Biological and Biochemical Characterization of Coronado Island Rattlesnake (Crotalus helleri caliginis) Venom and Antivenom Neutralization
Abstract
:1. Introduction
2. Results
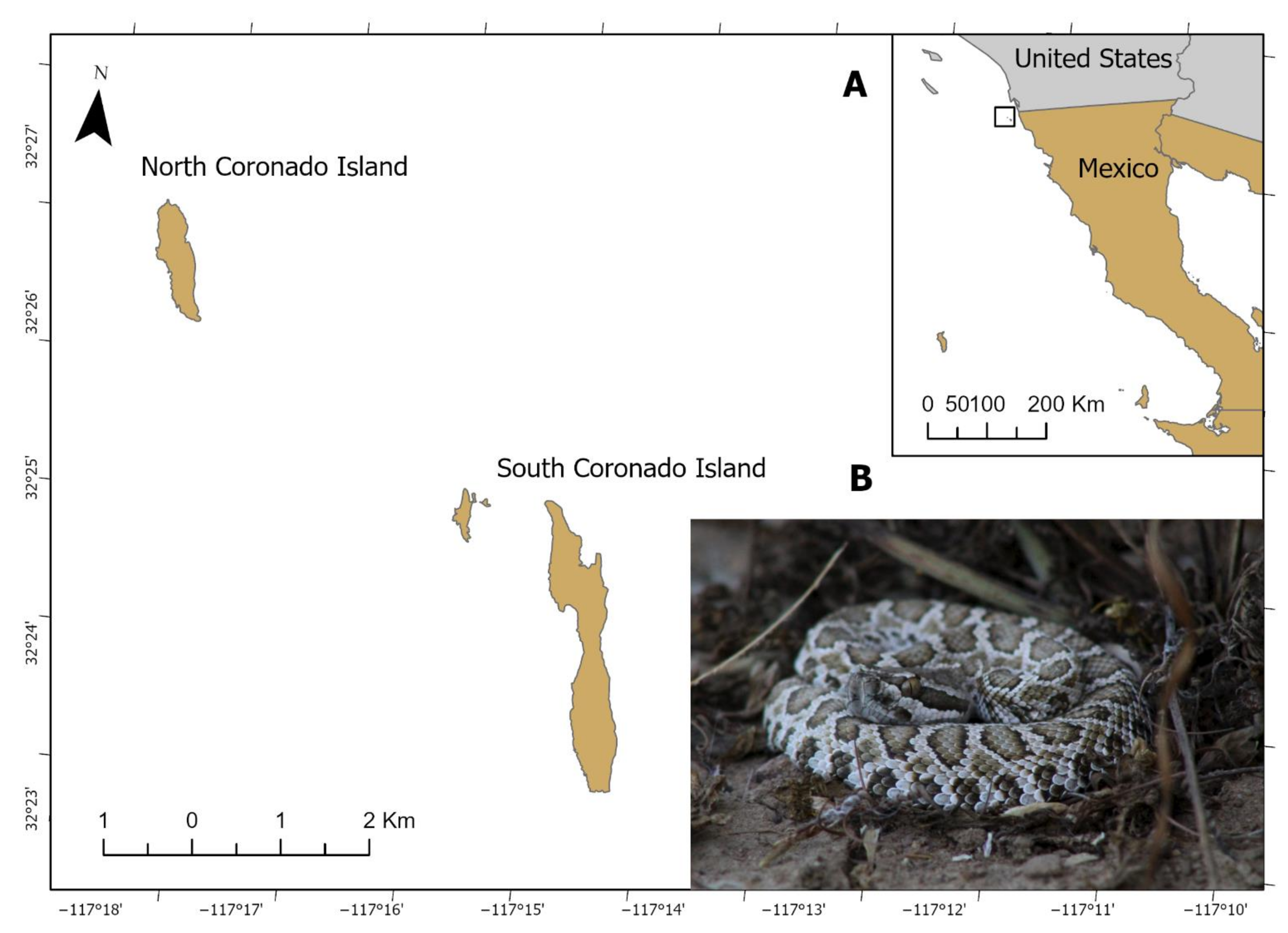
2.1. Crotalus helleri caliginis Samples
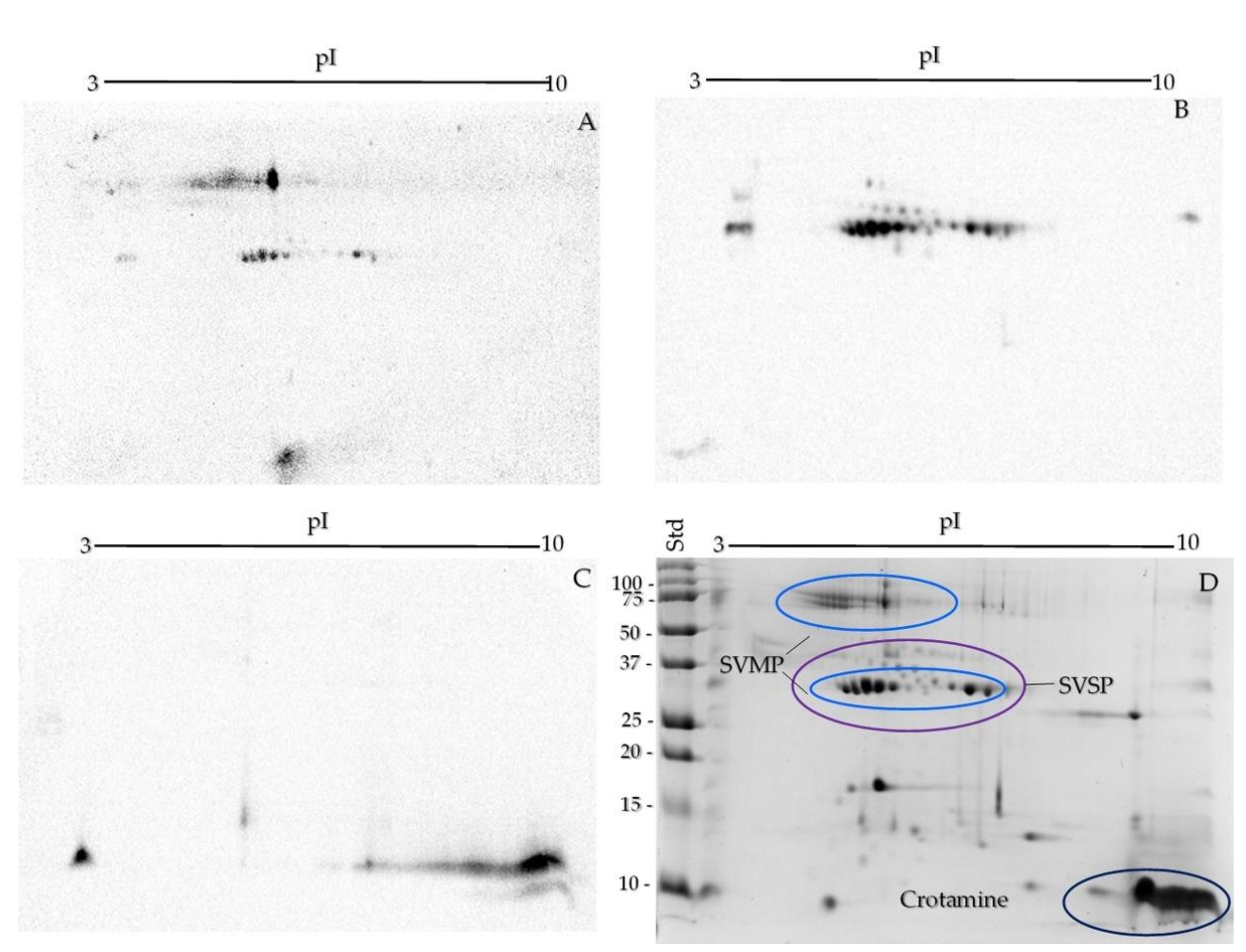
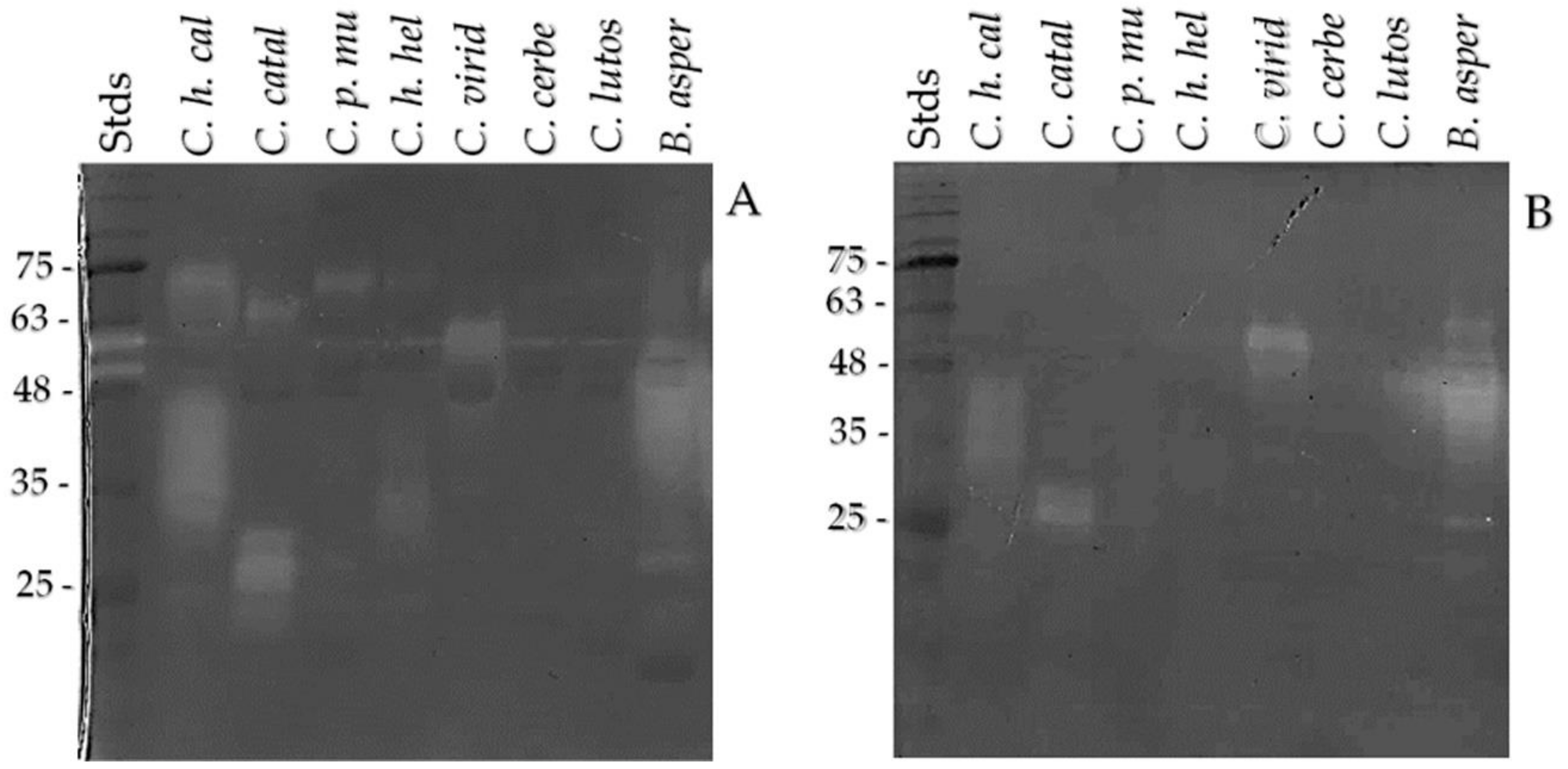
2.2. One- and Two-Dimensional SDS-PAGE
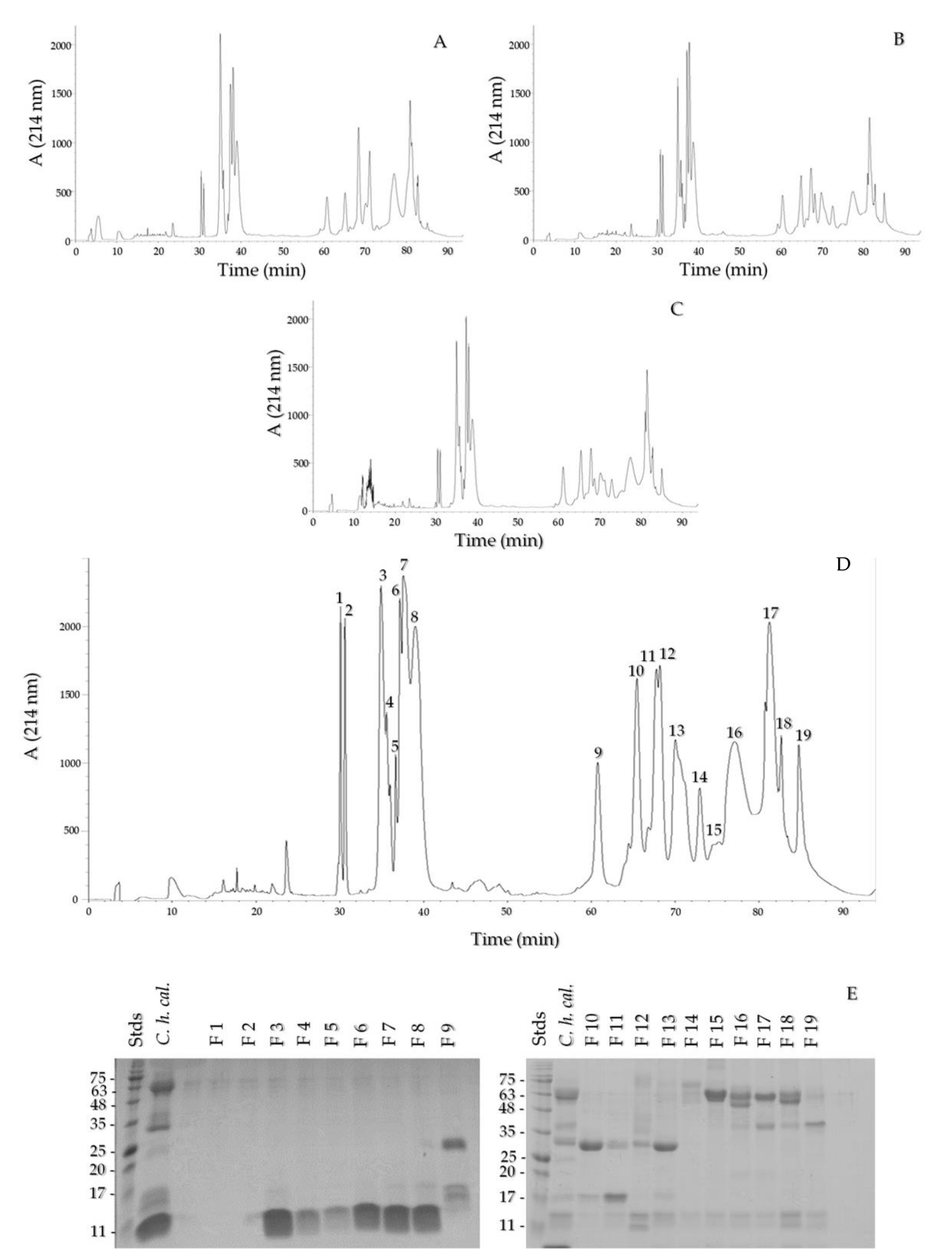
