The Therapeutic Strategies for Uremic Toxins Control in Chronic Kidney Disease
Abstract
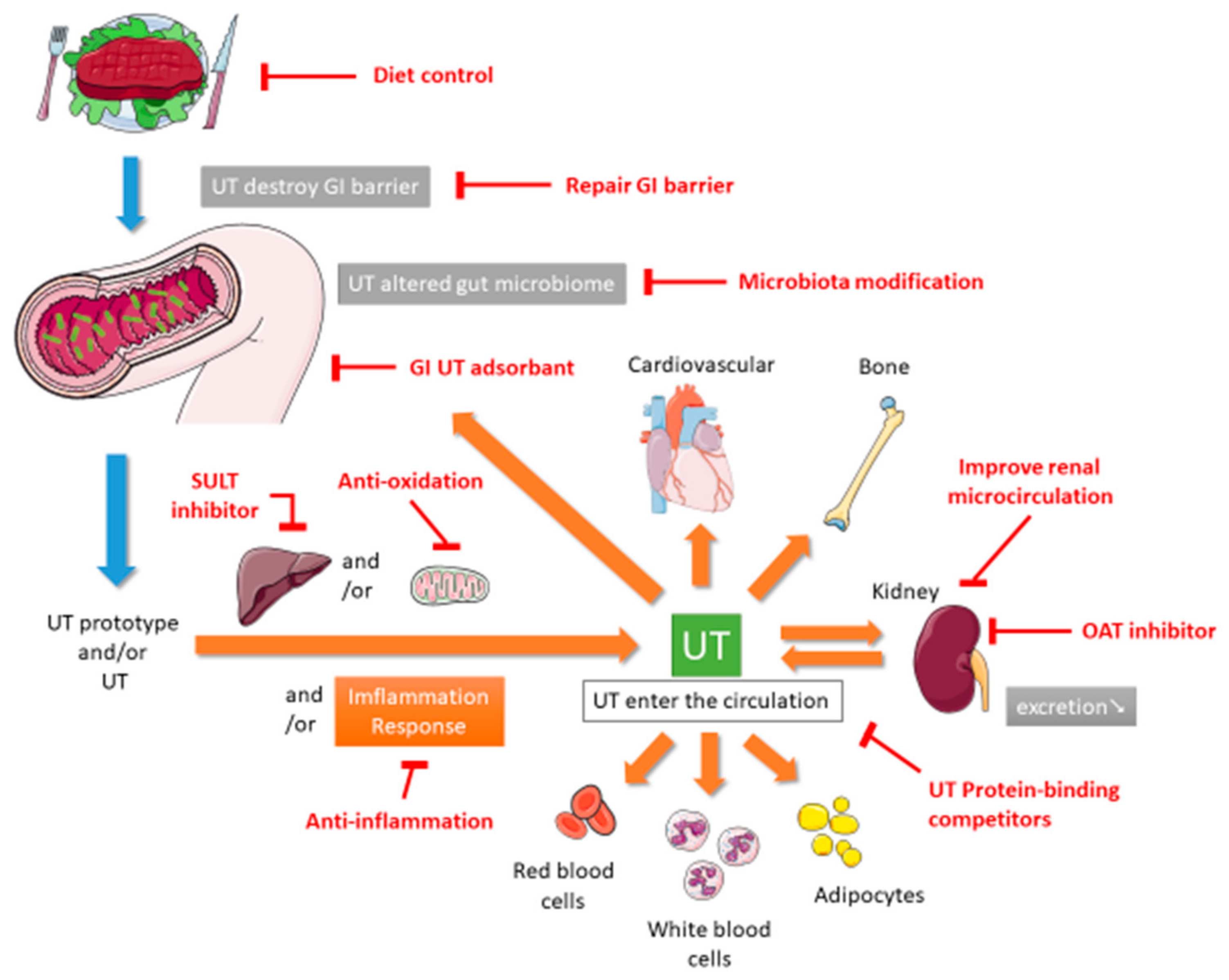
1. Introduction
2. Conventional Medication Therapy
2.1. Acarbose
2.2. AST-120
2.3. L-Carnitine
2.4. Cilastatin
2.5. Cyclosporine A
2.6. Enalapril
2.7. Folate and Methylcobalamin
2.8. Ketoacids
2.9. Meclofenamate
2.10. Reduced Glutathione
3. Diet Control and Diet Supplements
3.1. Diet® k/d®
3.2. Lingonberry
3.3. Mitoquinone
3.4. Prebiotic Oligofructose-Enriched Inulin
3.5. Probiotics
3.6. Short-Chain Fatty Acids
3.7. Soluble Fiber and Omega-3 Fatty Acids
3.8. Synbiotic
3.9. Vegetarian Diet
3.10. Vitamin D
4. Complementary and Alternative Medicine Therapy
4.1. Curcuma Longa and Boswellia Serrata
4.2. Dahuang Fuzi Decoction
4.3. Danhong Injection and Salvianolic Acids
4.4. Uremic Clearance Granule
4.5. Zhibai Dihuang Wan
4.6. Catechin Combined with Vitamin C and Vitamin E
4.7. Cyanidin-3-O-Glucoside
4.8. Epigallocatechin-3-Gallate
4.9. Gypenoside
4.10. Huangkui Capsule
4.11. Leonurine
4.12. Ligustrazine
4.13. Notoginsenoside R1
4.14. Osthole
4.15. Paeoniflorin
4.16. Resveratrol
4.17. Rhubarb
4.18. 10-(6′-Plastoquinonyl) Decylrhodamine 19
4.19. Tanshinone I
4.20. Acupuncture
4.21. Moxibustion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of chronic kidney disease in the United States. JAMA 2007, 298, 2038–2047. [Google Scholar] [CrossRef]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.; Bhave, N.; Bragg-Gresham, J.; Balkrishnan, R.; Dietrich, X.; Eckard, A.; Eggers, P.W. US renal data system 2017 annual data report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2018, 71, A7. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef]
- Hung, S.C.; Kuo, K.L.; Huang, H.L.; Lin, C.C.; Tsai, T.H.; Wang, C.H.; Chen, J.W.; Lin, S.J.; Huang, P.H.; Tarng, D.C. Indoxyl sulfate suppresses endothelial progenitor cell-mediated neovascularization. Kidney Int. 2016, 89, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Kazama, J.J.; Yamato, H.; Shimoda, H.; Fukagawa, M. Accumulated uremic toxins attenuate bone mechanical properties in rats with chronic kidney disease. Bone 2013, 57, 477–483. [Google Scholar] [CrossRef]
- Soulage, C.O.; Koppe, L.; Fouque, D. Protein-bound uremic toxins… new targets to prevent insulin resistance and dysmetabolism in patients with chronic kidney disease. J. Ren. Nutr. 2013, 23, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Savoj, J.; Nakata, M.B.; Vaziri, N.D. Altered microbiome in chronic kidney disease: Systemic effects of gut-derived uremic toxins. Clin. Sci. 2018, 132, 509–522. [Google Scholar] [CrossRef]
- Lu, P.-H.; Tai, Y.-C.; Yu, M.-C.; Lin, I.-H.; Kuo, K.-L. Western and complementary alternative medicine treatment of uremic pruritus: A literature review. Tzu Chi Med. J. 2021. [Google Scholar] [CrossRef]
- Kuo, K.-L.; Zhao, J.-F.; Huang, P.-H.; Guo, B.-C.; Tarng, D.-C.; Lee, T.-S. Indoxyl sulfate impairs valsartan-induced neovascularization. Redox Biol. 2020, 30, 101433. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Glorieux, G.; De Smet, R.; Lameire, N.; European Uremic Toxin Work Group. New insights in uremic toxins. Kidney Int. Suppl. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Rodríguez, E.; Pizarro-Sánchez, S.; Sanz, A.; Ramos, A.; Sanchez-Niño, M.; Martin-Cleary, C.; Fernandez-Fernandez, B.; Ortiz, A. Inflammatory Cytokines as Uremic Toxins: “Ni Son Todos Los Que Estan, Ni Estan Todos Los Que Son”. Toxins 2017, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.C.; Eloot, S.; Glorieux, G.L. Future Avenues to Decrease Uremic Toxin Concentration. Am. J. Kidney Dis. 2016, 67, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Fouque, D.; Soulage, C.O. The Role of Gut Microbiota and Diet on Uremic Retention Solutes Production in the Context of Chronic Kidney Disease. Toxins 2018, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Popkov, V.A.; Silachev, D.N.; Zalevsky, A.O.; Zorov, D.B.; Plotnikov, E.Y. Mitochondria as a Source and a Target for Uremic Toxins. Int. J. Mol. Sci. 2019, 20, 3094. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T. Indoxyl sulfate is a nephro-vascular toxin. J. Ren. Nutr. 2010, 20, S2–S6. [Google Scholar] [CrossRef]
- Saito, H.; Yoshimura, M.; Saigo, C.; Komori, M.; Nomura, Y.; Yamamoto, Y.; Sagata, M.; Wakida, A.; Chuman, E.; Nishi, K.; et al. Hepatic sulfotransferase as a nephropreventing target by suppression of the uremic toxin indoxyl sulfate accumulation in ischemic acute kidney injury. Toxicol. Sci. 2014, 141, 206–217. [Google Scholar] [CrossRef]
- Sumida, K.; Yamagata, K.; Kovesdy, C.P. Constipation in CKD. Kidney Int. Rep. 2020, 5, 121–134. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Y.-B. Intestinal microbiota and chronic constipation. SpringerPlus 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Meijers, B.; Glorieux, G.; Poesen, R.; Bakker, S.J. Nonextracorporeal methods for decreasing uremic solute concentration: A future way to go? Semin. Nephrol. 2014, 34, 228–243. [Google Scholar] [CrossRef]
- Enomoto, A.; Takeda, M.; Taki, K.; Takayama, F.; Noshiro, R.; Niwa, T.; Endou, H. Interactions of human organic anion as well as cation transporters with indoxyl sulfate. Eur. J. Pharmacol. 2003, 466, 13–20. [Google Scholar] [CrossRef]
- Fülöp, T.; Zsom, L.; Tapolyai, M.B.; Molnar, M.Z.; Salim, S.A.; Arany, I.; Hamrahian, M.; Rosivall, L. Peritoneal dialysis: The unique features by compartmental delivery of renal replacement therapy. Med. Hypotheses 2017, 108, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N.; Vanholder, R.; De Smet, R. Uremic toxins and peritoneal dialysis. Kidney Int. Suppl. 2001, 78, S292–S297. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Baurmeister, U.; Brunet, P.; Cohen, G.; Glorieux, G.; Jankowski, J. A bench to bedside view of uremic toxins. J. Am. Soc. Nephrol. 2008, 19, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, S.; Pillot, B.; Rognant, N.; Augeul, L.; Rayberin, M.; Varennes, A.; Laville, M.; Ovize, M.; Juillard, L. Postconditioning with cyclosporine a reduces early renal dysfunction by inhibiting mitochondrial permeability transition. Transplantation 2015, 99, 717–723. [Google Scholar] [CrossRef]
