Development of an Antifungal Device Based on Oriental Mustard Flour to Prevent Fungal Growth and Aflatoxin B1 Production in Almonds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
2.2. Oriental Mustard Flour (OMF) In Vitro Activity against A. flavus
2.3. Determination of the Fungal Population in Natural Almonds
2.4. Aflatoxin Determination
3. Conclusions
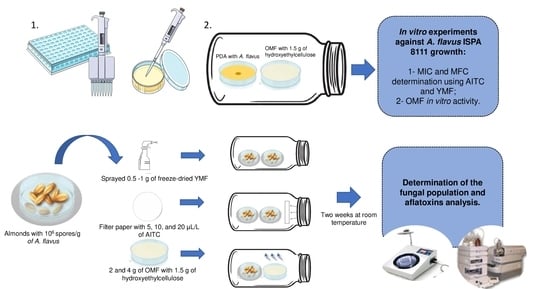
4. Materials and Methods
4.1. Chemicals
4.2. Microorganism and Culture Conditions
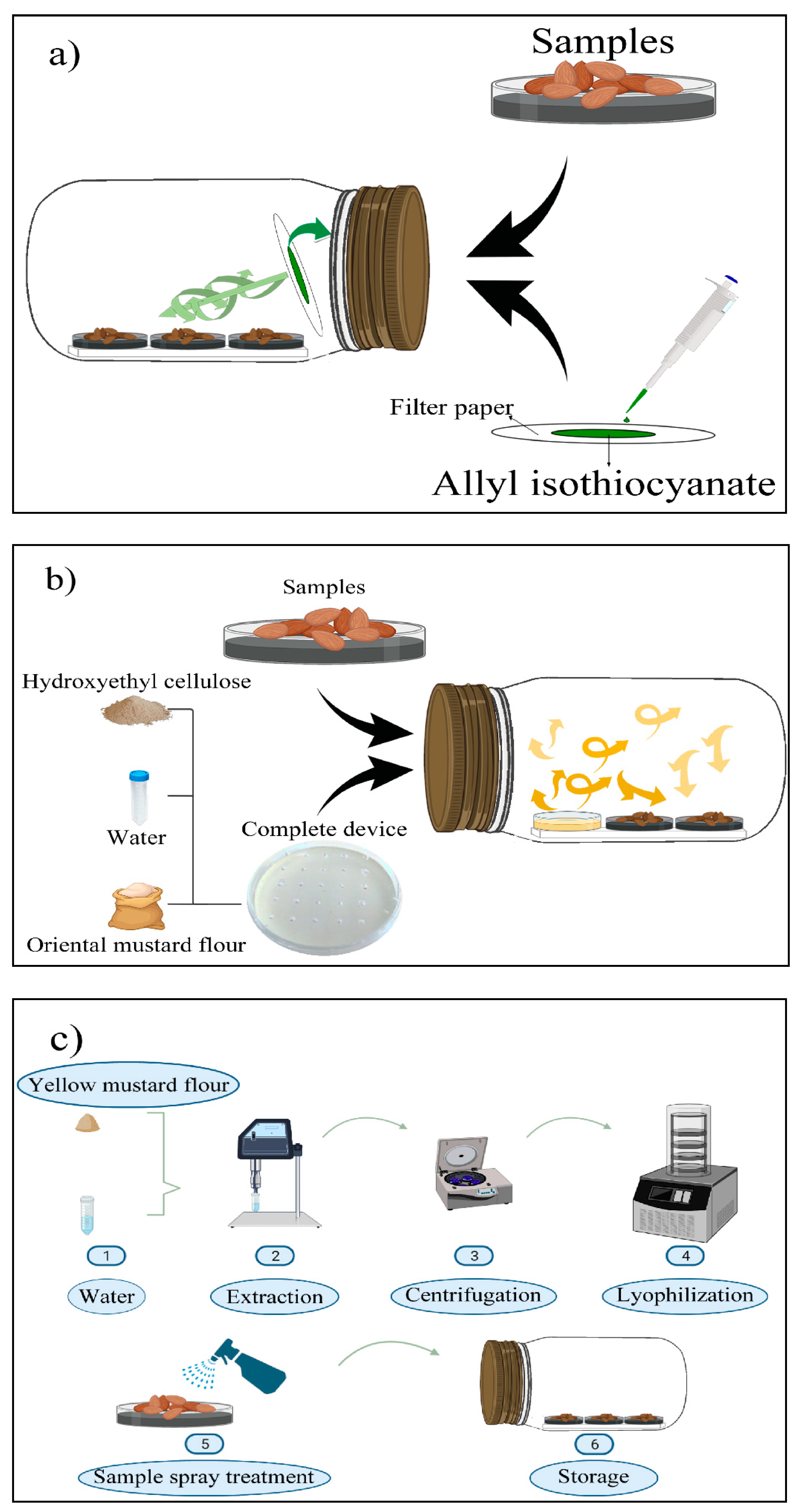
