Helicobacter pylori CagA EPIYA Motif Variations Affect Metabolic Activity in B Cells
Abstract
:1. Introduction
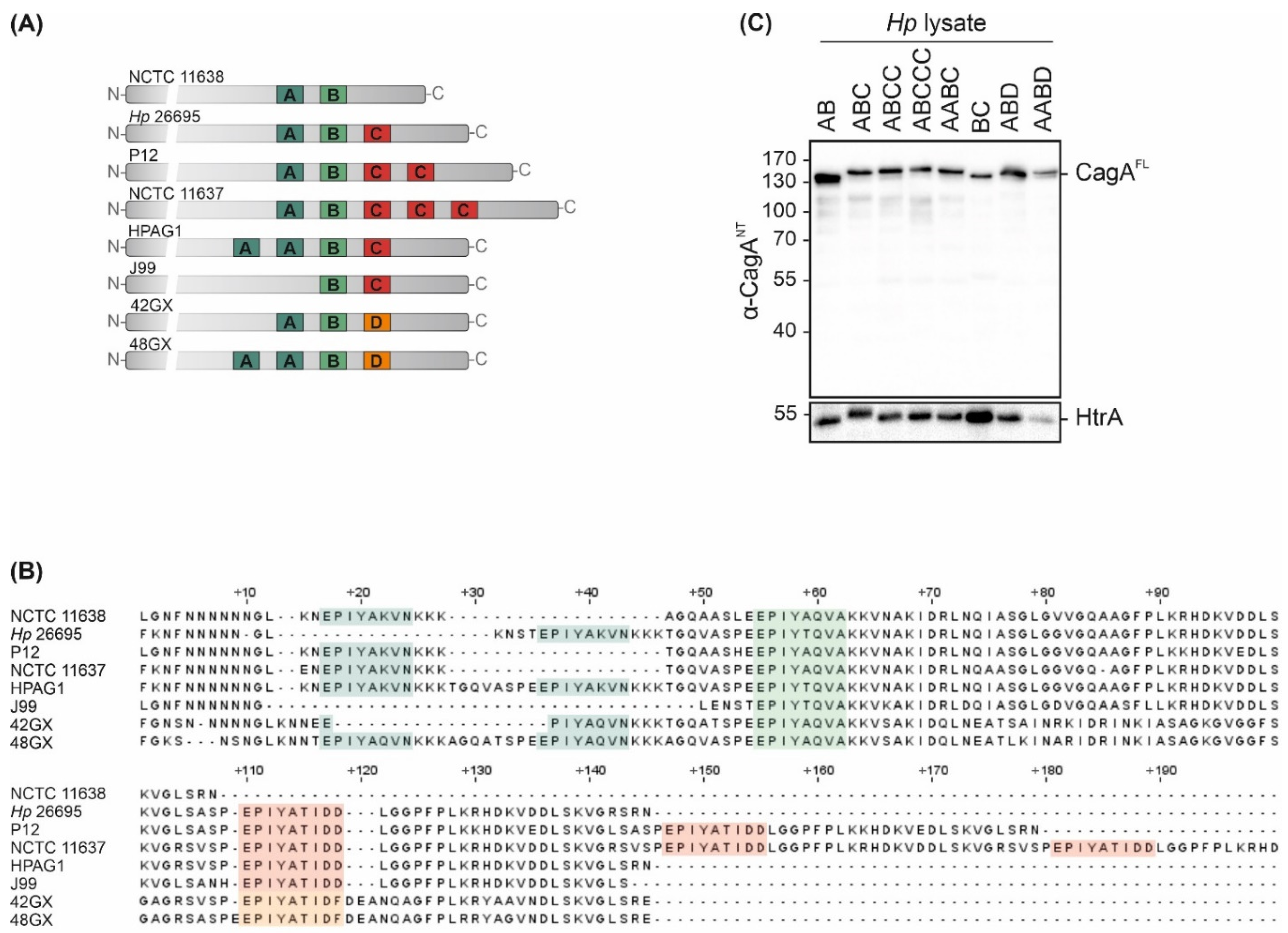
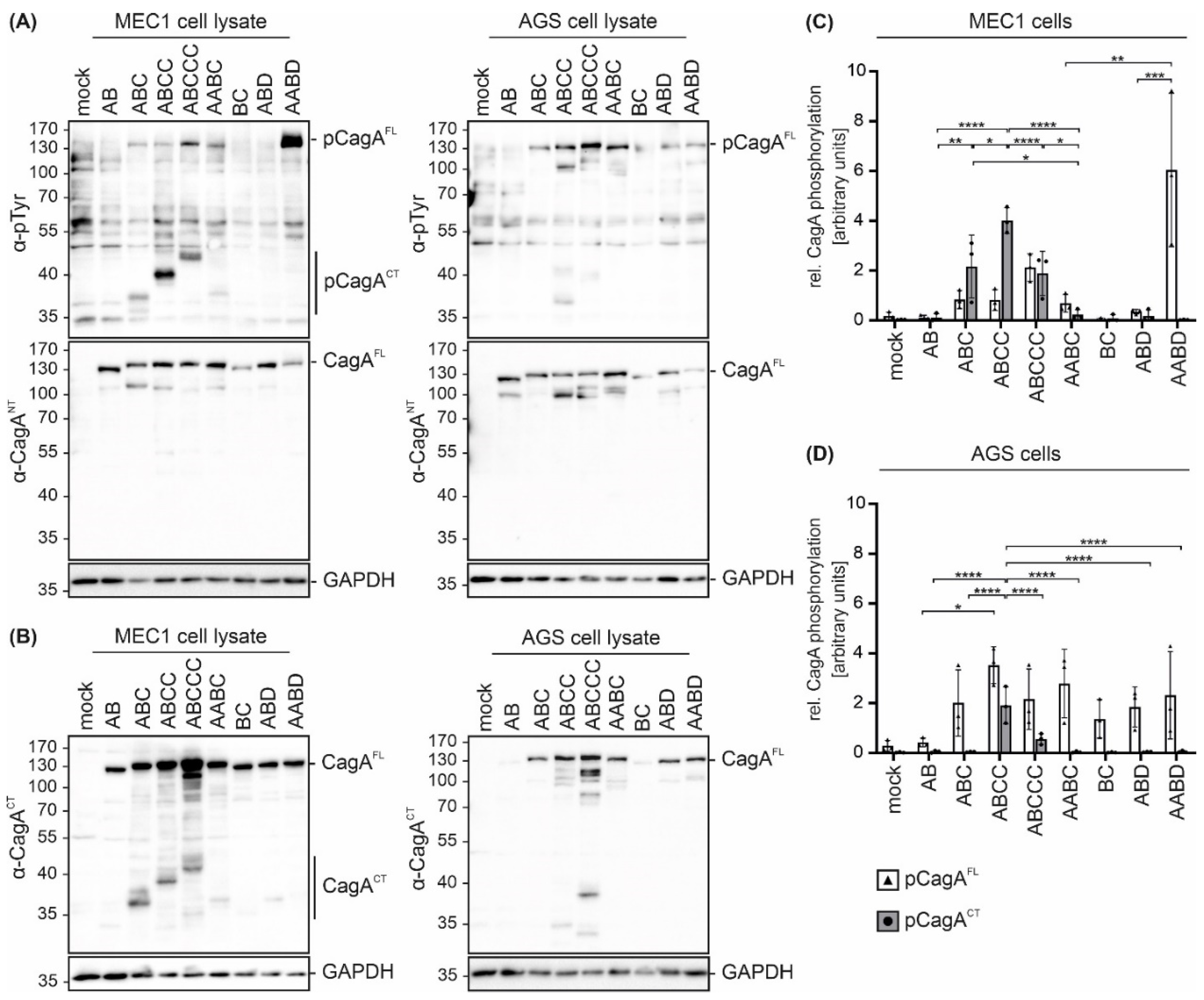
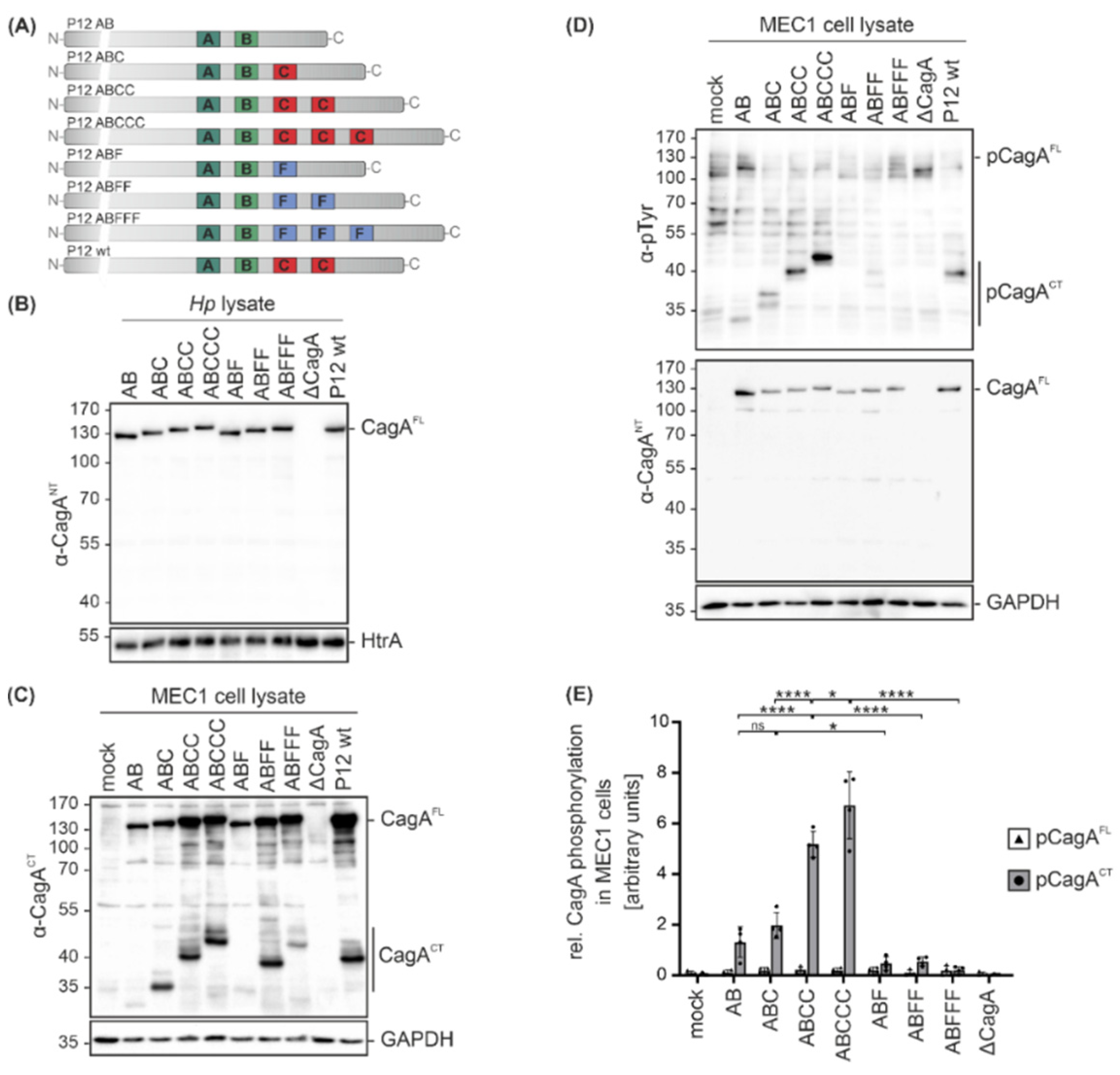
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacteria
5.2. Sequencing
5.3. Infection Experiments
5.4. Immunoblotting
5.5. Apoptosis Assays and Metabolic Activity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amieva, M.; Peek, R.M., Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef] [Green Version]
- Tegtmeyer, N.; Neddermann, M.; Asche, C.I.; Backert, S. Subversion of host kinases: A key network in cellular signaling hijacked by Helicobacter pylori CagA. Mol. Microbiol. 2017, 105, 358–372. [Google Scholar] [CrossRef] [Green Version]
- Eck, M.; Schmausser, B.; Haas, R.; Greiner, A.; Czub, S.; Müller-Hermelink, H.K. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology 1997, 112, 1482–1486. [Google Scholar] [CrossRef]
- Tsai, H.F.; Hsu, P.N. Interplay between Helicobacter pylori and immune cells in immune pathogenesis of gastric inflammation and mucosal pathology. Cell Mol. Immunol. 2010, 7, 255–259. [Google Scholar] [CrossRef] [Green Version]
- Hoy, B.; Löwer, M.; Weydig, C.; Carra, G.; Tegtmeyer, N.; Geppert, T.; Schröder, P.; Sewald, N.; Backert, S.; Schneider, G.; et al. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 2010, 11, 798–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tegtmeyer, N.; Wessler, S.; Necchi, V.; Rohde, M.; Harrer, A.; Rau, T.T.; Asche, C.I.; Boehm, M.; Loessner, H.; Figueiredo, C.; et al. Helicobacter pylori Employs a Unique Basolateral Type IV Secretion Mechanism for CagA Delivery. Cell Host Microbe 2017, 22, 552–560.e555. [Google Scholar] [CrossRef] [Green Version]