2.3. Reverse Phase HPLC
2.4. SVMP, SVSP, and Crotamine Detection by Western Blot
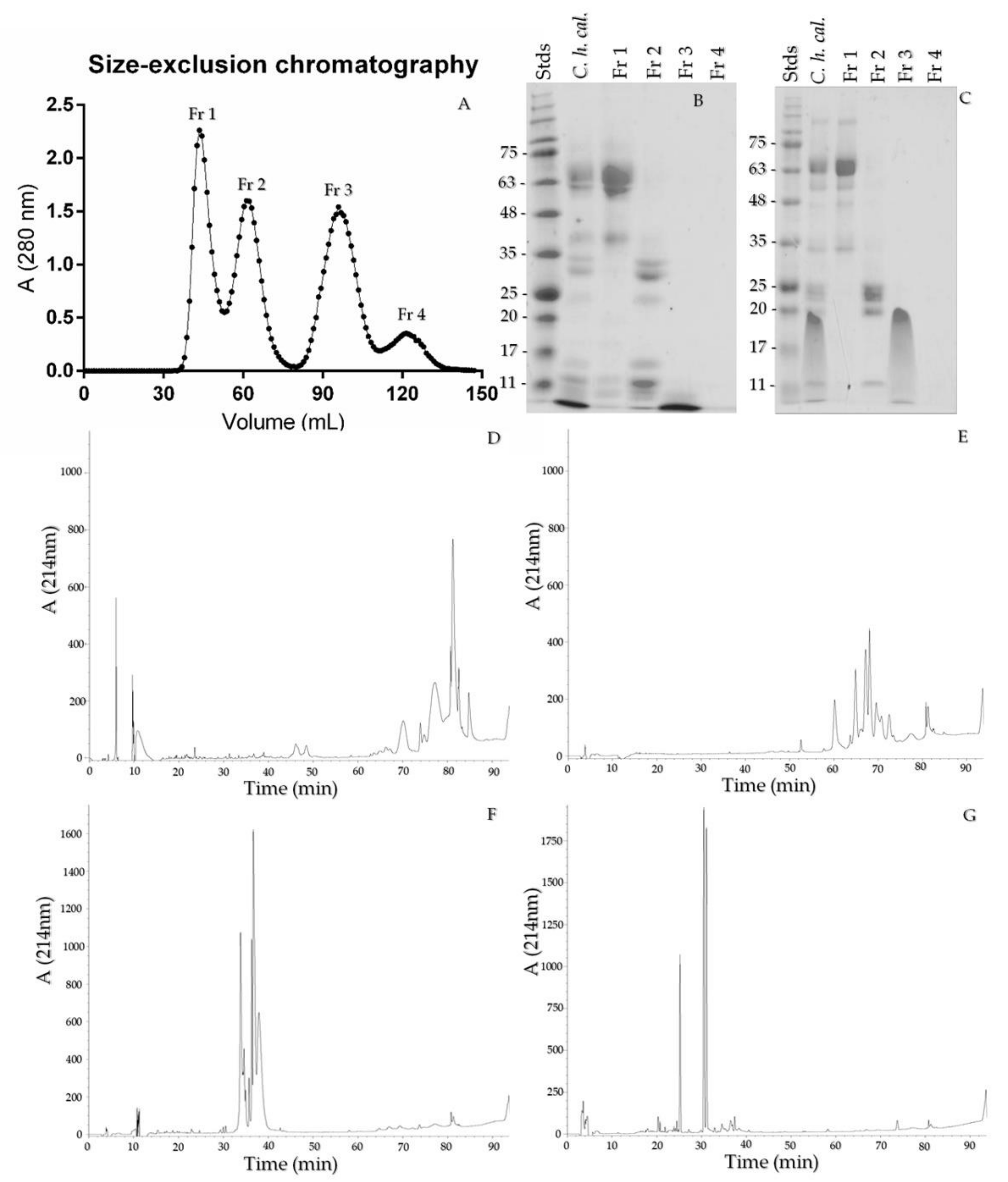
2.5. Size-Exclusion Chromatography and Crotamine Purification
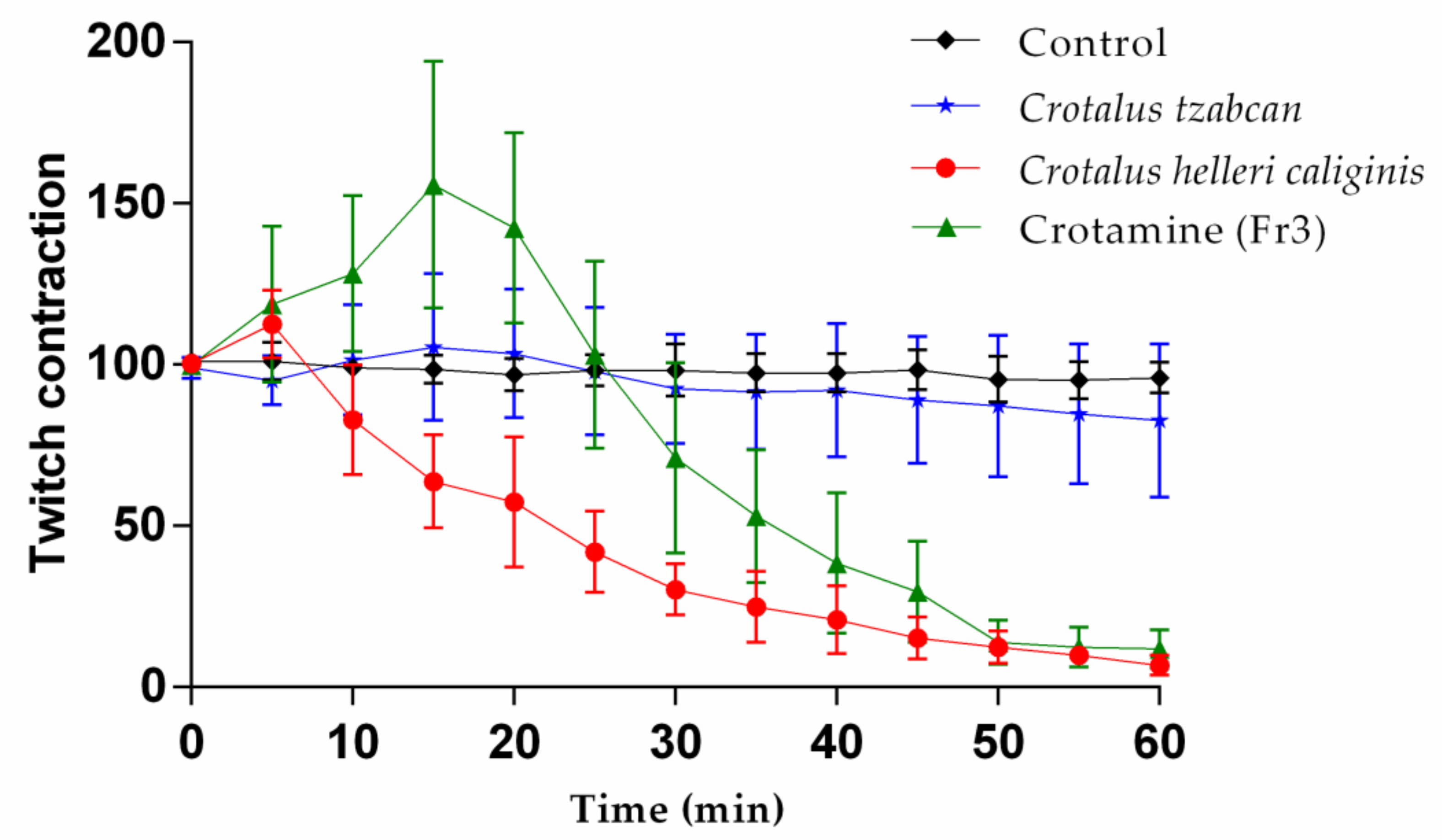
2.6. Mouse Phrenic Nerve–Hemidiaphragm Preparation
2.7. Biological, Biochemical, and Lethal Activity of C. helleri caliginis Venom
2.8. Zymogram on SDS-PAGE Copolymerized with Gelatin
2.9. Neutralization Capacity of Mexican Commercial Antivenoms
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethics Statement
5.2. Venoms
5.3. Protein Quantification
5.4. One-Dimensional SDS-PAGE
5.5. Two-Dimensional SDS-PAGE
5.6. Western Blot
5.7. Reverse Phase HPLC
5.8. Molecular Mass and N-Terminal Sequence Determination of Crotamine-Like Myotoxins
5.9. Size-Exclusion Chromatography and Crotamine Purification
5.10. Mouse Phrenic Nerve–Hemidiaphragm Neuromuscular Preparation
5.11. Median Lethal Dose (LD50)
5.12. PLA2 Activity
5.13. Proteolytic Activity
5.14. Fibrinolytic Activity
5.15. Detection of Crotoxin by ELISA
5.16. Zymograms
5.17. Antivenom Neutralization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uetz, P.; Freed, P.; Hosek, J. The Reptile Database. Available online: http://www.reptile-database.org/ (accessed on 26 April 2020).
