Bioaccessibility Study of Aflatoxin B1 and Ochratoxin A in Bread Enriched with Fermented Milk Whey and/or Pumpkin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bread Contamination and Analysis
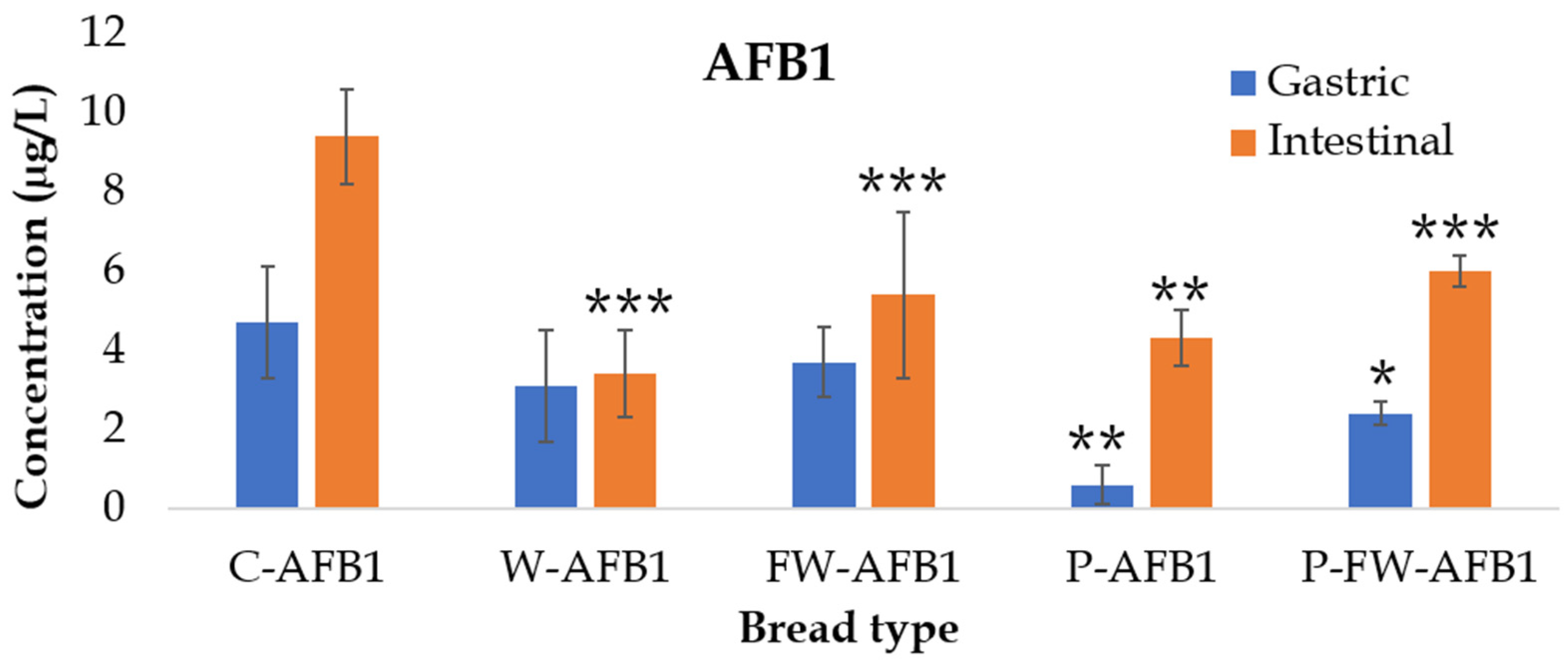
2.2. AFB1 Bioaccessibility
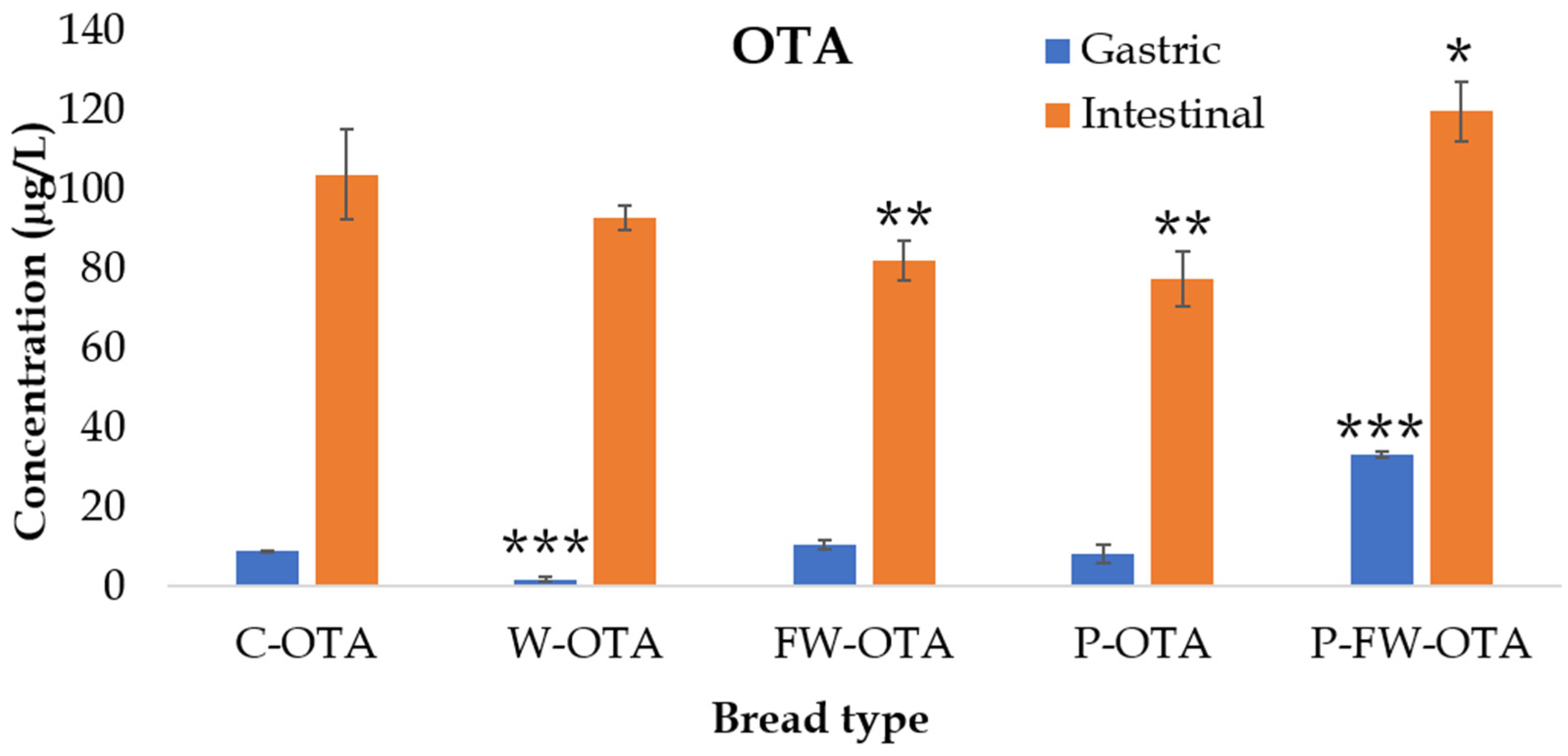
2.3. OTA Bioaccessibility
2.4. Effect of Bread Enrichment with Bioactive Ingredients on Mycotoxin Reduction
3. Conclusions
4. Material and Methods
4.1. Chemicals, Reagents and Biological Strains
4.2. Flour Contamination
4.3. Bread Natural Ingredients
4.4. Bread Preparation and Baking
4.5. Bread Analysis
4.6. HPLC–MS/qTOF Conditions
4.7. In Vitro Static Digestion Model
4.8. Gastrointestinal Extracts Analysis and Bioaccessibility
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC). Monographs. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993; Volume 56.