4.3. Preparation of the Freeze-Dried Yellow Mustard Extract (YMF-E)
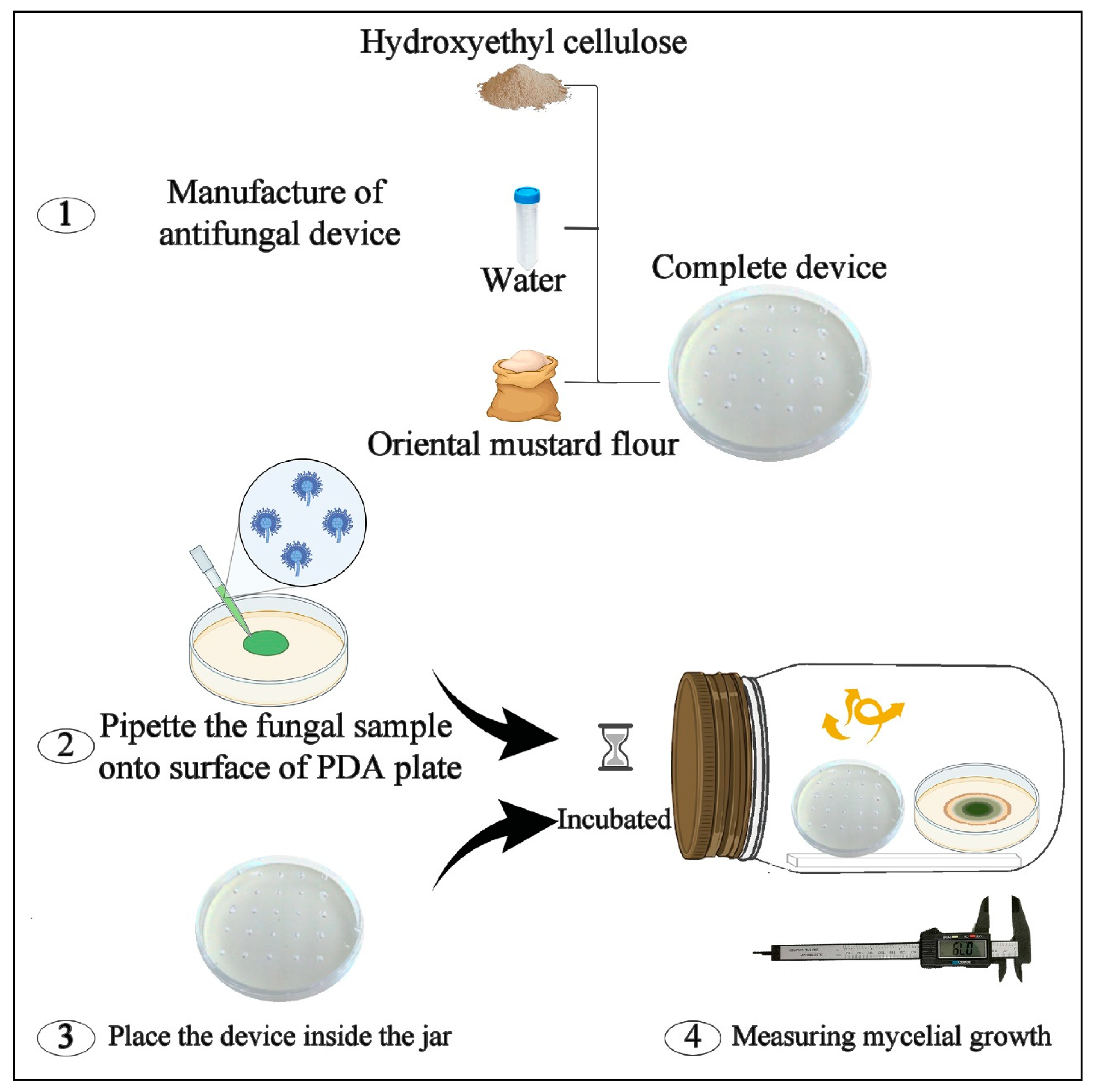
4.4. Manufacture of H-OMF Antifungal Device
4.5. Determination of the Minimum Inhibitory Concentration and the Minimum Fungicidal Concentration of AITC and YMF-E
4.6. In Vitro Antifungal Activity of H-OMF against A. flavus ISPA 8111
4.7. Microbiological Assay with Natural Almonds
- Filter papers (2.5 × 2.5 cm) containing AITC at 5.07, 10.13, and 20.26 mg/L (related to jar volume) were prepared and adhered to the lids of the jars (Figure 2a).