- Necchi, V.; Candusso, M.E.; Tava, F.; Luinetti, O.; Ventura, U.; Fiocca, R.; Ricci, V.; Solcia, E. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology 2007, 132, 1009–1023. [Google Scholar] [CrossRef]
- Lin, W.C.; Tsai, H.F.; Kuo, S.H.; Wu, M.S.; Lin, C.W.; Hsu, P.I.; Cheng, A.L.; Hsu, P.N. Translocation of Helicobacter pylori CagA into Human B lymphocytes, the origin of mucosa-associated lymphoid tissue lymphoma. Cancer Res. 2010, 70, 5740–5748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatakeyama, M. Helicobacter pylori CagA and gastric cancer: A paradigm for hit-and-run carcinogenesis. Cell Host Microbe 2014, 15, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Mueller, D.; Tegtmeyer, N.; Brandt, S.; Yamaoka, Y.; De Poire, E.; Sgouras, D.; Wessler, S.; Torres, J.; Smolka, A.; Backert, S. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J. Clin. Investig. 2012, 122, 1553–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backert, S.; Tegtmeyer, N. Type IV Secretion and Signal Transduction of Helicobacter pylori CagA through Interactions with Host Cell Receptors. Toxins 2017, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Selbach, M.; Paul, F.E.; Brandt, S.; Guye, P.; Daumke, O.; Backert, S.; Dehio, C.; Mann, M. Host cell interactome of tyrosine-phosphorylated bacterial proteins. Cell Host Microbe 2009, 5, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Papadakos, K.S.; Sougleri, I.S.; Mentis, A.F.; Sgouras, D.N. A mutagenesis method for the addition and deletion of highly repetitive DNA regions: The paradigm of EPIYA motifs in the cagA gene of Helicobacter pylori. Helicobacter 2013, 18, 229–241. [Google Scholar] [CrossRef]