- Koyama, K.; Ito, A.; Yamamoto, J.; Nishio, T.; Kajikuri, J.; Dohi, Y.; Ohte, N.; Sano, A.; Nakamura, H.; Kumagai, H.; et al. Randomized controlled trial of the effect of short-term coadministration of methylcobalamin and folate on serum ADMA concentration in patients receiving long-term hemodialysis. Am. J. Kidney Dis. 2010, 55, 1069–1078. [Google Scholar] [CrossRef]
- Evenepoel, P.; Bammens, B.; Verbeke, K.; Vanrenterghem, Y. Acarbose treatment lowers generation and serum concentrations of the protein-bound solute p-cresol: A pilot study. Kidney Int. 2006, 70, 192–198. [Google Scholar] [CrossRef]
- Goicoechea, M.; de Vinuesa, S.G.; Verdalles, U.; Ruiz-Caro, C.; Ampuero, J.; Rincón, A.; Arroyo, D.; Luño, J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin. J. Am. Soc. Nephrol. 2010, 5, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Saigo, C.; Nomura, Y.; Yamamoto, Y.; Sagata, M.; Matsunaga, R.; Jono, H.; Nishi, K.; Saito, H. Meclofenamate elicits a nephropreventing effect in a rat model of ischemic acute kidney injury by suppressing indoxyl sulfate production and restoring renal organic anion transporters. Drug Des. Dev. Ther. 2014, 8, 1073–1082. [Google Scholar] [CrossRef]
- Garibotto, G.; Sofia, A.; Parodi, E.L.; Ansaldo, F.; Bonanni, A.; Picciotto, D.; Signori, A.; Vettore, M.; Tessari, P.; Verzola, D. Effects of Low-Protein, and Supplemented Very Low-Protein Diets, on Muscle Protein Turnover in Patients With CKD. Kidney Int. Rep. 2018, 3, 701–710. [Google Scholar] [CrossRef]
- Huo, X.; Meng, Q.; Wang, C.; Zhu, Y.; Liu, Z.; Ma, X.; Ma, X.; Peng, J.; Sun, H.; Liu, K. Cilastatin protects against imipenem-induced nephrotoxicity via inhibition of renal organic anion transporters (OATs). Acta Pharm. Sin. B 2019, 9, 986–996. [Google Scholar] [CrossRef]
- Asai, M.; Kumakura, S.; Kikuchi, M. Review of the efficacy of AST-120 (KREMEZIN(®)) on renal function in chronic kidney disease patients. Ren. Fail. 2019, 41, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Wu, M.-Y.; Hu, P.-J.; Chen, T.-T.; Shen, W.-C.; Chang, W.-C.; Wu, M.-S. Effects and safety of an oral adsorbent on chronic kidney disease progression: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 1718. [Google Scholar] [CrossRef]
- Fatouros, I.G.; Douroudos, I.; Panagoutsos, S.; Pasadakis, P.; Nikolaidis, M.G.; Chatzinikolaou, A.; Sovatzidis, A.; Michailidis, Y.; Jamurtas, A.Z.; Mandalidis, D.; et al. Effects of L-carnitine on oxidative stress responses in patients with renal disease. Med. Sci. Sports Exerc. 2010, 42, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, H.; Schiel, A.; Freixas, E.; Díaz, M. Randomized trial of methylcobalamin and folate effects on homocysteine in hemodialysis patients. Nephron 2002, 91, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Dal Piaz, F.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Tartaglia, D.; Autore, G.; Di Iorio, B. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif. 2013, 35, 196–201. [Google Scholar] [CrossRef]
- Wang, Y.F. Analysis on the effects of reduced glutathione intervening in microinflammation of uremia patients with maintenance hemodialysis. Chin. J. Front. Med. Sci. 2016, 8, 101–104. [Google Scholar]
- Sato, E.; Saigusa, D.; Mishima, E.; Uchida, T.; Miura, D.; Morikawa-Ichinose, T.; Kisu, K.; Sekimoto, A.; Saito, R.; Oe, Y.; et al. Impact of the Oral Adsorbent AST-120 on Organ-Specific Accumulation of Uremic Toxins: LC-MS/MS and MS Imaging Techniques. Toxins 2017, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Paskaloglu, K.; Satiroglu, H.; Alican, I.; Kaçmaz, A.; Sakarcan, A. L-Carnitine Ameliorates Oxidative Damage due to Chronic Renal Failure in Rats. J. Cardiovasc. Pharmacol. 2004, 43, 698–705. [Google Scholar] [CrossRef]
- Konop, M.; Radkowski, M.; Grochowska, M.; Perlejewski, K.; Samborowska, E.; Ufnal, M. Enalapril decreases rat plasma concentration of TMAO, a gut bacteria-derived cardiovascular marker. Biomarkers 2018, 23, 380–385. [Google Scholar] [CrossRef]
- Akizawa, T.; Asano, Y.; Morita, S.; Wakita, T.; Onishi, Y.; Fukuhara, S.; Gejyo, F.; Matsuo, S.; Yorioka, N.; Kurokawa, K. Effect of a carbonaceous oral adsorbent on the progression of CKD: A multicenter, randomized, controlled trial. Am. J. Kidney Dis. 2009, 54, 459–467. [Google Scholar] [CrossRef]
- Cha, R.-H.; Kang, S.W.; Park, C.W.; Cha, D.R.; Na, K.Y.; Kim, S.G.; Yoon, S.A.; Han, S.Y.; Chang, J.H.; Park, S.K. A randomized, controlled trial of oral intestinal sorbent AST-120 on renal function deterioration in patients with advanced renal dysfunction. Clin. J. Am. Soc. Nephrol. 2016, 11, 559–567. [Google Scholar] [CrossRef]
- Armaly, Z.; Artol, S.; Jabbour, A.R.; Saffouri, A.; Habashi, N.; Abd Elkadir, A.; Ghattas, N.; Farah, R.; Kinaneh, S.; Nseir, W. Impact of pretreatment with carnitine and tadalafil on contrast-induced nephropathy in CKD patients. Ren. Fail. 2019, 41, 976–986. [Google Scholar] [CrossRef]
- Hornik, C.P.; Herring, A.H.; Benjamin, D.K., Jr.; Capparelli, E.V.; Kearns, G.L.; van den Anker, J.; Cohen-Wolkowiez, M.; Clark, R.H.; Smith, P.B. Adverse events associated with meropenem versus imipenem/cilastatin therapy in a large retrospective cohort of hospitalized infants. Pediatric Infect. Dis. J. 2013, 32, 748. [Google Scholar] [CrossRef]