- H-OMF antifungal device was manufactured by mixing 2 and 4 g of OMF with 10 mL of sterile distilled water and 1.5 g of hydroxyethyl-cellulose. The device was then placed into the jars, reaching a final concentration of 2000 and 4000 mg/L (related to jar volume) (Figure 2b). These values represented 80- and 160-folds of the minimal dose to avoid the mycelial growth of A. flavus significantly.
- A spray of YMF was prepared with 0.5 (32 folds MFC), 0.8 (51.2 folds MFC), and 1 g (64 folds MFC) of YMF-E with 5 mL of sterile distilled water. The treatment was carried out by spraying the extract on the surface of almonds to reach final concentrations of 100, 160, and 200 g/L (Figure 2c).
4.8. Determination of the Fungal Population in Natural Almonds
4.9. AFB1 Extraction
4.10. LC-MS/MS Analysis
4.11. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxin detection. Curr. Opin. Biotechnol. 2016, 37, 120–126. [Google Scholar] [CrossRef]
- Njobeh, P.B.; Dutton, M.F.; Koch, S.H.; Chuturgoon, A.A.; Stoev, S.D.; Mosonik, J.S. Simultaneous occurrence of mycotoxins in human food commodities from Cameroon. Mycotoxin Res. 2010, 26, 47–57. [Google Scholar] [CrossRef]
- Terzi, V.; Tumino, G.; Stanca, A.M.; Morcia, C. Reducing the incidence of cereal head infection and mycotoxins in small grain cereal species. J. Cereal Sci. 2014, 59, 284–293. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Alasalvar, C.; Salvadó, J.S.; Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Becerra-Tomás, N.; Paz-Graniel, I.; Kendall, C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: A meta-analysis of prospective cohort studies. Nutr. Rev. 2019, 77, 691–709. [Google Scholar] [CrossRef]
- Saladino, F.; Luz, C.; Manyes, L.; Fernández-Franzón, M.; Meca, G. In vitro antifungal activity of lactic acid bacteria against mycotoxigenic fungi and their application in loaf bread shelf life improvement. Food Control 2016, 67, 273–277. [Google Scholar] [CrossRef]
- Le Lay, C.; Coton, E.; Le Blay, G.; Chobert, J.M.; Haertlé, T.; Choiset, Y.; Van Long, N.N.; Meslet-Cladière, L.; Mounier, J. Identification and quantification of antifungal compounds produced by lactic acid bacteria and propionibacteria. Int. J. Food Microbiol. 2016, 239, 79–85. [Google Scholar] [CrossRef]
- Torrijos, R.; de Melo Nazareth, T.; Quiles, J.M.; Mañes, J.; Meca, G. Application of White Mustard Bran and Flour on Bread as Natural Preservative Agents. Foods 2021, 10, 431. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [Green Version]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H. Encapsulation of cardamom essential oil in chitosan nano-composites: In-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates—A review. LWT-Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Nadarajah, D.; Han, J.H.; Holley, R.A. Inactivation of Escherichia coli O157:H7 in packaged ground beef by allyl isothiocyanate. Int. J. Food Microbiol. 2005, 99, 269–279. [Google Scholar] [CrossRef]
- Ekanayake, A.; Zoutendam, P.H.; Strife, R.J.; Fu, X.; Jayatilake, G.S. Development of white mustard (Sinapis alba L.) essential oil, a food preservative. Food Chem. 2012, 133, 767–774. [Google Scholar] [CrossRef]
- Williams, J.R.; Rayburn, J.R.; Cline, G.R.; Sauterer, R.; Friedman, M. Effect of allyl isothiocyanate on developmental toxicity in exposed Xenopus laevis embryos. Toxicol. Rep. 2015, 2, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Hontanaya, C.; Meca, G.; Luciano, F.B.; Mañes, J.; Font, G. Inhibition of aflatoxin B1, B2, G1 and G2 production by Aspergillus parasiticus in nuts using yellow and oriental mustard flours. Food Control 2015, 47, 154–160. [Google Scholar] [CrossRef]