- Batista, S.A.; Rocha, G.A.; Rocha, A.M.; Saraiva, I.E.; Cabral, M.M.; Oliveira, R.C.; Queiroz, D.M. Higher number of Helicobacter pylori CagA EPIYA C phosphorylation sites increases the risk of gastric cancer, but not duodenal ulcer. BMC Microbiol. 2011, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Chichirau, B.E.; Scheidt, T.; Diechler, S.; Neuper, T.; Horejs-Hoeck, J.; Huber, C.G.; Posselt, G.; Wessler, S. Dissecting the Helicobacter pylori-regulated transcriptome of B cells. Pathog. Dis. 2020, 78. [Google Scholar] [CrossRef]
- Krisch, L.M.; Posselt, G.; Hammerl, P.; Wessler, S. CagA Phosphorylation in Helicobacter pylori-Infected B Cells Is Mediated by the Nonreceptor Tyrosine Kinases of the Src and Abl Families. Infect. Immun. 2016, 84, 2671–2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Court, M.; Jeremy, A.H.T.; Aboshkiwa, M.A.; Robinson, P.A.; Crabtree, J.E. Infection of Mongolian gerbils with Chinese Helicobacter pylori strains. FEMS Immunol. Med. Microbiol. 2003, 36, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Backert, S.; Moese, S.; Selbach, M.; Brinkmann, V.; Meyer, T.F. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol. Microbiol. 2001, 42, 631–644. [Google Scholar] [CrossRef]
- Poppe, M.; Feller, S.M.; Römer, G.; Wessler, S. Phosphorylation of Helicobacter pylori CagA by c-Abl leads to cell motility. Oncogene 2007, 26, 3462–3472. [Google Scholar] [CrossRef] [Green Version]