- European Commission (EC). No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on the risk assessment of ochratoxin A in food. EFSA J. 2020, 18, 6113. [CrossRef]
- Serrano, A.B.; Font, G.; Ruiz, M.J.; Ferrer, E. Co-occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chem. 2012, 135, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Saladino, F.; Quiles, J.M.; Mañes, J.; Fernández-Franzón, M.; Bittencourt, F.L.; Meca, G. Dietary exposure to mycotoxins through the consumption of commercial bread loaf in Valencia, Spain. LWT—Food Sci. Tech. 2017, 75, 697–701. [Google Scholar] [CrossRef]
- Coppa, S.C.C.F.; Khaneghah, A.M.; Alvito, P.; Assunção, R.; Martins, C.; Eş, I.; Gonçalves, B.L.; Valganon de Neeff, D.; Sant’Ana, A.S.; Corassin, C.H.; et al. The occurrence of mycotoxins in breast milk, fruit products and cereal-based infant formula: A review. Trends Food Sci. Techol. 2019, 92, 81–93. [Google Scholar] [CrossRef]
- Palumbo, R.; Crisci, A.; Venâncio, A.; Cortiñas Abrahantes, J.; Dorne, J.-L.; Battilani, P.; Toscano, P. Occurrence and co-occurrence of mycotoxins in cereal-based feed and food. Microorganisms 2020, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Maietti, A.; Tedeschi, P.; Catani, M.; Stevanin, C.; Pasti, L.; Cavazzini, A.; Marchetti, N. Nutrient composition and antioxidant performances of bread-making products. Foods 2021, 10, 938. [Google Scholar] [CrossRef]
- Bol, E.K.; Araujo, L.; Veras, F.V.; Welke, J.E. Estimated exposure to zearalenone, ochratoxin A and aflatoxin B1 through the consume of bakery products and pasta considering effects of food processing. Food Chem. Toxicol. 2016, 89, 85–91. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Stępniewska, S.; Modrzewska, M.; Waśkiewicz, A.; Szymczyk, K.; Szafrańska, A. Transformation of ochratoxin A during bread-making processes. Food Control 2021, 125, 107950. [Google Scholar] [CrossRef]
- Vidal, A.; Morales, H.; Sanchis, V.; Ramos, A.J.; Marín, S. Stability of DON and OTA during the bread making process and determination of process and performance criteria. Food Control 2014, 40, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. New mycotoxin adsorbents based on tri-octahedral bentonites for animal feed. Anim. Feed Sci. Technol. 2019, 255, 114228. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. Tri-octahedral bentonites as potential technological feed additive for Fusarium mycotoxin reduction. Food Addit. Contam. Part A Chem. Anal. Control 2020, 37, 1374–1387. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins affecting animals, foods, humans, and plants: Types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies—A revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef]
- Serrano-Niño, J.C.; Cavazos-Garduño, A.; Hernandez-Mendoza, A.; Applegate, B.; Ferruzzi, M.G.; San Martin-González, M.F.; García, H.S. Assessment of probiotic strains ability to reduce the bioaccessibility of aflatoxin M1 in artificially contaminated milk using an in vitro digestive model. Food Control 2013, 31, 202–207. [Google Scholar] [CrossRef]
- Saladino, F.; Posarelli, E.; Luz, C.; Luciano, F.B.; Rodriguez-Estrada, M.T.; Mañes, J.; Meca, G. Influence of probiotic microorganisms on aflatoxins B1 and B2 bioaccessibility evaluated with a simulated gastrointestinal digestion. J. Food Composit. Anal. 2018, 68, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Nazareth, T.D.M.; Luz, C.; Torrijos, R.; Quiles, J.M.; Luciano, F.B.; Mañes, J.; Meca, G. Potential application of lactic acid bacteria to reduce aflatoxin B1 and fumonisin B1 occurrence on corn kernels and corn ears. Toxins 2019, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Illueca, F.; Vila-Donat, P.; Calpe, J.; Luz, C.; Meca, G.; Quiles, J.M. Antifungal activity of biocontrol agents in vitro and potential application to reduce mycotoxins (aflatoxin B1 and ochratoxin A). Toxins 2021, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.M.; Manyes, L.; Luciano, F.B.; Mañes, J.; Meca, G. Effect of the oriental and yellow mustard flours as natural preservative against aflatoxins B1, B2, G1 and G2 production in wheat tortillas. J. Food Sci. Technol. 