laying down the health conditions for the production and the placing on the market of live bivalve molluscs. Off. J. Eur. Communities 1991, L268, 1–14. [Google Scholar]
- European Commission. Commission Decision 2002/225/EC laying down detailed rules for the implementation of Council Directive 91/492/EEC as regards the maximum levels and the methods of analysis of certain marine biotoxins in bivalve molluscs, echinoderms, tunicates and marine gastropods. Off. J. Eur. Communities 2002, L75, 62–64. [Google Scholar]
- European Parliament, Council of the European Union. Commission Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. Off. J. Eur. Union 2004, L39, 206–320. [Google Scholar]
- European Commission, Directorate-General for Health and Food Safety. Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as regards official controls. Off. J. Eur. Communities 2019, L131, 51–100. [Google Scholar]
- Fernández, R.; Mamán, L.; Jaén, D.; Fuentes, L.F.; Ocaña, M.A.; Gordillo, M.M. Dinophysis species and diarrhetic shellfish toxins: 20 years of monitoring program in Andalusia, South of spain. Toxins 2019, 11, 189. [Google Scholar] [CrossRef]
- Blanco, J.; Arévalo, F.; Correa, J.; Moroño, Á. Lipophilic toxins in Galicia (NW Spain) between 2014 and 2017: Incidence on the main molluscan species and analysis of the monitoring efficiency. Toxins 2019, 11, 612. [Google Scholar] [CrossRef]
- Botelho, M.J.; Vale, C.; Ferreira, J.G. Seasonal and multi-annual trends of bivalve toxicity by PSTs in Portuguese marine waters. Sci. Total Environ. 2019, 664, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Salas, R.; Clarke, D. Review of DSP toxicity in Ireland: Long-term trend impacts, biodiversity and toxin profiles from a monitoring perspective. Toxins 2019, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Van der Fels-Klerx, H.; Adamse, P.; Goedhart, P.; Poelman, M.; Pol-Hofstad, I.; Van Egmond, H.; Gerssen, A. Monitoring phytoplankton and marine biotoxins in production waters of the Netherlands: Results after one decade. Food Addit. Contam. Part A 2012, 29, 1616–1629. [Google Scholar] [CrossRef]
- Tibiriçá, C.E.J.d.A.; Fernandes, L.F.; Mafra Junior, L.L. Seasonal and spatial patterns of toxigenic species of Dinophysis and Pseudo-nitzschia in a subtropical Brazilian estuary. Braz. J. Oceanogr. 2015, 63, 17–32. [Google Scholar] [CrossRef]
- Lee, T.C.H.; Fong, F.L.Y.; Ho, K.C.; Lee, F.W.F. The mechanism of diarrhetic shellfish poisoning toxin production in Prorocentrum spp.: Physiological and molecular perspectives. Toxins 2016, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.C.; Reis Costa, P.; Vinga, S.; Krippahl, L.; Lopes, M.B. A Review of recent machine learning advances for forecasting harmful algal blooms and shellfish contamination. J. Mar. Sci. Eng. 2021, 9, 283. [Google Scholar] [CrossRef]
- Wang, X.; Bouzembrak, Y.; Marvin, H.J.; Clarke, D.; Butler, F. Bayesian Networks modeling of diarrhetic shellfish poisoning in Mytilus edulis harvested in Bantry Bay, Ireland. Harmful Algae 2022, 112, 102171. [Google Scholar] [CrossRef]
- Velo-Suárez, L.; Gutiérrez-Estrada, J. Artificial neural network approaches to one-step weekly prediction of Dinophysis acuminata blooms in Huelva (Western Andalucía, Spain). Harmful Algae 2007, 6, 361–371. [Google Scholar] [CrossRef]
- Schmidt, W.; Evers-King, H.L.; Campos, C.J.; Jones, D.B.; Miller, P.I.; Davidson, K.; Shutler, J.D. A generic approach for the development of short-term predictions of Escherichia coli and biotoxins in shellfish. Aquac. Environ. Interact. 2018, 10, 173–185. [Google Scholar] [CrossRef]
- Raine, R.; McDermott, G.; Silke, J.; Lyons, K.; Nolan, G.; Cusack, C. A simple short range model for the prediction of harmful algal events in the bays of southwestern Ireland. J. Mar. Syst. 2010, 83, 150–157. [Google Scholar] [CrossRef]
- Tong, T.T.V.; Le, T.H.H.; Tu, B.M.; Le, D.C. Spatial and seasonal variation of diarrheic shellfish poisoning (DSP) toxins in bivalve mollusks from some coastal regions of Vietnam and assessment of potential health risks. Mar. Pollut. Bull. 2018, 133, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, K.J.; Suzuki, T.; Kang, Y.S.; Kim, P.H.; Song, K.C.; Lee, T.S. Seasonal variability of lipophilic shellfish toxins in bivalves and waters, and abundance of Dinophysis spp. in Jinhae Bay, Korea. J. Shellfish Res. 2010, 29, 1061–1067. [Google Scholar] [CrossRef]
- Naustvoll, L.J.; Gustad, E.; Dahl, E. Monitoring of Dinophysis species and diarrhetic shellfish toxins in Flødevigen Bay, Norway: Inter-annual variability over a 25-year time-series. Food Addit. Contam. Part A 2012, 29, 1605–1615. [Google Scholar] [CrossRef]
- Vale, P.; Botelho, M.J.; Rodrigues, S.M.; Gomes, S.S.; Sampayo, M.A.d.M. Two decades of marine biotoxin monitoring in bivalves from Portugal (1986–2006): A review of exposure assessment. Harmful Algae 2008, 7, 11–25. [Google Scholar] [CrossRef]
- Karlson, B.; Rehnstam-Holm, A.S.; Loo, L.O. Temporal and Spatial Distribution of Diarrhetic Shellfish Toxins in Blue Mussels, Mytilus edulis (L.), on the Swedish West Coast, NE Atlantic, 1988–2005; Sveriges Meteorologiska och Hydrologiska Institut (SMHI): Norrköping, Sweden, 2007.