- Tunc, S.; Chollet, E.; Chalier, P.; Preziosi-Belloy, L.; Gontard, N. Combined effect of volatile antimicrobial agents on the growth of Penicillium notatum. Int. J. Food Microbiol. 2007, 113, 263–270. [Google Scholar] [CrossRef]
- Nazareth, T.M.; Bordin, K.; Manyes, L.; Meca, G.; Mañes, J.; Luciano, F.B. Gaseous allyl isothiocyanate to inhibit the production of aflatoxins, beauvericin and enniatins by Aspergillus parasiticus and Fusarium poae in wheat flour. Food Control 2016, 62, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Tracz, B.L.; Bordin, K.; de Nazareth, T.M.; Costa, L.B.; de Macedo, R.E.F.; Meca, G.; Luciano, F.B. Assessment of allyl isothiocyanate as a fumigant to avoid mycotoxin production during corn storage. LWT 2017, 75, 692–696. [Google Scholar] [CrossRef]
- Andini, S.; Araya-Cloutier, C.; Lay, B.; Vreeke, G.; Hageman, J.; Vincken, J.P. QSAR-based physicochemical properties of isothiocyanate antimicrobials against gram-negative and gram-positive bacteria. LWT 2021, 144, 111222. [Google Scholar] [CrossRef]
- Drakopoulos, D.; Meca, G.; Torrijos, R.; Marty, A.; Kägi, A.; Jenny, E.; Forrer, H.R.; Six, J.; Vogelgsang, S. Control of Fusarium graminearum in Wheat With Mustard-Based Botanicals: From in vitro to in planta. Front. Microbiol. 2020, 11, 1595. [Google Scholar] [CrossRef]
- Nielsen, P.V.; Rios, R. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int. J. Food Microbiol. 2000, 60, 219–229. [Google Scholar] [CrossRef]
- Clemente, I.; Aznar, M.; Nerín, C. Synergistic properties of mustard and cinnamon essential oils for the inactivation of foodborne moulds in vitro and on Spanish bread. Int. J. Food Microbiol. 2019, 298, 44–50. [Google Scholar] [CrossRef]
- Quiles, J.M.; Torrijos, R.; Luciano, F.B.; Mañes, J.; Meca, G. Aflatoxins and A. flavus reduction in loaf bread through the use of natural ingredients. Molecules 2018, 23, 1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiles, J.M.; Manyes, L.; Luciano, F.B.; Mañes, J.; Meca, G. Effect of the oriental and yellow mustard flours as natural preservative against aflatoxins B1, B2, G1 and G2 production in wheat tortillas. J. Food Sci. Technol. 2015, 52, 8315–8321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manyes, L.; Luciano, F.B.; Mañes, J.; Meca, G. In vitro antifungal activity of allyl isothiocyanate (AITC) against Aspergillus parasiticus and Penicillium expansum and evaluation of the AITC estimated daily intake. Food Chem. Toxicol. 2015, 83, 293–299. [Google Scholar] [CrossRef]
- Saladino, F.; Bordin, K.; Manyes, L.; Luciano, F.B.; Mañes, J.; Fernández-Franzón, M.; Meca, G. Reduction of the aflatoxins B1, B2, G1and G2in Italian piadina by isothiocyanates. LWT-Food Sci. Technol. 2016, 70, 302–308. [Google Scholar] [CrossRef]
- Luciano, F.B.; Belland, J.; Holley, R.A. Microbial and chemical origins of the bactericidal activity of thermally treated yellow mustard powder toward Escherichia coli O157:H7 during dry sausage ripening. Int. J. Food Microbiol. 2011, 145, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bahmid, N.A.; Pepping, L.; Dekker, M.; Fogliano, V.; Heising, J. Using particle size and fat content to control the release of Allyl isothiocyanate from ground mustard seeds for its application in antimicrobial packaging. Food Chem. 2020, 308, 125573. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Lim, L.T. Release of allyl isothiocyanate from mustard seed meal powder. J. Food Sci. 2014, 79, E47–E53. [Google Scholar] [CrossRef] [PubMed]