- Moese, S.; Selbach, M.; Zimny-Arndt, U.; Jungblut, P.R.; Meyer, T.F.; Backert, S. Identification of a tyrosine-phosphorylated 35 kDa carboxy-terminal fragment (p35CagA) of the Helicobacter pylori CagA protein in phagocytic cells: Processing or breakage? Proteomics 2001, 1, 618–629. [Google Scholar] [CrossRef]
- Odenbreit, S.; Gebert, B.; Püls, J.; Fischer, W.; Haas, R. Interaction of Helicobacter pylori with professional phagocytes: Role of the cag pathogenicity island and translocation, phosphorylation and processing of CagA. Cell Microbiol. 2001, 3, 21–31. [Google Scholar] [CrossRef]
- Papadakos, K.S.; Sougleri, I.S.; Mentis, A.F.; Hatziloukas, E.; Sgouras, D.N. Presence of terminal EPIYA phosphorylation motifs in Helicobacter pylori CagA contributes to IL-8 secretion, irrespective of the number of repeats. PLoS ONE 2013, 8, e56291. [Google Scholar] [CrossRef]
- Umehara, S.; Higashi, H.; Ohnishi, N.; Asaka, M.; Hatakeyama, M. Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes, the origin of MALT lymphoma. Oncogene 2003, 22, 8337–8342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomb, J.F.; White, O.; Kerlavage, A.R.; Clayton, R.A.; Sutton, G.G.; Fleischmann, R.D.; Ketchum, K.A.; Klenk, H.P.; Gill, S.; Dougherty, B.A.; et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 1997, 388, 539–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, W.; Haas, R. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: Structural similarities with the IgA protease type of exported protein. Mol. Microbiol. 1994, 12, 307–319. [Google Scholar] [CrossRef]