- Klöpper, S.; Scharek, R.; Gerdts, G. Diarrhetic shellfish toxicity in relation to the abundance of Dinophysis spp. in the German Bight near Helgoland. Mar. Ecol. Prog. Ser. 2003, 259, 93–102. [Google Scholar] [CrossRef]
- Yasumoto, T.; Oshima, Y.; Sugawara, W.; Fukuyo, Y.; Oguri, H.; Igarashi, T.; Fujita, N. Identification of Dinophysis fortii as the Causative Organism of Diarrhetic Shellfish Poisoning. Bull. Jpn. Soc. Sci. Fish. 1980, 46, 1405–1411. [Google Scholar] [CrossRef]
- Ninčević-Gladan, Ž.; Skejić, S.; Bužančić, M.; Marasović, I.; Arapov, J.; Ujević, I.; Bojanić, N.; Grbec, B.; Kušpilić, G.; Vidjak, O. Seasonal variability in Dinophysis spp. abundances and diarrhetic shellfish poisoning outbreaks along the eastern Adriatic coast. Bot. Mar. 2008, 51, 449–463. [Google Scholar] [CrossRef]
- Mar—Instituto Português do Mar e da Atmosfera, I. P. Despacho n.º 4362/2020, de 9 de abril. In Diário da República n.º 71/2020; Série II de 2020-04-09; 2020; pp. 105–114. Available online: https://dre.pt/dre/detalhe/despacho/4362-2020-131292633 (accessed on 29 July 2022).
- Copernicus. Available online: https://www.copernicus.eu/ (accessed on 29 July 2022).
- IPMA—Bivalves. Available online: https://www.ipma.pt/pt/bivalves/ (accessed on 29 July 2022).
- Sousa, R.; Amado, C.; Henriques, R. AutoMTS: Fully autonomous processing of multivariate time series data from heterogeneous sensor networks. In Proceedings of the International Conference on Heterogeneous Networking for Quality, Reliability, Security and Robustness, Virtual Event, 29–30 November 2020; pp. 154–178. [Google Scholar]
- Mockus, J.; Tiesis, V.; Zilinskas, A. The application of Bayesian methods for seeking the extremum. Towards Glob. Optim. 1978, 2, 2. [Google Scholar]
- West, M. Time series decomposition. Biometrika 1997, 84, 489–494. [Google Scholar] [CrossRef]
- Benesty, J.; Chen, J.; Huang, Y.; Cohen, I. Pearson correlation coefficient. In Noise Reduction in Speech Processing; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–4. [Google Scholar]
- Yuan, N.; Fu, Z.; Zhang, H.; Piao, L.; Xoplaki, E.; Luterbacher, J. Detrended partial-cross-correlation analysis: A new method for analyzing correlations in complex system. Sci. Rep. 2015, 5, 8143. [Google Scholar] [CrossRef]
- Github—Andrempp/Time-Lagged-Correlation-in-Bivalves. Available online: https://github.com/Andrempp/time-lagged-correlation-in-bivalves (accessed on 29 July 2022).