- Vitt, L.J.; Caldwell, J.P. Squamates-Snakes. In Herpetology: An Introductory Biology of Amphibians and Reptiles, 4th ed.; Elsevier: London, UK, 2014; pp. 597–628. [Google Scholar]
- Mackessy, S.P. The field of reptile toxinology, snakes, lizards, and their venoms. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2010; pp. 3–24. [Google Scholar]
- Grismer, L.L. Amphibians and Reptiles of Baja California, including Its Pacific Islands, and the Islands in the Sea of Cortés; University of California Press: Los Angeles, CA, USA, 2002; pp. 257–342. [Google Scholar]
- Meik, J.M.; Schaack, S.; Flores-Villela, O.; Streicher, J.W. Integrative taxonomy at the nexus of population divergence and speciation in insular speckled rattlesnakes. J. Nat. Hist. 2018, 52, 989–1016. [Google Scholar] [CrossRef]
- Sedlock, R.L. Geology and tectonics of the Baja California Peninsula and adjacent areas. Geol. Soc. Am. Spec. Pap. 2003, 374, 1–42. [Google Scholar]
- SEMARNAT. Programa de Acción para la Conservación de las Especies: Serpientes de Cascabel (Crotalus spp.); SEMARNAT/CONABIO: Ciudad de México, México, 2018; pp. 19–116. [Google Scholar]
- Kuper, H.T.; Hart, M.W. Natural History of the Coronado Islands, Baja California, Mexico; San Diego Association of Geologists: San Diego, CA, USA, 1978; pp. 7–53. [Google Scholar]
- Klauber, L.M. Some new and revived subspecies of rattlesnakes; San Diego Society of Natural History: San Diego, CA, USA, 1949; Volume 11, pp. 61–116. [Google Scholar] [CrossRef]
- Pook, C.E.; Wüster, W.; Thorpe, R.S. Historical biogeography of the western rattlesnake (Serpentes: Viperidae: Crotalus viridis), inferred from mitochondrial DNA sequence information. Mol. Phylogenet. Evol. 2000, 15, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Douglas, M.E.; Douglas, M.R.; Schuett, G.W.; Porras, L.W.; Holycross, A.T. Phylogeography of the western rattlesnake (Crotalus viridis) complex, with emphasis on the Colorado Plateau. In Biology of the Vipers; Schuett, G.W., Hoggern, M., Douglas, M.E., Greene, H.W., Eds.; Eagle Mountain Publishing: Eagle Mountain, UT, USA, 2002; pp. 11–50. [Google Scholar]
- Ashton, K.G.; de Queiroz, A. Molecular systematics of the western rattlesnake, Crotalus viridis (Viperidae), with comments on the utility of the D-loop in phylogenetic studies of snakes. Mol. Phylogenet. Evol. 2001, 21, 176–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.A.; Douglas, M.R.; Collyer, M.L.; Douglas, M.E. Deconstructing a species-complex: Geometric morphometric and molecular analyses define species in the western rattlesnake (Crotalus viridis). PLoS ONE 2016, 11, e0146166. [Google Scholar]
- Grismer, L.L. An evolutionary classification and checklist of amphibians and reptiles on the Pacific Islands of Baja California, Mexico. Bull. South. Calif. Acad. Sci. 2001, 100, 12. [Google Scholar]
- Nasim, F.; Das, S.; Mishra, R.; Mishra, R. Hematological alterations and splenic T lymphocyte polarization at the crest of snake venom induced acute kidney injury in adult male mice. Toxicon 2017, 134, 57–63. [Google Scholar] [CrossRef]
- Jiménez, N.; Escalante, T.; Gutiérrez, J.M.; Rucavado, A. Skin pathology induced by snake venom metalloproteinase: Acute damage, revascularization, and re-epithelization in a mouse ear model. J. Investig. Dermatol. 2008, 128, 2421–2428. [Google Scholar] [CrossRef]
- Herrera, C.; Macêdo, J.K.A.; Feoli, A.; Escalante, T.; Rucavado, A.; Gutiérrez, J.M.; Fox, J.W. Muscle tissue damage induced by the venom of Bothrops asper: Identification of early and late pathological events through proteomic analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004599. [Google Scholar] [CrossRef] [Green Version]
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [Green Version]
- Mackessy, S. Venom Composition in Rattlesnakes: Trends and Biological Significance. In The Biology of Rattlesnakes; Hayes, W.K., Beaman, K.R., Cardwell, M.D., Bush, S.P., Eds.; Loma Linda University Press: Loma Linda, CA, USA, 2008; pp. 495–510. [Google Scholar]
- French, W.J.; Hayes, W.K.; Bush, S.P.; Cardwell, M.D.; Bader, J.O.; Rael, E.D. Mojave toxin in venom of Crotalus helleri (Southern Pacific Rattlesnake): Molecular and geographic characterization. Toxicon 2004, 44, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Viala, V.L.; Hildebrand, D.; Fucase, T.M.; Sciani, J.M.; Prezotto-Neto, J.P.; Riedner, M.; Sanches, L.; Nishimura, P.J.; Oguiura, N.; Pimenta, D.C.; et al. Proteomic analysis of the rare Uracoan rattlesnake Crotalus vegrandis venom: Evidence of a broad arsenal of toxins. Toxicon 2015, 107, 234–251. [Google Scholar] [CrossRef] [Green Version]