- Enroth, H.; Kraaz, W.; Engstrand, L.; Nyrén, O.; Rohan, T. Helicobacter pylori strain types and risk of gastric cancer: A case-control study. Cancer Epidemiol. Biomark. Prev. 2000, 9, 981–985. [Google Scholar]
- Alm, R.A.; Ling, L.S.; Moir, D.T.; King, B.L.; Brown, E.D.; Doig, P.C.; Smith, D.R.; Noonan, B.; Guild, B.C.; de Jonge, B.L.; et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 1999, 397, 176–180. [Google Scholar] [CrossRef]
- Abfalter, C.M.; Schubert, M.; Götz, C.; Schmidt, T.P.; Posselt, G.; Wessler, S. HtrA-mediated E-cadherin cleavage is limited to DegP and DegQ homologs expressed by gram-negative pathogens. Cell Commun. Signal. 2016, 14, 30. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diechler, S.; Chichirau, B.E.; Posselt, G.; Sgouras, D.N.; Wessler, S. Helicobacter pylori CagA EPIYA Motif Variations Affect Metabolic Activity in B Cells. Toxins 2021, 13, 592. https://doi.org/10.3390/toxins13090592
Diechler S, Chichirau BE, Posselt G, Sgouras DN, Wessler S. Helicobacter pylori CagA EPIYA Motif Variations Affect Metabolic Activity in B Cells. Toxins. 2021; 13(9):592. https://doi.org/10.3390/toxins13090592
Chicago/Turabian StyleDiechler, Sebastian, Bianca E. Chichirau, Gernot Posselt, Dionyssios N. Sgouras, and Silja Wessler. 2021. "Helicobacter pylori CagA EPIYA Motif Variations Affect Metabolic Activity in B Cells" Toxins 13, no. 9: 592. https://doi.org/10.3390/toxins13090592
APA StyleDiechler, S., Chichirau, B. E., Posselt, G., Sgouras, D. N., & Wessler, S. (2021). Helicobacter pylori CagA EPIYA Motif Variations Affect Metabolic Activity in B Cells. Toxins, 13(9), 592. https://doi.org/10.3390/toxins13090592