- Vale, P. Differential dynamics of dinophysistoxins and pectenotoxins between blue mussel and common cockle: A phenomenon originating from the complex toxin profile of Dinophysis acuta. Toxicon 2004, 44, 123–134. [Google Scholar] [CrossRef]
- Vale, P.; Sampayo, M.A.d.M. Esterification of DSP toxins by Portuguese bivalves from the Northwest coast determined by LC-MS—A widespread phenomenon. Toxicon 2002, 40, 33–42. [Google Scholar] [CrossRef]
- Wu, H.; Yao, J.; Guo, M.; Tan, Z.; Zhou, D.; Zhai, Y. Distribution of marine lipophilic toxins in shellfish products collected from the Chinese market. Mar. Drugs 2015, 13, 4281–4295. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Mok, J.S.; Song, K.C.; Yu, H.; Jung, J.H.; Kim, J.H. Geographical and annual variation in lipophilic shellfish toxins from oysters and mussels along the south coast of Korea. J. Food Prot. 2011, 74, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.L.; Vershinin, A.; Smith, L.L.; Leighfield, T.A.; Pankov, S.; Quilliam, M.A. Seasonality of Dinophysis spp. and Prorocentrum lima in Black Sea phytoplankton and associated shellfish toxicity. Harmful Algae 2009, 8, 629–636. [Google Scholar] [CrossRef]
- Vale, P.; Sampayo, M.A.d.M. Seasonality of diarrhetic shellfish poisoning at a coastal lagoon in Portugal: Rainfall patterns and folk wisdom. Toxicon 2003, 41, 187–197. [Google Scholar] [CrossRef]
- Peperzak, L.; Snoeijer, G.; Dijkema, R.; Gieskes, W.; Joordens, J.; Peeters, J.; Schol, C.; Vrieling, E.; Zevenboom, W. Development of a Dinophysis Acuminata Bloom in the River Rifine Plume (North Sea); Intergovernmental Oceanographic Commission of UNESCO: Sendai, Japan, 1996. [Google Scholar]
- Miller, C.A.; Yang, S.; Love, B.A. Moderate increase in TCO2 enhances photosynthesis of seagrass Zostera japonica, but not Zostera marina: Implications for acidification mitigation. Front. Mar. Sci. 2017, 4, 228. [Google Scholar] [CrossRef]
| (a) Diarrhetic Shellfish Poisoning (DSP) toxins dataset. | ||||
| Description | Range | Mean | Type | |
| Date | Date of the measurement | 2015-01-05 to 2020-12-29 | - | Date |
| Production Area | Production area, as defined by IPMA, where the measurement was performed | - | - | Categorical |
| Species | Species analysed | - | - | Categorical |
| DSP Toxins | Concentration of Diarrhetic Shellfish Poisoning toxins ( OA eq. −1) | 9 to 1945 | 110.58 | Numerical |
| (b) Toxic phytoplankton cell counts dataset. | ||||
| Description | Range | Mean | Type | |
| Date | Date of the measurement | 2015-01-05 to 2020-12-29 | - | Date |
| Production Area | Production area, as defined by IPMA, where the measurement was performed | - | - | Categorical |
| DSP Toxins Producers | Toxic phytoplankton abundances (cell/L) | 20 to 1907840 | 1772.27 | Numerical |
| (c) Oceanographic and meteorological dataset. | ||||
| Description | Range | Mean | Type | |
| Date | Date of the measurement | 2015-01-05 to 2020-12-29 | - | Date |
| Production Area | Production area, as defined by IPMA, where the measurement was performed | - | - | Categorical |
| Mean SST | Weekly mean Sea Surface Temperature (SST) obtained from Copernicus (Kelvin) | 285 to 297 | 290.08 | Numerical |
| Mean Chlorophyll-a | Weekly mean of chlorophyll-a concentration obtained from Copernicus (mg/L) | 0.23 to 27.25 | 3.74 | Numerical |
| Mean Air Temperature | Weekly mean air temperature (Celsius) at 1.5 m of altitude | 3.93 to 28.69 | 16.34 | Numerical |
| Mean Wind Intensity | Weekly mean wind speed (km/h) | 0.33 to 8.75 | 2.82 | Numerical |
| Rainfall | Weekly mean of accumulated precipitation (millimeters) | 0 to 24.59 | 1.57 | Numerical |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrício, A.; Lopes, M.B.; Costa, P.R.; Costa, R.S.; Henriques, R.; Vinga, S. Time-Lagged Correlation Analysis of Shellfish Toxicity Reveals Predictive Links to Adjacent Areas, Species, and Environmental Conditions. Toxins 2022, 14, 679. https://doi.org/10.3390/toxins14100679
Patrício A, Lopes MB, Costa PR, Costa RS, Henriques R, Vinga S. Time-Lagged Correlation Analysis of Shellfish Toxicity Reveals Predictive Links to Adjacent Areas, Species, and Environmental Conditions. Toxins. 2022; 14(10):679. https://doi.org/10.3390/toxins14100679
Chicago/Turabian StylePatrício, André, Marta B. Lopes, Pedro Reis Costa, Rafael S. Costa, Rui Henriques, and Susana Vinga. 2022. "Time-Lagged Correlation Analysis of Shellfish Toxicity Reveals Predictive Links to Adjacent Areas, Species, and Environmental Conditions" Toxins 14, no. 10: 679. https://doi.org/10.3390/toxins14100679
APA StylePatrício, A., Lopes, M. B., Costa, P. R., Costa, R. S., Henriques, R., & Vinga, S. (2022). Time-Lagged Correlation Analysis of Shellfish Toxicity Reveals Predictive Links to Adjacent Areas, Species, and Environmental Conditions. Toxins, 14(10), 679. https://doi.org/10.3390/toxins14100679