- Koyama, K.; Usami, T.; Takeuchi, O.; Morozumi, K.; Kimura, G. Efficacy of methylcobalamin on lowering total homocysteine plasma concentrations in haemodialysis patients receiving high-dose folic acid supplementation. Nephrol. Dial. Transplant. 2002, 17, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Martí-Carvajal, A.J.; Solà, I.; Lathyris, D.; Dayer, M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 2017, 8, CD006612. [Google Scholar] [CrossRef]
- Shah, A.P.; Kalantar-Zadeh, K.; Kopple, J.D. Is there a role for ketoacid supplements in the management of CKD? Am. J. Kidney Dis. 2015, 65, 659–673. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.C.; Kuo, K.-L. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Ceballos-Picot, I.; Witko-Sarsat, V.; Merad-Boudia, M.; Nguyen, A.T.; Thévenin, M.; Jaudon, M.C.; Zingraff, J.; Verger, C.; Jingers, P.; Descamps-Latscha, B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic. Biol. Med. 1996, 21, 845–853. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J.; Choi, J. γ-Glutamyl transpeptidase in glutathione biosynthesis. Methods Enzymol. 2005, 401, 468–483. [Google Scholar]
- Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol. 2015, 6, 198–205. [Google Scholar] [CrossRef]
- Snelson, M.; Biruete, A.; McFarlane, C.; Campbell, K. A Renal Clinician’s Guide to the Gut Microbiota. J. Ren. Nutr. 2020, 30, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.R.; Rocchetti, M.T.; De Angelis, M.; Cosola, C.; Marzocco, S.; Di Micco, L.; di Bari, I.; Accetturo, M.; Vacca, M.; Gobbetti, M.; et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J. Clin. Med. 2019, 8, 1424. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yadav, A.K.; Lal, A.; Kumar, V.; Singhal, M.; Billot, L.; Gupta, K.L.; Banerjee, D.; Jha, V. A randomized trial of vitamin D supplementation on vascular function in CKD. J. Am. Soc. Nephrol. 2017, 28, 3100–3108. [Google Scholar] [CrossRef]
- Meijers, B.K.; De Preter, V.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol. Dial. Transplant. 2010, 25, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, N.; Ranganathan, P.; Friedman, E.A.; Joseph, A.; Delano, B.; Goldfarb, D.S.; Tam, P.; Rao, A.V.; Anteyi, E.; Musso, C.G. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv. Ther. 2010, 27, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Taki, K.; Takayama, F.; Niwa, T. Beneficial effects of Bifidobacteria in a gastroresistant seamless capsule on hyperhomocysteinemia in hemodialysis patients. J. Ren. Nutr. 2005, 15, 77–80. [Google Scholar] [CrossRef]
- Marzocco, S.; Fazeli, G.; Di Micco, L.; Autore, G.; Adesso, S.; Dal Piaz, F.; Heidland, A.; Di Iorio, B. Supplementation of Short-Chain Fatty Acid, Sodium Propionate, in Patients on Maintenance Hemodialysis: Beneficial Effects on Inflammatory Parameters and Gut-Derived Uremic Toxins, A Pilot Study (PLAN Study). J. Clin. Med. 2018, 7, 315. [Google Scholar] [CrossRef]
- Nakabayashi, I.; Nakamura, M.; Kawakami, K.; Ohta, T.; Kato, I.; Uchida, K.; Yoshida, M. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: A preliminary study. Nephrol. Dial. Transplant. 2010, 26, 1094–1098. [Google Scholar] [CrossRef]
- Kandouz, S.; Mohamed, A.S.; Zheng, Y.; Sandeman, S.; Davenport, A. Reduced protein bound uraemic toxins in vegetarian kidney failure patients treated by haemodiafiltration. Hemodial. Int. 2016, 20, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Fritsch, D.A.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Jewell, D.E. A longitudinal study on the acceptance and effects of a therapeutic renal food in pet dogs with IRIS-Stage 1 chronic kidney disease. J. Anim. Physiol. Anim. Nutr. 2018, 102, 297–307. [Google Scholar] [CrossRef]
- Madduma Hewage, S.; Prashar, S.; Debnath, S.C.; Karmin, O.; Siow, Y.L. Inhibition of Inflammatory Cytokine Expression Prevents High-Fat Diet-Induced Kidney Injury: Role of Lingonberry Supplementation. Front. Med. 2020, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ren, J.; Li, G.; Wu, J.; Wu, X.; Wang, G.; Gu, G.; Ren, H.; Hong, Z.; Li, J. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis. 2018, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Ephraim, E.; Jackson, M.I.; Yerramilli, M.; Jewell, D.E. Soluble fiber and omega-3 fatty acids reduce levels of advanced glycation end products and uremic toxins in senior dogs by modulating the gut microbiome. J. Food Sci. Nutr. Res. 2020, 3, 18–33. [Google Scholar] [CrossRef]
- Isaak, C.K.; Wang, P.; Prashar, S.; Karmin, O.; Brown, D.C.; Debnath, S.C.; Siow, Y.L. Supplementing diet with Manitoba lingonberry juice reduces kidney ischemia-reperfusion injury. J. Sci. Food Agric. 2017, 97, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Ouchfoun, M.; Brault, A.; Vallerand, D.; Musallam, L.; Arnason, J.T.; Haddad, P.S. Lingonberry (Vaccinium vitis-idaea L.) exhibits antidiabetic activities in a mouse model of diet-induced obesity. Evid. Based Complementary Altern. Med. 2014, 2014, 645812. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Lopez, I.; Diaz-Morales, N.; Rovira-Llopis, S.; de Marañon, A.M.; Orden, S.; Alvarez, A.; Bañuls, C.; Rocha, M.; Murphy, M.P.; Hernandez-Mijares, A. The mitochondria-targeted antioxidant MitoQ modulates oxidative stress, inflammation and leukocyte-endothelium interactions in leukocytes isolated from type 2 diabetic patients. Redox Biol. 2016, 10, 200–205. [Google Scholar] [CrossRef]
- Dare, A.J.; Bolton, E.A.; Pettigrew, G.J.; Bradley, J.A.; Saeb-Parsy, K.; Murphy, M.P. Protection against renal ischemia–reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol. 2015, 5, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Montemurno, E.; Cosola, C.; Dalfino, G.; Daidone, G.; De Angelis, M.; Gobbetti, M.; Gesualdo, L. What would you like to eat, Mr CKD microbiota? A Mediterranean diet, please! Kidney Blood Press. Res. 2014, 39, 114–123. [Google Scholar] [CrossRef]
- Murthy, M.; Venkitanarayan, K.; Rangavajhyala, N.; Shahani, K. Delineation of beneficial characteristics of effective probiotics. JAMA 2000, 3, 38–43. [Google Scholar]