2015, 52, 8315–8321. [Google Scholar] [CrossRef] [Green Version]
- Quiles, J.M.; Torrijos, R.; Luciano, F.B.; Mañes, J.; Meca, G. Aflatoxins and A. flavus reduction in loaf bread through the use of natural ingredients. Molecules 2018, 4, 1638. [Google Scholar] [CrossRef] [Green Version]
- Torrijos, R.; Nazareth, T.M.; Pérez, J.; Mañes, J.; Meca, G. Development of a bioactive sauce based on oriental mustard flour with antifungal properties for pita bread shelf life improvement. Molecules 2019, 24, 1019. [Google Scholar] [CrossRef] [Green Version]
- Luz, C.; Izzo, L.; Ritieni, A.; Mañes, J.; Meca, G. Antifungal and antimycotoxigenic activity of hydrolyzed goat whey on Penicillium spp: An application as biopreservation agent in pita bread. LWT 2020, 118, 108717. [Google Scholar] [CrossRef]
- Bergantin, C.; Maietti, A.; Tedeschi, P.; Font, G.; Manyes, L.; Marchetti, N. HPLC-UV/Vis-APCI-MS/MS determination of major carotenoids and their bioaccessibility from “Delica” (Cucurbita maxima) and “Violina” (Cucurbita moschata) pumpkins as food traceability markers. Molecules 2018, 23, 2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Garrido, M.; Tedeschi, P.; Maietti, A.; Font, G.; Marchetti, N.; Manyes, L. Mitochondrial transcriptional study of the effect of aflatoxins, enniatins and carotenoids in vitro in a blood brain barrier model. Food Chem. Toxicol. 2020, 137, 111077. [Google Scholar] [CrossRef]
- Alonso-Garrido, M.; Frangiamone, M.; Font, G.; Cimbalo, A.; Manyes, L. In vitro blood brain barrier exposure to mycotoxins and carotenoids pumpkin extract alters mitochondrial gene expression and oxidative stress. Food Chem. Toxicol. 2020, 153, 112261. [Google Scholar] [CrossRef]
- Yilmaz, S.; Kaya, E.; Karaca, A.; Karatas, O. Aflatoxin B1 induced renal and cardiac damage in rats: Protective effect of lycopene. Res. Vet. Sci. 2018, 119, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Chatzipaschali, A.A.; Stamatis, A.G. Biotechnological utilization with a focus on anaerobic treatment of cheese whey: Current status and prospects. Energies 2012, 5, 3492–3525. [Google Scholar] [CrossRef] [Green Version]
- Escrivá, L.; Manyes, L.; Vila-Donat, P.; Font, G.; Meca, G.; Lozano, M. Bioaccessibility and bioavailability of bioactive compounds from yellow mustard flour and milk whey fermented with lactic acid bacteria. Food Funct. 2021, 12, 11250. [Google Scholar] [CrossRef]
- Coppa, S.C.C.F.; Cirelli, A.C.; Gonçalves, B.L.; Barnabé, E.M.B.; Khaneghah, A.M.; Corassin, C.H.; Oliveira, C.A.F. Dietary exposure assessment and risk characterization of mycotoxins in lactating women: Case study of São Paulo state, Brazil. Food Res. Int. 2020, 134, 109272. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Brera, C.; Faid, M.; Benlemlih, M.; Miraglia, M. Ochratoxin A and fusarium toxins in cereals from Morocco. Cah. Agric. 2017, 16, 11–15. [Google Scholar]