- Madey, J.J.; Price, A.B.; Dobson, J.V.; Stickler, D.E.; McSwain, S.D. Facial diplegia, pharyngeal paralysis, and ophthalmoplegia after a timber rattlesnake envenomation. Pediatr. Emerg. Care 2013, 29, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, W.L.G.; Noronha-Matos, J.B.; Timóteo, M.A.; Fontes, M.R.M.; Gallacci, M.; Correia-de-Sá, P. Neuromuscular paralysis by the basic phospholipase A2 subunit of crotoxin from Crotalus durissus terrificus snake venom needs its acid chaperone to concurrently inhibit acetylcholine release and produce muscle blockage. Toxicol. Appl. Pharmacol. 2017, 334, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Borja, M.; Neri-Castro, E.; Castañeda-Gaytán, G.; Strickland, J.L.; Parkinson, C.L.; Castañeda-Gaytán, J.; Ponce-López, R.; Lomonte, B.; Olvera-Rodríguez, A.; Alagón, A.; et al. Biological and proteolytic variation in the venom of Crotalus scutulatus scutulatus from Mexico. Toxins 2018, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Calvete, J.J.; Pérez, A.; Lomonte, B.; Sánchez, E.E.; Sanz, L. Snake venomics of Crotalus tigris: The minimalist toxin arsenal of the deadliest neartic rattlesnake venom. Evolutionary clues for generating a pan-specific antivenom against crotalid type II venoms. J. Proteome Res. 2012, 11, 1382–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zancolli, G.; Baker, T.G.; Barlow, A.; Bradley, R.K.; Calvete, J.J.; Carter, K.C.; De Jager, K.; Owens, J.B.; Price, J.F.; Sanz, L.; et al. Is hybridization a source of adaptive venom variation in rattlesnakes? A test, using a Crotalus scutulatus × viridis hybrid zone in Southwestern New Mexico. Toxins 2016, 8, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, E.N.; Lomonte, B.; del Carmen Gutiérrez, M.; Alagón, A.; Gutiérrez, J.M. Intraspecies variation in the venom of the rattlesnake Crotalus simus from Mexico: Different expression of crotoxin results in highly variable toxicity in the venoms of three subspecies. J. Proteomics 2013, 87, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.B.; Scott-Davey, T. Secreted phospholipases A2 of snake venoms: Effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins 2013, 5, 2533–2571. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Clemetson, J.M.; Clemetson, K.J. Snake venoms and hemostasis. J. Thromb. Haemost. 2005, 3, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Borja, M.; Neri-Castro, E.; Pérez-Morales, R.; Strickland, J.; Ponce-López, R.; Parkinson, C.; Espinosa-Fematt, J.; Sáenz-Mata, J.; Flores-Martínez, E.; Alagón, A.; et al. Ontogenetic Change in the Venom of Mexican Black-Tailed Rattlesnakes (Crotalus molossus nigrescens). Toxins 2018, 10, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowsky, E.R.; Mackessy, S.P. Predator-prey interactions and venom composition in a high elevation lizard specialist, Crotalus pricei (Twin-spotted Rattlesnake). Toxicon 2019, 170, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P.; Williams, K.; Ashton, K.G. Ontogenetic variation in venom composition and diet of Crotalus oreganus concolor: A case of venom paedomorphosis? Copeia 2003, 2003, 769–782. [Google Scholar] [CrossRef]
- Mackessy, S.P. Venom ontogeny in the pacific rattlesnakes Crotalus viridis helleri and C. v. oreganus. Copeia 1988, 1998, 92. [Google Scholar] [CrossRef]
- Saviola, A.J.; Pla, D.; Sanz, L.; Castoe, T.A.; Calvete, J.J.; Mackessy, S.P. Comparative venomics of the prairie rattlesnake (Crotalus viridis viridis) from Colorado: Identification of a novel pattern of ontogenetic changes in venom composition and assessment of the immunoreactivity of the commercial antivenom CroFab®. J. Proteom. 2015, 121, 28–43. [Google Scholar] [CrossRef]
- Mackessy, S.P.; Leroy, J.; Mociño-Deloya, E.; Setser, K.; Bryson, R.W.; Saviola, A.J. Venom ontogeny in the mexican lance-headed rattlesnake (Crotalus polystictus). Toxins 2018, 10, 271. [Google Scholar] [CrossRef] [Green Version]
- Holding, M.L.; Biardi, J.E.; Gibbs, H.L. Coevolution of venom function and venom resistance in a rattlesnake predator and its squirrel prey. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152841. [Google Scholar] [CrossRef]
- Smiley-Walters, S.A.; Farrell, T.M.; Gibbs, H.L. Evaluating local adaptation of a complex phenotype: Reciprocal tests of pigmy rattlesnake venoms on treefrog prey. Oecologia 2017, 184, 739–748. [Google Scholar] [CrossRef]
- Holding, M.L.; Strickland, J.L.; Rautsaw, R.M.; Hofmann, E.P.; Mason, A.J.; Hogan, M.P.; Nystrom, G.S.; Ellsworth, S.A.; Colston, T.J.; Borja, M.; et al. Phylogenetically diverse diets favor more complex venoms in North American pitvipers. Proc. Natl. Acad. Sci. USA 2021, 118, e2015579118. [Google Scholar] [CrossRef]
- Neri-Castro, E.; Bénard-Valle, M.; Gil, G.; Borja, M.; de León, J.L.; Alagón, A. Venomous snakes in Mexico: A review of the study of venoms, antivenom and epidemiology. Rev. Latinoam. Herpetol. 2020, 3, 5–22. [Google Scholar]
- Glenn, J.L.; Straight, R.C. Venom properties of the rattlesnakes (Crotalus) inhabiting the Baja California region of Mexico. Toxicon 1985, 23, 769–775. [Google Scholar] [CrossRef]
- Arnaud-Franco, G.; Cordero-Tapia, A.; Ortíz-Ávila, V.; Moctezuma-González, C.L.; Tejocote-Pérez, M.; Carbajal-Saucedo, A. Comparison of biological and biochemical characteristics of venom from rattlesnakes in the southern Baja California Peninsula. Toxicon 2018, 148, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P. Evolutionary trends in venom composition in the western rattlesnakes (Crotalus viridis sensu lato): Toxicity vs. tenderizers. Toxicon 2010, 55, 1463–1474. [Google Scholar] [CrossRef]
- Fiero, M.K.; Seifert, M.W.; Weaver, T.J.; Bonilla, C.A. Comparative study of juvenile and adult prairie rattlesnake (Crotalus viridis viridis) venoms. Toxicon 1972, 10, 81–82. [Google Scholar] [CrossRef]