- Lee, Y.-K.; Salminen, S. The coming of age of probiotics. Trends Food Sci. Technol. 1995, 6, 241–245. [Google Scholar] [CrossRef]
- Reddy, B.S. Possible mechanisms by which pro-and prebiotics influence colon carcinogenesis and tumor growth. J. Nutr. 1999, 129, 1478S–1482S. [Google Scholar] [CrossRef]
- Puddu, A.; Sanguineti, R.; Montecucco, F.; Viviani, G.L. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediat. Inflamm. 2014, 2014, 162021. [Google Scholar] [CrossRef] [PubMed]
- Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation. FAO Food Nutr. Pap. 1998, 66, 1–140.
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Pisano, A.; D’Arrigo, G.; Coppolino, G.; Bolignano, D. Biotic Supplements for Renal Patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1224. [Google Scholar] [CrossRef]
- Friedman, A.; Moe, S. Review of the effects of omega-3 supplementation in dialysis patients. Clin. J. Am. Soc. Nephrol. 2006, 1, 182–192. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Uwaezuoke, S.N.; Muoneke, U.V.; Mbanefo, N.R. The supportive treatment of IgA nephropathy and idiopathic nephrotic syndrome: How useful are omega-3 polyunsaturated fatty acids? Int. J. Nephrol. Renov. Dis. 2020, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Bouzidi, N.; Mekki, K.; Boukaddoum, A.; Dida, N.; Kaddous, A.; Bouchenak, M. Effects of omega-3 polyunsaturated fatty-acid supplementation on redox status in chronic renal failure patients with dyslipidemia. J. Ren. Nutr. 2010, 20, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, V.M.R.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef]
- De Vrese, M.; Schrezenmeir, J. Probiotics, prebiotics, and synbiotics. Food Biotechnol. 2008, 111, 1–66. [Google Scholar]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Meirlaen, L.; Levy, E.I.; Vandenplas, Y. Prevention and Management with Pro-, Pre and Synbiotics in Children with Asthma and Allergic Rhinitis: A Narrative Review. Nutrients 2021, 13, 934. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, P.; Koppe, L.; Combe, C.; Lasseur, C.; Trolonge, S.; Aparicio, M. Vegetarian diets and chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Tsai, W.H.; Liu, J.S.; Kuo, K.L. Association of Vegetarian Diet with Chronic Kidney Disease. Nutrients 2019, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Cases, A.; Cigarran-Guldris, S.; Mas, S.; Gonzalez-Parra, E. Vegetable-Based Diets for Chronic Kidney Disease? It is Time to Reconsider. Nutrients 2019, 11, 1263. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Iodice, S.; Zittermann, A.; Grant, W.B.; Gandini, S. Vitamin D status and mortality risk in CKD: A meta-analysis of prospective studies. Am. J. Kidney Dis. 2011, 58, 374–382. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Jiang, C.-M.; Sun, C.; Tang, T.-F.; Jin, B.; Cao, D.-W.; He, J.-S.; Zhang, M. Hypovitaminosis D is associated with endothelial dysfunction in patients with non-dialysis chronic kidney disease. J. Nephrol. 2015, 28, 471–476. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Shergis, J.; Zhang, L.; Zhang, A.L.; Guo, X.; Qin, X.; Johnson, D.; Liu, X.; Lu, C. Chinese herbal medicine for diabetic kidney disease: A systematic review and meta-analysis of randomised placebo-controlled trials. BMJ Open 2019, 9, e025653. [Google Scholar] [CrossRef]
- Moreillon, J.J.; Bowden, R.G.; Deike, E.; Griggs, J.; Wilson, R.; Shelmadine, B.; Cooke, M.; Beaujean, A. The use of an anti-inflammatory supplement in patients with chronic kidney disease. J. Complement. Integr. Med. 2013, 10, 143–152. [Google Scholar] [CrossRef]
- Yu, J.S.; Ho, C.H.; Wang, H.Y.; Chen, Y.H.; Hsieh, C.L. Acupuncture on Renal Function in Patients with Chronic Kidney Disease: A Single-Blinded, Randomized, Preliminary Controlled Study. J. Altern. Complement. Med. 2017, 23, 624–631. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, Q.; Wang, Y.; Ren, Q.; Zhu, W.; Yao, Z.; Chen, J. Moxibustion as an Adjuvant Therapy for Chronic Kidney Disease: A Systematic Review and Meta-Analysis of 23 Randomized Controlled Trials. Evid. Based Complementary Altern. Med. 2020, 2020, 6128673. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-D.; Meng, X.-M.; Huang, C.; Zhang, L.; Lv, X.-W.; Li, J. Application of Herbal Traditional Chinese Medicine in the Treatment of Acute Kidney Injury. Front. Pharmacol. 2019, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cai, G.-Y.; He, L.-Q.; Lin, H.-L.; Cheng, X.-H.; Wang, N.-S.; Jian, G.-H.; Liu, X.-S.; Liu, Y.-N.; Ni, Z.-H. Efficacy and safety of Niaoduqing particles for delaying moderate-to-severe renal dysfunction: A randomized, double-blind, placebo-controlled, multicenter clinical study. Chin. Med. J. 2017, 130, 2402. [Google Scholar] [CrossRef]
- Tu, Y.; Sun, W.; Wan, Y.G.; Gao, K.; Liu, H.; Yu, B.Y.; Hu, H.; Huang, Y.R. Dahuang Fuzi Decoction ameliorates tubular epithelial apoptosis and renal damage via inhibiting TGF-beta1-JNK signaling pathway activation in vivo. J. Ethnopharmacol. 2014, 156, 115–124. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Xu, X.; Cao, W.; Shen, Z.; Wang, N.; Leng, J.; Zou, N.; Shang, E.; Zhu, Z.; et al. Improved dialysis removal of protein-bound uremic toxins by salvianolic acids. Phytomedicine 2019, 57, 166–173. [Google Scholar] [CrossRef]
- Huang, Y.-R.; Wei, Q.-X.; Wan, Y.-G.; Sun, W.; Mao, Z.-M.; Chen, H.-L.; Meng, X.-J.; Shi, X.-M.; Tu, Y.; Zhu, Q. Ureic clearance granule, alleviates renal dysfunction and tubulointerstitial fibrosis by promoting extracellular matrix degradation in renal failure rats, compared with enalapril. J. Ethnopharmacol. 2014, 155, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-H.; Chen, T.-H.; Wu, M.-Y.; Lin, Y.-F.; Chen, W.-L.; Cheng, T.-H.; Chen, C.-H. Protective effects of Zhibai Dihuang Wan on renal tubular cells affected with gentamicin-induced apoptosis. J. Ethnopharmacol. 2014, 151, 635–642. [Google Scholar] [CrossRef]