- Nogueira, A.; Oliveira, R.; Steel, C. Protein enrichment of wheat flour doughs: Empirical rheology using protein hydrolysates. Food Sci. Technol. 2020, 4, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Rakcejeva, T.; Galoburda, R.; Cude, L.; Strautniece, E. Use of dried pumpkins in wheat bread production. Procedia Food Sci. 2011, 1, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Assunção, R.; Martins, C.; Dupont, D.; Alvito, P. Patulin and ochratoxin A co-occurrence and their bioaccessibility in processed cereal-based foods: A contribution for Portuguese children risk assessment. Food Chem. Toxicol. 2016, 96, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Versantvoort, C.H.M.; Oomen, A.G.; Van de Kamp, E.; Rompelberg, C.J.M.; Sips, A.J.A.M. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.; Mykkanen, H.; Ouwehand, A.C.; Juvonen, R.; Salminen, S.; El-Nezami, H. Intestinal mucus alters the ability of probiotic bacteria to bind aflatoxin B1 in vitro. Appl. Environ. Microbiol. 2004, 70, 6306–6308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabak, B.; Brandon, F.A.E.; Var, I.; Blokland, M.; Sips, J.A. Effects of probiotic bacteria on the bioaccessibility of aflatoxin B1 and ochratoxin A using an in vitro digestion model under fed conditions. J. Environ. Sci. Health Part B 2009, 44, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B.; Ozbey, F. Assessment of the bioaccessibility of aflatoxins from various food matrices using an in vitro digestion model, and the efcacy of probiotic bacteria in reducing bioaccessibility. J. Food Compost. Anal. 2012, 27, 21–31. [Google Scholar] [CrossRef]
- Nogueira, W.V.; Oliveira, F.K.; Sibaja, K.V.M.; Garcia, S.O.; Kupski, L.; Souza, M.M.; Tesser, M.B.; Garda-Buffon, J. Occurrence and bioacessibility of mycotoxins in fish feed. Food Addit. Contam. Part B 2020, 13, 244–251. [Google Scholar] [CrossRef]
- Saddiq, A.A.N.; Awedh, M.H. Pumpkin (Cucurbita moschata) against Aspergillus flavus and aflatoxin B1 induced lung cyto-morphological damage in rats. Pak. J. Pharm. Sci. 2019, 32, 575–579. [Google Scholar]
- Marin, D.E.; Bulgaru, C.V.; Anghel, C.A.; Pistol, G.C.; Dore, M.I.; Palade, M.L.; Taranu, I. Grape seed waste counteracts aflatoxin B1 toxicity in piglet mesenteric lymph nodes. Toxins 2020, 12, 800. [Google Scholar] [CrossRef]
- Jin, S.; Yang, H.; Liu, F.; Pang, Q.; Shan, A.; Feng, X. Effect of dietary curcumin supplementation on duck growth performance, antioxidant capacity and breast meat quality. Foods 2021, 10, 2981. [Google Scholar] [CrossRef]
- Reddy, L.; Odhav, B.; Bhoola, K. Aflatoxin B1-induced toxicity in HepG2 cells inhibited by carotenoids: Morphology, apoptosis and DNA damage. Biol. Chem. 2006, 387, 87–93. [Google Scholar] [CrossRef]
- Ferrer, E.; Manyes, L.; Mañes, J.; Meca, G. Influence of prebiotics, probiotics and protein ingredients on mycotoxins bioaccessibility. Food Funct. 2015, 6, 987–994. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Greco, G.; Damascelli, A.; Solfrizzo, M.; Visconti, A. Assessment of multi-mycotoxin adsorption efficacy of grape pomace. J. Agric. Food Chem. 2014, 62, 497–507. [Google Scholar] [CrossRef]
- González-Arias, C.A.; Piquer-Garcia, I.; Marin, S.; Sanchis, V.; Ramos, A.J. Bioaccessibility of ochratoxin A from red wine in an in vitro dynamic gastrointestinal model. World Mycotoxin J. 2015, 8, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Mujahid, H.; Hashmi, A.S.M.Z.; Tayyab, M.; Shehzad, W. Protective effect of yeast sludge and whey powder against ochratoxicosis in broiler chicks. Pak. Vet. J. 2019, 39, 588–592. [Google Scholar] [CrossRef]
- Mansour, T.A.; Safinaz, G.; Soliman, M.K.; Eglal, A.; Srour, T.M.; Mona, S.Z.; Shahinaz, M.H. Ameliorate the drastic effect of ochratoxin A by using yeast and whey in cultured Oreochromus niloticus in Egypt. Life Sci. J. 2011, 8, 68–81. [Google Scholar]