- Turgis, M.; Han, J.; Caillet, S.; Lacroix, M. Antimicrobial activity of mustard essential oil against Escherichia coli O157:H7 and Salmonella typhi. Food Control 2009, 20, 1073–1079. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troncoso, R.; Espinoza, C.; Sánchez-Estrada, A.; Tiznado, M.E.; García, H.S. Analysis of the isothiocyanates present in cabbage leaves extract and their potential application to control Alternaria rot in bell peppers. Food Res. Int. 2005, 38, 701–708. [Google Scholar] [CrossRef]
- Lopes, L.F.; Bordin, K.; de Lara, G.H.C.; Saladino, F.; Quiles, J.M.; Meca, G.; Luciano, F.B. Fumigation of Brazil nuts with allyl isothiocyanate to inhibit the growth of Aspergillus parasiticus and aflatoxin production. J. Sci. Food Agric. 2018, 98, 792–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazareth, T.M.; Corrêa, J.A.F.; Pinto, A.C.S.M.; Palma, J.B.; Meca, G.; Bordin, K.; Luciano, F.B. Evaluation of gaseous allyl isothiocyanate against the growth of mycotoxigenic fungi and mycotoxin production in corn stored for 6 months. J. Sci. Food Agric. 2018, 98, 5235–5241. [Google Scholar] [CrossRef]
- Quiles, J.M.; de Melo Nazareth, T.; Luz, C.; Luciano,, F.B.; Mañes, J.; Meca, G. Development of an antifungal and antimycotoxigenic device containing allyl isothiocyanate for silo fumigation. Toxins 2019, 11, 137. [Google Scholar] [CrossRef] [Green Version]
- Suhr, K.I.; Nielsen, P.V. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J. Appl. Microbiol. 2003, 94, 665–674. [Google Scholar] [CrossRef]
- Quiles, J.M.; Manyes, L.; Luciano, F.; Mañes, J.; Meca, G. Influence of the antimicrobial compound allyl isothiocyanate against the Aspergillus parasiticus growth and its aflatoxins production in pizza crust. Food Chem. Toxicol. 2015, 83, 222–228. [Google Scholar] [CrossRef]
- Peromingo, B.; Rodríguez, M.; Delgado, J.; Andrade, M.J.; Rodríguez, A. Gene expression as a good indicator of aflatoxin contamination in dry-cured ham. Food Microbiol. 2017, 67, 31–40. [Google Scholar] [CrossRef]
- Nazareth, T.D.M.; Alonso-Garrido, M.; Stanciu, O.; Mañes, J.; Manyes, L.; Meca, G. Effect of allyl isothiocyanate on transcriptional profile, aflatoxin synthesis, and Aspergillus flavus growth. Food Res. Int. 2020, 128. [Google Scholar] [CrossRef]
- García-Pascual, P.; Mateos, M.; Carbonell, V.; Salazar, D.M. Influence of storage conditions on the quality of shelled and roasted almonds. Biosyst. Eng. 2003, 84, 201–209. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Approved Standard. CLSI document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Espinel-Ingroff, A.; Cantón, E.; Pemán, J. Antifungal susceptibility testing of filamentous fungi. Curr. Fungal Infect. Rep. 2012, 6, 41–50. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Raeisi, S. Evaluation of antifungal and antioxidant properties of edible coating based on apricot (Prunus armeniaca) gum containing Satureja intermedia extract in fresh wild almond (Amygdalus scoparia) kernels. J. Food Meas. Charact. 2018, 12, 362–369. [Google Scholar] [CrossRef]