- Lu, P.-H.; Lee, H.-Y.; Liou, Y.-L.; Tung, S.-F.; Kuo, K.-L.; Chen, Y.-H. Nephroprotective Role of Zhibai Dihuang Wan in Aristolochic Acid-Intoxicated Zebrafish. BioMed Res. Int. 2020, 2020, 5204348. [Google Scholar] [CrossRef]
- Korish, A.A.; Arafah, M.M. Catechin combined with vitamins C and E ameliorates insulin resistance (IR) and atherosclerotic changes in aged rats with chronic renal failure (CRF). Arch. Gerontol. Geriatr. 2008, 46, 25–39. [Google Scholar] [CrossRef]
- Qin, Y.; Zhai, Q.; Li, Y.; Cao, M.; Xu, Y.; Zhao, K.; Wang, T. Cyanidin-3-O-glucoside ameliorates diabetic nephropathy through regulation of glutathione pool. Biomed. Pharm. 2018, 103, 1223–1230. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Du, F.; Su, X.; Sun, G.; Zhou, G.; Bian, X.; Liu, N. Epigallocatechin-3-Gallate Attenuates Oxidative Stress and Inflammation in Obstructive Nephropathy via NF-κB and Nrf2/HO-1 Signalling Pathway Regulation. Basic Clin. Pharm. Toxicol. 2015, 117, 164–172. [Google Scholar] [CrossRef]
- Ye, Q.; Zhu, Y.I.; Ye, S.; Liu, H.; She, X.; Niu, Y.; Ming, Y. Gypenoside attenuates renal ischemia/reperfusion injury in mice by inhibition of ERK signaling. Exp. Ther. Med. 2016, 11, 1499–1505. [Google Scholar] [CrossRef]
- Cai, H.-D.; Su, S.-L.; Qian, D.-W.; Guo, S.; Tao, W.-W.; Cong, X.D.; Tang, R.; Duan, J.-A. Renal protective effect and action mechanism of Huangkui capsule and its main five flavonoids. J. Ethnopharmacol. 2017, 206, 152–159. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Li, J.-P.; Lu, J.-B.; Li, C.-X.; Yu, J.-G.; Zhang, S.; Jiang, S.; Guo, J.-M.; Duan, J.-A. Effect and mechanism of Huangkui capsule on reduction of uremic toxin accumulation in an animal model of chronic kidney disease. Acta Pharm. Sin. 2019, 54, 10. [Google Scholar]
- Xu, D.; Chen, M.; Ren, X.; Ren, X.; Wu, Y. Leonurine ameliorates LPS-induced acute kidney injury via suppressing ROS-mediated NF-kappaB signaling pathway. Fitoterapia 2014, 97, 148–155. [Google Scholar] [CrossRef]
- Feng, L.; Ke, N.; Cheng, F.; Guo, Y.; Li, S.; Li, Q.; Li, Y. The protective mechanism of ligustrazine against renal ischemia/reperfusion injury. J. Surg. Res. 2011, 166, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Tang, H.T.; Jia, Y.T.; Ma, B.; Fu, J.F.; Wang, Y.; Lv, K.Y.; Xia, Z.F. Notoginsenoside R1 attenuates renal ischemia-reperfusion injury in rats. Shock 2010, 34, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-N.; Xie, D.Q.; Zhang, X.G.; Jiang, R. Osthole decreases renal ischemia-reperfusion injury by suppressing JAK2/STAT3 signaling activation. Exp. Ther. Med. 2016, 12, 2009–2014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, C.; Cheng, Z.; Wang, Y.; Dai, X.; Zhang, J.; Xue, D. Paeoniflorin exerts a nephroprotective effect on concanavalin A-induced damage through inhibition of macrophage infiltration. Diagn. Pathol. 2015, 10, 120. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210-15. [Google Scholar] [CrossRef]
- Feng, C.; Xie, X.; Wu, M.; Li, C.; Gao, M.; Liu, M.; Qi, X.; Ren, J. Tanshinone I protects mice from aristolochic acid I-induced kidney injury by induction of CYP1A. Environ. Toxicol. Pharmacol. 2013, 36, 850–857. [Google Scholar] [CrossRef]
- Lu, Z.; Zeng, Y.; Lu, F.; Liu, X.; Zou, C. Rhubarb Enema Attenuates Renal Tubulointerstitial Fibrosis in 5/6 Nephrectomized Rats by Alleviating Indoxyl Sulfate Overload. PLoS ONE 2015, 10, e0144726. [Google Scholar] [CrossRef]
- Ji, C.; Deng, Y.; Yang, A.; Lu, Z.; Chen, Y.; Liu, X.; Han, L.; Zou, C. Rhubarb Enema Improved Colon Mucosal Barrier Injury in 5/6 Nephrectomy Rats May Associate With Gut Microbiota Modification. Front. Pharmacol. 2020, 11, 1092. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Chupyrkina, A.A.; Jankauskas, S.S.; Pevzner, I.B.; Silachev, D.N.; Skulachev, V.P.; Zorov, D.B. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim. Biophys. Acta 2011, 1812, 77–86. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.; Torti, S. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Usharani, P.; Mateen, A.; Naidu, M.; Raju, Y.; Chandra, N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus. Drugs R D 2008, 9, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Massey, H.D.; Krieg, R.; Fazelbhoy, Z.A.; Ghosh, S.; Sica, D.A.; Fakhry, I.; Gehr, T.W. Curcumin ameliorates renal failure in 5/6 nephrectomized rats: Role of inflammation. Am. J. Physiol. Ren. Physiol. 2009, 296, F1146–F1157. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, K.; Alluri, K.V.; Satish, A.R.; Mishra, S.; Golakoti, T.; Sarma, K.V.; Dey, D.; Raychaudhuri, S.P. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin® for treatment of osteoarthritis of the knee. Arthritis Res. Ther. 2008, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Madisch, A.; Miehlke, S.; Eichele, O.; Mrwa, J.; Bethke, B.; Kuhlisch, E.; Bästlein, E.; Wilhelms, G.; Morgner, A.; Wigginghaus, B. Boswellia serrata extract for the treatment of collagenous colitis. A double-blind, randomized, placebo-controlled, multicenter trial. Int. J. Colorectal Dis. 2007, 22, 1445–1451. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, Q.; Tong, X. The clinical application and pharmacological research progress of Dahuang fuzi Decoction. Chin. Arch. Tradit. Chin. Med. 2010, 28, 1848–1851. [Google Scholar]
- Li, J.-P.; Guo, J.-M.; Hua, Y.-Q.; Zhu, K.Y.; Tang, Y.-P.; Zhao, B.-C.; Jia, L.-F.; Zhao, J.; Tang, Z.-S.; Duan, J.-A. The mixture of Salvia miltiorrhiza–Carthamus tinctorius (Danhong injection) alleviates low-dose aspirin induced gastric mucosal damage in rats. Phytomedicine 2016, 23, 662–671. [Google Scholar] [CrossRef]