- Maeda, N.; Tamiya, N.; Pattabhiraman, T.R.; Russell, F.E. Some chemical properties of the venom of the rattlesnake, Crotalus viridis helleri. Toxicon 1978, 16, 431–441. [Google Scholar] [CrossRef]
- Neri-Castro, E.; Bénard-Valle, M.; Paniagua, D.; Boyer, L.V.; Possani, L.D.; López-Casillas, F.; Olvera, A.; Romero, C.; Zamudio, F.; Alagón, A. Neotropical Rattlesnake (Crotalus simus) venom pharmacokinetics in lymph and blood using an ovine model. Toxins 2020, 12, 455. [Google Scholar] [CrossRef]
- Ponce López, J.R. Evaluación del Nivel de Reconocimiento y Potencia Neutralizante del Suero de Conejos Hiperinmunizados con el Veneno de Crotalus simus. Bachelor’s Thesis, Instituto de biotecnología, UNAM, Cuernavaca, Morelos, Mexico, February 2018. [Google Scholar]
- Margres, M.J.; Patton, A.; Wray, K.P.; Hassinger, A.T.B.; Ward, M.J.; Lemmon, E.M.; Lemmon, A.R.; Rokyta, D.R. Tipping the scales: The migration–selection balance leans toward selection in snake venoms. Mol. Biol. Evol. 2019, 36, 271–282. [Google Scholar] [CrossRef]
- Jurado, J.D.; Rael, E.D.; Lieb, C.S.; Nakayasu, E.; Hayes, W.K.; Bush, S.P.; Ross, J.A. Complement inactivating proteins and intraspecies venom variation in Crotalus oreganus helleri. Toxicon 2007, 49, 339–350. [Google Scholar] [CrossRef]
- Chang, C.C.; Hong, S.J.; Su, M.J. A study on the membrane depolarization of skeletal muscles caused by a scorpion toxin, sea anemone toxin II and crotamine and the interaction between toxins. Br. J. Pharmacol. 1983, 79, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Ponce-Soto, L.A.; Martins-de-Souza, D.; Marangoni, S. Structural and pharmacological characterization of the crotamine isoforms III-4 (MYX4_CROCu) and III-7 (MYX7_CROCu) isolated from the Crotalus durissus cumanensis venom. Toxicon 2010, 55, 1443–1452. [Google Scholar] [CrossRef]
- Smith, C.F.; Mackessy, S.P. The effects of hybridization on divergent venom phenotypes: Characterization of venom from Crotalus scutulatus scutulatus × Crotalus oreganus helleri hybrids. Toxicon 2016, 120, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Toyama, M.H.; Carneiro, E.M.; Marangoni, S.; Barbosa, R.L.; Corso, G.; Boschero, A.C. Biochemical characterization of two crotamine isoforms isolated by a single step RP-HPLC from Crotalus durissus terrificus (South American Rattlesnake) venom and their action on insulin secretion by pancreatic islets. Biochim. Biophys. Acta Gen. Subj. 2000, 1474, 56–60. [Google Scholar] [CrossRef]
- Chang, C.C.; Tseng, K.H. Effect of crotamine, a toxin of south american rattlesnake venom, on the sodium channel of murine skeletal muscle. Br. J. Pharmacol. 1978, 63, 551–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, S.d.C.; Porta, L.d.C.; Lima, Á.d.C.; Campeiro, J.D.; Meurer, Y.; Teixeira, N.B.; Duarte, T.; Oliveira, E.B.; Picolo, G.; Godinho, R.O.; et al. Pharmacological characterization of crotamine effects on mice hind limb paralysis employing both ex vivo and in vivo assays: Insights into the involvement of voltage-gated ion channels in the crotamine action on skeletal muscles. PLoS Negl. Trop. Dis. 2018, 12, e0006700. [Google Scholar] [CrossRef] [Green Version]
- Brazil, O.V.; Prado-Franceschi, J.; Laure, C.J. Repetitive muscle responses induced by crotamine. Toxicon 1979, 17, 61–67. [Google Scholar] [CrossRef]
- Giglio, J. Analytical studies on crotamine hydrochloride. Anal. Biochem. 1975, 69, 207–221. [Google Scholar] [CrossRef]
- Glenn, J.L.; Straight, R.C. Intergradation of two different venom populations of the Mojave rattlesnake (Crotalus scutulatus scutulatus) in Arizona. Toxicon 1989, 27, 411–418. [Google Scholar] [CrossRef]
- Dowell, N.L.; Giorgianni, M.W.; Griffin, S.; Kassner, V.A.; Selegue, J.E.; Sanchez, E.E.; Carroll, S.B. Extremely Divergent Haplotypes in Two Toxin Gene Complexes Encode Alternative Venom Types within Rattlesnake Species. Curr. Biol. 2018, 28, 1016–1026. [Google Scholar] [CrossRef] [Green Version]
- Sunagar, K.; Undheim, E.A.B.; Scheib, H.; Gren, E.C.K.; Cochran, C.; Person, C.E.; Koludarov, I.; Kelln, W.; Hayes, W.K.; King, G.F.; et al. Intraspecific venom variation in the medically significant Southern Pacific Rattlesnake (Crotalus oreganus helleri): Biodiscovery, clinical and evolutionary implications. J. Proteom. 2014, 99, 68–83. [Google Scholar] [CrossRef]
- Bastos, V.; Gomes-Neto, F.; Perales, J.; Neves-Ferreira, A.; Valente, R. Natural inhibitors of snake venom metalloendopeptidases: History and current challenges. Toxins 2016, 8, 250. [Google Scholar] [CrossRef] [Green Version]
- Durban, J.; Sanz, L.; Trevisan-Silva, D.; Neri-Castro, E.; Alagón, A.; Calvete, J.J. Integrated venomics and venom gland transcriptome analysis of juvenile and adult mexican rattlesnakes Crotalus simus, C. tzabcan, and C. culminatus revealed miRNA-modulated ontogenetic shifts. J. Proteome Res. 2017, 16, 3370–3390. [Google Scholar] [CrossRef]
- Strickland, J.L.; Smith, C.F.; Mason, A.J.; Schield, D.R.; Borja, M.; Castañeda-Gaytán, G.; Spencer, C.L.; Smith, L.L.; Trápaga, A.; Bouzid, N.M.; et al. Evidence for divergent patterns of local selection driving venom variation in Mojave Rattlesnakes (Crotalus scutulatus). Sci. Rep. 2018, 8, 17622. [Google Scholar] [CrossRef] [PubMed]