- Huang, B.; Han, Z.; Cai, Z.; Wu, Y.; Ren, Y. Simultaneous determination of aflatoxins B1, B2, G1, G2, M1 and M2 in peanuts and their derivative products by ultra-high-performance liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2010, 662, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.M.; Saladino, F.; Mañes, J.; Fernández-Franzón, M.; Meca, G. Occurrence of mycotoxins in refrigerated pizza dough and risk assessment of exposure for the Spanish population. Food Chem. Toxicol. 2016, 94, 19–24. [Google Scholar] [CrossRef] [PubMed]
| Minimum Inhibitory Concentration/Minimum Fungicidal Concentration | ||||
|---|---|---|---|---|
| Fungi | Compounds | |||
| AITC (mg/L) | YMF-E (mg/L) | |||
| MIC | MFC | MIC | MFC | |
| Aspergillus flavus | 7.90 | 31.61 | 390 | 3130 |
| Mycelial Growth (mm of Diameter) after Fumigation by H-OMF | |||
|---|---|---|---|
| Treatment | Days | ||
| 3 | 5 | 7 | |
| Control | 25 ± 1.2 A | 50 ± 2.5 A | 50 ± 5.5 A |
| 30 mg/L | ND | ND | ND |
| 25 mg/L | 5 ± 1.3 B | 25 ± 3.4 B | 37 ± 5.1 B |
| 12.5 mg/L | 9 ± 0.8 C | 34 ± 2.2 C | 38 ± 4.2 B |
| 6.2 mg/L | 9 ± 1.1 C | 48 ± 1.6 A | 50 ± 6.1 A |
| Fungal Population in log CFU/g (Mean ± SD) | ||||
|---|---|---|---|---|
| Treatment | Concentration | Days | ||
| 0 | 7 | 15 | ||
| Control | - | 5.11 ± 0.26 A | 9.24 ± 0.34 A | 10.52 ± 0.08 A |
| AITC | 5.07 mg/L | 5.10 ± 0.16 A | ≤1.22 ± 0.00 B | ≤1.22 ± 0.00 B |
| 10.13 mg/L | 5.10 ± 0.11 A | ≤1.22 ± 0.00 B | ≤1.22 ± 0.00 B | |
| 20.26 mg/L | 5.70 ± 0.51 A | ≤1.22 ± 0.00 B | ≤1.22 ± 0.00 B | |
| H-OMF | 2000 mg/L | 5.90 ± 0.20 A | ≤1.22 ± 0.00 B | ≤1.22 ± 0.00 B |
| 4000 mg/L | 5.80 ± 0.34 A | ≤1.22 ± 0.00 B | ≤1.22 ± 0.00 B | |
| YMF-E | 100 g/L | 5.60 ± 0.27 A | 8.91 ± 0.29 A | 10.31 ± 0.04 A |
| 160 g/L | 5.40 ± 0.53 A | 9.96 ± 0.10 A | 10.68 ± 0.16 A | |
| 200 g/L | 5.70 ± 0.21 A | 8.96 ± 0.17 A | 10.15 ± 0.12 A | |
| The Concentration of AFB1 in µg/kg (Mean ± SD) | |||
|---|---|---|---|
| Treatment | Concentration | Days | |
| 7 | 15 | ||
| Control | - | 36.22 ± 4.21 A | 71.67 ± 2.84 A |
| AITC | 5.07 mg/L | ≤LOD C | ≤LOD C |
| 10.13 mg/L | ≤LOD C | ≤LOD C | |
| 20.26 mg/L | ≤LOD C | ≤LOD C | |
| H-OMF | 2000 mg/L | ≤LOD C | ≤LOD C |
| 4000 mg/L | ≤LOD C | ≤LOD C | |
| YMF-E | 100 g/L | 267.38 ± 43.09 B | 276.43 ± 186.83 B |
| 160 g/L | 314.13 ± 131.49 B | 364.41 ± 77.78 B | |
| 200 g/L | 395.21 ± 143.14 B | 575.90 ± 169.61 B | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazareth, T.M.; Torrijos, R.; Bocate, K.P.; Mañes, J.; Luciano, F.B.; Meca, G.; Vila-Donat, P. Development of an Antifungal Device Based on Oriental Mustard Flour to Prevent Fungal Growth and Aflatoxin B1 Production in Almonds. Toxins 2022, 14, 5. https://doi.org/10.3390/toxins14010005
Nazareth TM, Torrijos R, Bocate KP, Mañes J, Luciano FB, Meca G, Vila-Donat P. Development of an Antifungal Device Based on Oriental Mustard Flour to Prevent Fungal Growth and Aflatoxin B1 Production in Almonds. Toxins. 2022; 14(1):5. https://doi.org/10.3390/toxins14010005
Chicago/Turabian StyleNazareth, Tiago Melo, Raquel Torrijos, Karla Paiva Bocate, Jordi Mañes, Fernando Bittencourt Luciano, Giuseppe Meca, and Pilar Vila-Donat. 2022. "Development of an Antifungal Device Based on Oriental Mustard Flour to Prevent Fungal Growth and Aflatoxin B1 Production in Almonds" Toxins 14, no. 1: 5. https://doi.org/10.3390/toxins14010005
APA StyleNazareth, T. M., Torrijos, R., Bocate, K. P., Mañes, J., Luciano, F. B., Meca, G., & Vila-Donat, P. (2022). Development of an Antifungal Device Based on Oriental Mustard Flour to Prevent Fungal Growth and Aflatoxin B1 Production in Almonds. Toxins, 14(1), 5. https://doi.org/10.3390/toxins14010005