- Wang, X.; Yu, S.; Jia, Q.; Chen, L.; Zhong, J.; Pan, Y.; Shen, P.; Shen, Y.; Wang, S.; Wei, Z. NiaoDuQing granules relieve chronic kidney disease symptoms by decreasing renal fibrosis and anemia. Oncotarget 2017, 8, 55920. [Google Scholar] [CrossRef]
- Yin, X.-F.; Han, L.-L. Effect of uremic clearance granule on the systemic micro-inflammatory state of patients after peritoneal dialysis. Pract. Pharm. Clin. Remedies 2013, 2, 125–126. [Google Scholar]
- Ye, M.-Y.; Zheng, J.; Zhang, J. The Influence of Niaoduqing Particle on the Calcium and Phosphorus Metabolism and FGF23 in Patients with Chronic Kidney Disease. Med. Innov. China 2015, 12, 16–18. [Google Scholar]
- Wojcikowski, K.; Johnson, D.W.; Gobe, G. Herbs or natural substances as complementary therapies for chronic kidney disease: Ideas for future studies. J. Lab. Clin. Med. 2006, 147, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Suzukawa, M.; Ito, T.; Yoshida, H.; Ayaori, M.; Nishiwaki, M.; Yonemura, A.; Hara, Y.; Nakamura, H. Effect of tea flavonoid supplementation on the susceptibility of low-density lipoprotein to oxidative modification. Am. J. Clin. Nutr. 1997, 66, 261–266. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C.; Feskens, E.J.; Bueno de Mesquita, H.B.; Kromhout, D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: The Zutphen Elderly Study. Am. J. Clin. Nutr. 2001, 74, 227–232. [Google Scholar] [CrossRef]
- Chander, V.; Singh, D.; Chopra, K. Catechin, a natural antioxidant protects against rhabdomyolysis-induced myoglobinuric acute renal failure. Pharmacol. Res. 2003, 48, 503–509. [Google Scholar] [CrossRef]
- Sumien, N.; Forster, M.J.; Sohal, R.S. Supplementation with vitamin E fails to attenuate oxidative damage in aged mice. Exp. Gerontol. 2003, 38, 699–704. [Google Scholar] [CrossRef]
- Mahfouz, M.; Kummerow, F. Vitamin C or vitamin B6 supplementation prevent the oxidative stress and decrease of prostacyclin generation in homocysteinemic rats. Int. J. Biochem. Cell Biol. 2004, 36, 1919–1932. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Peterson, J.J.; Patel, R.; Jacques, P.F.; Shah, R.; Dwyer, J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 2012, 95, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Li, Q.S.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R. Antiviral Effects of Green Tea EGCG and Its Potential Application against COVID-19. Molecules 2021, 26, 3962. [Google Scholar] [CrossRef] [PubMed]
- Hodges, J.K.; Sasaki, G.Y.; Bruno, R.S. Anti-inflammatory activities of green tea catechins along the gut-liver axis in nonalcoholic fatty liver disease: Lessons learned from preclinical and human studies. J. Nutr. Biochem. 2020, 85, 108478. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Malavolti, M.; Borrelli, F.; Izzo, A.A.; Fairweather-Tait, S.J.; Horneber, M.; Vinceti, M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2020, 3, CD005004. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Ha, T.K.Q.; Yang, J.L.; Pham, H.T.T.; Oh, W.K. Triterpenoids from the genus Gynostemma: Chemistry and pharmacological activities. J. Ethnopharmacol. 2021, 268, 113574. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Yang, Y.; Wang, H.; Shu, B.; Gong, Q.H.; Qian, M. Gypenosides attenuate cholesterol-induced DNA damage by inhibiting the production of reactive oxygen species in human umbilical vein endothelial cells. Mol. Med. Rep. 2015, 11, 2845–2851. [Google Scholar] [CrossRef]
- Li, N.; Tang, H.; Wu, L.; Ge, H.; Wang, Y.; Yu, H.; Zhang, X.; Ma, J.; Gu, H.F. Chemical constituents, clinical efficacy and molecular mechanisms of the ethanol extract of Abelmoschus manihot flowers in treatment of kidney diseases. Phytother. Res. 2021, 35, 198–206. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, G.; Sun, X.; Chen, X. Treatment of chronic kidney disease using a traditional Chinese medicine, Flos Abelmoschus manihot (Linnaeus) Medicus (Malvaceae). Clin. Exp. Pharmacol. Physiol. 2016, 43, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, P.; Xing, C.Y.; Zhao, J.Y.; He, Y.N.; Wang, J.Q.; Wu, X.F.; Liu, Z.S.; Zhang, A.P.; Lin, H.L.; et al. Efficacy and safety of Abelmoschus manihot for primary glomerular disease: A prospective, multicenter randomized controlled clinical trial. Am. J. Kidney Dis. 2014, 64, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lin, H.; Ni, Z.; Zhan, Y.; He, Y.; Yang, H.; Fang, J.; Wang, N.; Li, W.; Cai, G.; et al. Efficacy and safety of Abelmoschus manihot for IgA nephropathy: A multicenter randomized clinical trial. Phytomedicine 2020, 76, 153231. [Google Scholar] [CrossRef] [PubMed]
- Wojtyniak, K.; Szymański, M.; Matławska, I. Leonurus cardiaca L. (motherwort): A review of its phytochemistry and pharmacology. Phytother. Res. 2013, 27, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Pan, L.L.; Yang, H.B.; Gong, Q.H.; Zhu, Y.Z. Leonurine attenuates lipopolysaccharide-induced inflammatory responses in human endothelial cells: Involvement of reactive oxygen species and NF-κB pathways. Eur. J. Pharmacol. 2012, 680, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Yu, S.-Z.; Wang, Z.-T.; Zhao, B.-L.; Hou, J.-W.; Yang, F.-J.; Xin, W.-J. Scavenging effects of tetramethylpyrazine on active oxygen free radicals. Zhongguo Yao Li Xue Bao = Acta Pharmacol. Sin. 1994, 15, 229–231. [Google Scholar]
- Wu, W.; Qiu, F. Experimental study on ischemia and reperfusion injury of rat liver and effects of ligustrazine and salvia compound. Chin. Med. Sci. J. = Chung-Kuo I Hsueh K’o Hsueh Tsa Chih 1994, 9, 162–166. [Google Scholar]
- Feng, L.; Xiong, Y.; Cheng, F.; Zhang, L.; Li, S.; Li, Y. Effect of ligustrazine on ischemia-reperfusion injury in murine kidney. Transpl. Proc. 2004, 36, 1949–1951. [Google Scholar] [CrossRef]
- Li, F.; Gong, Q.; Wang, L.; Shi, J. Osthole attenuates focal inflammatory reaction following permanent middle cerebral artery occlusion in rats. Biol. Pharm. Bull. 2012, 35, 1686–1690. [Google Scholar] [CrossRef]