- Mansour, T.A.; Omar, A.E.; Soliman, K.M.; Snour, M.T.; Nour, M.A. The antagonistic effect of whey on ochratoxin a toxicity on the growth performance, feed utilization, liver and kidney functions of Nile tilapia (Oreochromis niloticus). Middle East. J. Appl. Sci. 2015, 5, 176–183. [Google Scholar]
- Izzo, L.; Luz, C.; Ritieni, A.; Mañes, J.; Meca, G. Whey fermented by using Lactobacillus plantarum strains: A promising approach to increase the shelf life of pita bread. J. Dairy Sci. 2020, 103, 5906–5915. [Google Scholar] [CrossRef] [PubMed]
- Manzini, M.; Rodriguez-Estrada, M.T.; Meca, G.; Mañes, J. Reduction of beauvericin and enniatins bioaccessibility by prebiotic compounds: Evaluated in static and dynamic simulated gastrointestinal digestion. Food Control 2015, 47, 203–211. [Google Scholar] [CrossRef]
| Bread Concentration | ||
|---|---|---|
| (µg/kg) | ||
| AFB1 | C-AFB1 | 78 ± 3 |
| W-AFB1 | 92 ± 12 | |
| FW-AFB1 | 85 ± 6 | |
| P-AFB1 | 148 ± 5 | |
| FW-P AFB1 | 164 ± 16 | |
| OTA | C-OTA | 1184 ± 118 |
| W-OTA | 1258 ± 220 | |
| FW-OTA | 1173 ± 78 | |
| P-OTA | 1336 ± 202 | |
| FW-P-OTA | 1540 ± 291 |
| Bread Type | Gastric Bioaccessibility (%) | Intestinal Bioaccessibility (%) |
|---|---|---|
| C-AFB1 | 61 ± 19 | 114 ± 9 |
| W-AFB1 | 34 ± 15 | 41 ± 13 *** |
| FW-AFB1 | 43 ± 11 | 52 ± 5 *** |
| P-AFB1 | 4 ± 2 * | 29 ± 5 *** |
| P-FW-AFB1 | 15 ± 2 * | 37 ± 3 *** |
| Bread Type | Gastric Bioaccessibility (%) | Intestinal Bioaccessibility (%) |
|---|---|---|
| C-OTA | 7.7 ± 0.1 | 88 ± 10 |
| W-OTA | 1.4 ± 0.5 ** | 74 ± 2 * |
| FW-OTA | 9.0 ± 1.1 | 70 ± 4 * |
| P-OTA | 6.1 ± 1.8 | 58 ± 5 *** |
| P-FW-OTA | 21.6 ± 0.6 *** | 78 ± 5 |
| Bread Ingredient | Intestinal Bioaccessibility Reduction (%) | |
|---|---|---|
| AFB1 | OTA | |
| W bread | 64 ± 11 | 16 ± 3 |
| FW bread | 57 ± 4 | 20 ± 5 |
| P bread | 74 ± 4 | 34 ± 6 |
| P-FW bread | 68 ± 2 | 11 ± 6 |
| Bread Type |
| Control Bread |
| Bread (C) |
| Bread + AFB1 (C-AFB1) |
| Bread + OTA (C-OTA) |
| Whey bread |
| Bread + whey (W) |
| Bread + whey + AFB1 (W-AFB1) |
| Bread + whey + OTA (W-OTA) |
| Fermented whey bread |
| Bread + fermented whey (FW) |
| Bread + fermented whey + AFB1 (FW-AFB1) |
| Bread + fermented whey + OTA (FW-OTA) |
| Pumpkin bread |
| Bread + pumpkin (P) |
| Bread + pumpkin + AFB1 (P-AFB1) |
| Bread + pumpkin + OTA (P-OTA) |
| Fermented whey-Pumpkin bread |
| Bread + fermented whey + pumpkin (FW-P) |
| Bread + fermented whey + pumpkin +AFB1 (FW-P-AFB1) |
| Bread + fermented whey+ pumpkin + OTA (FW-P-OTA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escrivá, L.; Agahi, F.; Vila-Donat, P.; Mañes, J.; Meca, G.; Manyes, L. Bioaccessibility Study of Aflatoxin B1 and Ochratoxin A in Bread Enriched with Fermented Milk Whey and/or Pumpkin. Toxins 2022, 14, 6. https://doi.org/10.3390/toxins14010006
Escrivá L, Agahi F, Vila-Donat P, Mañes J, Meca G, Manyes L. Bioaccessibility Study of Aflatoxin B1 and Ochratoxin A in Bread Enriched with Fermented Milk Whey and/or Pumpkin. Toxins. 2022; 14(1):6. https://doi.org/10.3390/toxins14010006
Chicago/Turabian StyleEscrivá, Laura, Fojan Agahi, Pilar Vila-Donat, Jordi Mañes, Giuseppe Meca, and Lara Manyes. 2022. "Bioaccessibility Study of Aflatoxin B1 and Ochratoxin A in Bread Enriched with Fermented Milk Whey and/or Pumpkin" Toxins 14, no. 1: 6. https://doi.org/10.3390/toxins14010006
APA StyleEscrivá, L., Agahi, F., Vila-Donat, P., Mañes, J., Meca, G., & Manyes, L. (2022). Bioaccessibility Study of Aflatoxin B1 and Ochratoxin A in Bread Enriched with Fermented Milk Whey and/or Pumpkin. Toxins, 14(1), 6. https://doi.org/10.3390/toxins14010006