- Ponce-López, R.; Neri-Castro, E.; Borja, M.; Strickland, J.L.; Alagón, A. Neutralizing potency and immunochemical evaluation of an anti-Crotalus mictlantecuhtli experimental serum. Toxicon 2020, 187, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Román-Domínguez, L.; Neri-Castro, E.; Vázquez López, H.; García-Osorio, B.; Archundia, I.G.; Ortiz-Medina, J.A.; Petricevich, V.L.; Alagón, A.; Bénard-Valle, M. Biochemical and immunochemical characterization of venoms from snakes of the genus Agkistrodon. Toxicon X 2019, 4, 100013. [Google Scholar] [CrossRef] [PubMed]
- Bulbring, E. Observations on the isolated phrenic nerve diaphragm preparation of the rat. Br. J. Pharmacol. Chemother. 1946, 1, 38–61. [Google Scholar] [CrossRef] [PubMed]
- Neri-Castro, E.; Lomonte, B.; Valdés, M.; Ponce-López, R.; Bénard-Valle, M.; Borja, M.; Strickland, J.L.; Jones, J.M.; Grünwald, C.; Zamudio, F.; et al. Venom characterization of the three species of Ophryacus and proteomic profiling of O. sphenophrys unveils Sphenotoxin, a novel Crotoxin-like heterodimeric β-neurotoxin. J. Proteom. 2019, 192, 196–207. [Google Scholar] [CrossRef]
- Shiloan, J.; Klibansky, C.; de Vries, A.; Berger, A. Phospholipase B activity of a purified phospholipase A from Vipera palestinae venom. J. Lipid Res. 1973, 14, 267–278. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Sanz, L.; Escolano, J.; Fernández, J.; Lomonte, B.; Angulo, Y.; Rucavado, A.; Warrell, D.A.; Calvete, J.J. Snake venomics of the lesser antillean pit vipers Bothrops caribbaeus and Bothrops lanceolatus: Correlation with toxicological activities and immunoreactivity of a heterologous antivenom. J. Proteome Res. 2008, 7, 4396–4408. [Google Scholar] [CrossRef]
- Rivas, E.; Neri-Castro, E.; Bénard-Valle, M.; Hernánez-Dávila, A.I.; Zamudio, F.; Alagón, A. General characterization of the venoms from two species of rattlesnakes and an intergrade population (C. lepidus × aquilus) from Aguascalientes and Zacatecas, Mexico. Toxicon 2017, 138, 191–195. [Google Scholar] [CrossRef]
- Neri-Castro, E.; Hernández-Dávila, A.; Olvera-Rodríguez, A.; Cardoso-Torres, H.; Bénard-Valle, M.; Bastiaans, E.; López-Gutierrez, O.; Alagón, A. Detection and quantification of a β-neurotoxin (crotoxin homologs) in the venom of the rattlesnakes Crotalus simus, C. culminatus and C. tzabcan from Mexico. Toxicon 2019, 2, 100007. [Google Scholar] [CrossRef] [PubMed]

| Species | Geographic Location | LD50 (µg/g) | PLA2 (U/mg) * | Proteolysis (U/mg) * | Fibrinolytic Activity ** | Crotoxin Detection | ||
|---|---|---|---|---|---|---|---|---|
| Rat | Mouse | Chicken | ||||||
| C. helleri caliginis | South Coronado Island | 0.54 (0.5–0.6) | 0.58 (0.5–0.65) | 0.62 (0.55–0.7) | 28 ± 3 | 0.53 ± 0.06 | α | Negative |
| C. helleri helleri | Mainland | *** | 0.26 | *** | 32 ± 2.5 | 0.94 ± 0.05 | α, β | Positive |
| C. catalinensis | Santa Catalina Island | *** | 1.47 (1.4–1.5) | *** | 145 ± 14 | 1.4 ± 0.07 | α, β | Negative |
| C. pyrrhus muertensis | El Muerto Island | *** | 1.16 (1.0–1.3) | *** | 165 ± 12.5 | 1.98 ± 0.16 | α, β | Negative |
| Antivenom | Batch | ED50 (µLAV/3LD50) | mgAV/mgV | LD50/Vial |
|---|---|---|---|---|
| Antivipmyn® | B-8K-31 | 40 (25.8–55.6) * | 6.4 | 789 |
| Faboterapico polivalente antiviperino® | FV044A | 34.3 (27.8–40.9) * | 29 | 875 |
| Inoserp® | 8805181002 | 92.4 (90.6–94.3) * | 21.7 | 324 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-Servín, C.; Neri-Castro, E.; Bénard-Valle, M.; Alagón, A.; Rosales-García, R.A.; Guerrero-Alba, R.; Poblano-Sánchez, J.E.; Silva-Briano, M.; Guerrero-Barrera, A.L.; Sigala-Rodríguez, J.J. Biological and Biochemical Characterization of Coronado Island Rattlesnake (Crotalus helleri caliginis) Venom and Antivenom Neutralization. Toxins 2021, 13, 582. https://doi.org/10.3390/toxins13080582
Franco-Servín C, Neri-Castro E, Bénard-Valle M, Alagón A, Rosales-García RA, Guerrero-Alba R, Poblano-Sánchez JE, Silva-Briano M, Guerrero-Barrera AL, Sigala-Rodríguez JJ. Biological and Biochemical Characterization of Coronado Island Rattlesnake (Crotalus helleri caliginis) Venom and Antivenom Neutralization. Toxins. 2021; 13(8):582. https://doi.org/10.3390/toxins13080582
Chicago/Turabian StyleFranco-Servín, Cristian, Edgar Neri-Castro, Melisa Bénard-Valle, Alejandro Alagón, Ramsés Alejandro Rosales-García, Raquel Guerrero-Alba, José Emanuel Poblano-Sánchez, Marcelo Silva-Briano, Alma Lilián Guerrero-Barrera, and José Jesús Sigala-Rodríguez. 2021. "Biological and Biochemical Characterization of Coronado Island Rattlesnake (Crotalus helleri caliginis) Venom and Antivenom Neutralization" Toxins 13, no. 8: 582. https://doi.org/10.3390/toxins13080582
APA StyleFranco-Servín, C., Neri-Castro, E., Bénard-Valle, M., Alagón, A., Rosales-García, R. A., Guerrero-Alba, R., Poblano-Sánchez, J. E., Silva-Briano, M., Guerrero-Barrera, A. L., & Sigala-Rodríguez, J. J. (2021). Biological and Biochemical Characterization of Coronado Island Rattlesnake (Crotalus helleri caliginis) Venom and Antivenom Neutralization. Toxins, 13(8), 582. https://doi.org/10.3390/toxins13080582