- Zhang, J.; Dou, W.; Zhang, E.; Sun, A.; Ding, L.; Wei, X.; Chou, G.; Mani, S.; Wang, Z. Paeoniflorin abrogates DSS-induced colitis via a TLR4-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G27–G36. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Lu, Y.; Yang, Z.; Lv, Z.; Xu, Q.; Pan, Q.; Lu, L. Paeoniflorin regulates macrophage activation in dimethylnitrosamine-induced liver fibrosis in rats. BMC Complementary Altern. Med. 2012, 12, 1–11. [Google Scholar] [CrossRef]
- Chung, J.H.; Manganiello, V.; Dyck, J.R.B. Resveratrol as a calorie restriction mimetic: Therapeutic implications. Trends Cell Biol. 2012, 22, 546–554. [Google Scholar] [CrossRef]
- Larrosa, M.; Yañéz-Gascón, M.J.; Selma, M.V.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Effect of a Low Dose of Dietary Resveratrol on Colon Microbiota, Inflammation and Tissue Damage in a DSS-Induced Colitis Rat Model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef]
- Rakici, O.; Kiziltepe, U.; Coskun, B.; Aslamaci, S.; Akar, F. Effects of resveratrol on vascular tone and endothelial function of human saphenous vein and internal mammary artery. Int. J. Cardiol. 2005, 105, 209–215. [Google Scholar] [CrossRef]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Fan, L.; Wang, L.; Zhou, Y.; Qin, D.; Sun, Q.; Wu, J.; Cao, S. Free total rhubarb anthraquinones protect intestinal injury via regulation of the intestinal immune response in a rat model of severe acute pancreatitis. Front. Pharmacol. 2018, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, Y.-L.; Zhang, Z.; Lin, Z.-F.; Chen, D.-C. Rhubarb monomers protect intestinal mucosal barrier in sepsis via junction proteins. Chin. Med. J. 2017, 130, 1218. [Google Scholar] [CrossRef] [PubMed]
- Fetisova, E.K.; Avetisyan, A.V.; Izyumov, D.S.; Korotetskaya, M.V.; Chernyak, B.V.; Skulachev, V.P. Mitochondria-targeted antioxidant SkQR1 selectively protects MDR (Pgp 170)-negative cells against oxidative stress. FEBS Lett. 2010, 584, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Silachev, D.N.; Plotnikov, E.Y.; Pevzner, I.B.; Zorova, L.D.; Balakireva, A.V.; Gulyaev, M.V.; Pirogov, Y.A.; Skulachev, V.P.; Zorov, D.B. Neuroprotective effects of mitochondria-targeted plastoquinone in a rat model of neonatal hypoxic–ischemic brain injury. Molecules 2018, 23, 1871. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, W.; Chen, Z.; Shi, Z.; He, C.; Chen, M. Recent insights into the biological activities and drug delivery systems of tanshinones. Int. J. Nanomed. 2016, 11, 121. [Google Scholar]
- Zhou, J.; Jiang, Y.Y.; Chen, H.; Wu, Y.C.; Zhang, L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020, 53, e12739. [Google Scholar] [CrossRef]
- World Health Organization. Acupuncture: Review and Analysis of Reports on Controlled Clinical Trials; World Health Organization: Geneve, Switzerland, 2002. [Google Scholar]
- Cabýoglu, M.T.; Ergene, N.; Tan, U. The mechanism of acupuncture and clinical applications. Int. J. Neurosci. 2006, 116, 115–125. [Google Scholar] [CrossRef]
- Xiong, W.; He, F.F.; You, R.Y.; Xiong, J.; Wang, Y.M.; Zhang, C.; Meng, X.F.; Su, H. Acupuncture Application in Chronic Kidney Disease and its Potential Mechanisms. Am. J. Chin. Med. 2018, 46, 1169–1185. [Google Scholar] [CrossRef]
- Huang, Q.-F.; Wu, H.-G.; Liu, J.; Hong, J. Bibliometric analysis of diseases spectrum of moxibustion therapy. J. Acupunct. Tuina Sci. 2012, 10, 342–348. [Google Scholar] [CrossRef]
- Lin, J.-G.; Li, T.; Hsu, S. Newly Edited Color Book of Acupuncture and Moxibustion; JYIN Publishing Company: Taipei, Taiwan, 2009; pp. 107–108. [Google Scholar]
- Cardini, F.; Weixin, H. Moxibustion for correction of breech presentation: A randomized controlled trial. JAMA 1998, 280, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.-H. How does moxibustion possibly work? Evid. Based Complementary Altern. Med. 2013, 2013, 198584. [Google Scholar] [CrossRef]
- Deng, H.; Shen, X. The mechanism of moxibustion: Ancient theory and modern research. Evid. Based Complementary Altern. Med. 2013, 2013, 379291. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Zhang, C.; Wang, K.; Shen, P.; Huang, D.; Ma, W.; Zhang, J.; Li, L.; He, L. Moxibustion alleviates injury in a rat focal segmental glomerulosclerosis model. Evid. Based Complementary Altern. Med. 2017, 2017, 7169547. [Google Scholar] [CrossRef] [PubMed]
| Intervention | Route, Dosage and Frequency | Author/Year | Mechanism/Usage | Study Design | Subjects | Subject Number | Result |
|---|---|---|---|---|---|---|---|
| Clinical Studies | |||||||
| Acarbose | Oral, 100 mg, TID | Evenepoel et al., 2006 [26] | Changes in bacterial amino acid metabolism | Clinical trial | Healthy people | 9 | PCS ↘ |
| AST-120 | Oral, 2.7 to 9 g/day | Chen et al., 2019 [32] | UT adsorbent | Meta-analysis | Patients with CKD | 3349 | IS ↘ |
| L-carnitine | i.v., 20 mg/kg, 3 times/week | Fatouros et al., 2010 [33] | Antioxidation | Clinical trial | Patients undergoing HD | 12 | MDA ↘ |
| Folate | Oral, 10 mg, QD | Trimarchi et al., 2002 [34] | Metabolic degradation of UT | RCT | Patients undergoing HD | 62 | Hcy ↘ |
| Folate and Methylcobami | i.v. methylcobalami 500 µg, 3 times/week and oral folate 15 mg, QD | Koyama et al., 2010 [25] | Metabolic degradation of UT | RCT | Patients undergoing HDs | 40 | ADMA ↘, Hcy ↘ |
| Ketoacid and LPD | Oral, 1 pill/5 kg, QD | Marzocco et al., 2013 [35] | Decreased amino acid degradation/protein carbamylation | RCT | CKD stage 3 adults | 32 | IS ↘ |
| Ketoacid and LPD | Oral, 0.1 g/kg, TID | Garibotto et al., 2018 [29] | Decreased amino acid degradation/protein carbamylation | RCT | Patients with CKD | 17 | Urea ↘ |
| Reduced glutathione | Oral, 400 mg, TID | Wang et al., 2016 [36] | Antioxidation | RCT | Patients undergoing HD | 150 | IL-6 ↘, TNF-α ↘ |
| Animal Studies | |||||||
| AST-120 | Oral, 8% w/w, QD | Sato et al., 2017 [37] | UT adsorbent | Animal | Adenine-induced CKD mice | 24 | IS ↘, PCS ↘ |
| L-carnitine | i.p., 500 mg/kg, QD | Sener et al., 2004 [38] | Antioxidation | Animal | Right nephrectomy rats | 16 | BUN ↘, Cr ↘, MDA ↘ |
| Cilastatin | i.v., 200 mg/kg, once | Huo et al., 2019 [30] | OAT inhibitor | Animal | Imipenem-induced nephrotoxicity rabbits | 4 | BUN ↘, Cr ↘ |
| cyclosporine | i.v., 3 mg/kg, once | Lemoine et al., 2015 [24] | Antioxidation | Animal | I/R mice | 22 | BUN ↘, Cr ↘ |
| Enalapril | Oral, 12.6 mg/kg, QD | Marek et al., 2018 [39] | ACEI, increased glomerular filtration, and urine output | Animal | Wistar rats | 27 | TMAO ↘ |
| meclofenamate | i.v., 10 mg/kg, TID | Saigo et al., 2014 [28] | SULT inhibitors | Animal | Renal I/R rats | 9 | BUN ↘, Cr ↘, IS ↘ |
| Probenecid | i.v., 50 mg/kg, once | Huo et al., 2019, [30] | OAT inhibitor | Animal | Imipenem-induced nephrotoxicity rabbits | 12 | BUN ↘, Cr ↘ |
| Intervention | Route, Dosage and Frequency | Author/Year | Mechanism/Usage | Study Design | Subjects | Subject Number | Result |
|---|---|---|---|---|---|---|---|
| Clinical Studies | |||||||
| Prebiotics—OF-IN | Oral, 10 g, BID | Meijers et al., 2010 [54] | Modulating intestinal microbiota | Open-label phase I/II study | Patients undergoing HD | 22 | PCS ↘ |
| Probiotics: L. acidophilus KB27, B. longum KB31, and S. thermophilus KB19 | Oral, 2 capsules, TID | Ranganathan et al., 2010 [55] | Modulating intestinal microbiota | RCT | Patients with CKD stages 3 and 4 | 46 | BUN ↘, Cr ↘, UA ↘ |
| Probiotics: B. longum | Oral, 3–12 × 109 CFU/day | Taki et al., 2005 [56] | Modulating intestinal microbiota | Case series | Patients undergoing HD | 27 | Hcy ↘, IS ↘ |
| SCFA: sodium propionate | Oral, 1 g, QD | Marzocco et al., 2018 [57] | Anti-inflammation and antioxidation | Clinical trial | Patients undergoing HD | 20 | IS ↘, MDA ↘, PCS ↘ |
| Synbiotic: L. casei, B. breve, and galactooligosaccharides | Oral, 1 pack, TID | Nakabayashi el et al., 2011 [58] | Modulating intestinal microbiota | Clinical trial or case series | Patients undergoing HD | 9 | p-Cresol ↘ |
| Vegetarian | Oral | Kandouz et al., 2016 [59] | Improvement of metabolic acidosis, modification of intestinal microbiota | Cohort | Patients in hemodiafiltration | 138 | IS ↘, PCS ↘, Urea ↘ |
| Vitamin D | Oral, 300,000 IU, QD | Kumar et al., 2017 [53] | Anti-inflammation | RCT | Patients with nondiabetic CKD and vitamin D deficiency | 120 | IL-6 ↘, UA ↘ |
| Animal Studies | |||||||
| Diet® k/d® | Oral, 1.6 RER, QD | Hall et al., 2018 [60] | Anti-inflammation | Animal | CKD dogs | 36 | BUN ↘, Cr ↘, SDMA ↘ |
| Lingonberry | Oral, 5% w/w, QD | Madduma Hewageet al., 2020 [61] | Anti-inflammation | Animal | HFD-induced kidney injury mice | 30 | BUN ↘, Cr ↘, IL-6 ↘, TNF-α ↘ |
| MitoQ | i.v., 4 mg/kg, once | Hu et al., 2018 [62] | Antioxidation through reducing mitochondrial ROS | Animal | I/R mice | 24 | Cr ↘, IL-1β ↘, IL-6 ↘, TNF-α ↘ |
| Soluble Fiber and Omega-3 | Oral, 3666 kcal/kg, QD | Ephraim et al., 2020 [63] | Modulating intestinal microbiota | Animal | Dogs aged older than 7 years | 36 | phenolic UTs ↘, SDMA ↘ |
| Intervention | Route, Dosage, and Frequency | Author/Year | Mechanism/Usage | Study Design | Subjects | Subject Number | Result |
|---|---|---|---|---|---|---|---|
| Clinical Studies | |||||||
| Curcuma longa and Boswellia serrata | Oral, 1 capsule, BID | Moreillon et al., 2013 [91] | Anti-inflammation, inhibition of NF-Κb and MAPK | RCT | Patients with CKD | 16 | IL-6 ↘ |
| UCG | Oral, 5 g, TID and 10 g HS | Zheng et al., 2017 [95] | Anti-inflammation and antifibrosis | RCT | Patients with CKD | 292 | Cr ↘ |
| Acupuncture | External, LI4, ST36 and KI3, 1 time/week | Yu et al., 2017 [92] | Improving renal local microcirculation | RCT | Patients with CKD | 59 | Cr ↘ |
| Moxibustion | External, 0.5~7 sessions/week | Zhou et al., 2020 [93] | Dilating local renal capillaries, alleviating kidney podocyte injury | MA | Patients with CKD | 1571 | BUN ↘, Cr ↘ |
| Animal Studies | |||||||
| DFD | Gastric gavage, 2.5 g/kg, QD | Tu et al., 2014 [96] | Inhibiting apoptosis by blocking TGF-b1-JNK | Animal | Adenine-induced renal injury rats | 27 | BUN ↘, Cr ↘, UA ↘ |
| DHI and salvianolic acids | Extracorporeal, DHI 4.16 mL/kg or LA 24.69 mg/kg, once | Li et al., 2019 [97] | Protein-binding competitors | Animal | CKD rats with accumulated IS and pCS | 16 | Enhanced dialysis removal of IS and pCS |
| UCG | Gastric gavage, 5 g/kg, QD | Huang et al., 2014 [98] | Antifibrosis, regulation of ECM degradation | Animal | Adenine and UUO-induced renal failure rats | 26 | BUN ↘, Cr ↘, UA ↘ |
| ZDW | i.p., 2 g/kg, once | Hsu et al., 2014 [99] | Attenuation of apoptosis through limiting of caspase-3 activation | Animal | Gentamicin-induced renal injury rat | 12 | BUN ↘Cr ↘ |
| ZDW | Embryo exposure, 100 ppm, once | Lu et al., 2020 [100] | Suppression of proinflammatory gene expression | Animal | AA-intoxicated zebrafish embryos | 150 | tnf-α ↘ |
| Catechin | Oral, 100 mg/kg, QD | Korish et al., 2008 [101] | Antioxidation | Animal | 5/6 nephrectomy rats | 40 | ADMA ↘ |
| Cyanidin-3-O-glucoside (C3G) | i.p., 20 mg/kg, QD | Qin et al., 2018 [102] | Antioxidation | Animal | db/db mice with DN | 60 | BUN ↘, Cr ↘ |
| EGCG | i.p., 50 mg/kg, QD | Wang et al., 2015 [103] | Anti-inflammation and antioxidation through inhibition of the NF-κB signaling pathway and activation of the Nrf2-Keap1 pathway | Animal | UUO mice | 24 | BUN ↘, Cr ↘ |
| Gypenoside (GP) | i.v., 50 mg/kg, once | Ye et al., 2016 [104] | Attenuating inflammatory and oxidative stress by inhibiting ERK signaling | Animal | I/R-induced renal injury mice | 30 | BUN ↘, Cr ↘, IL-1β ↘, IL-6 ↘, MDA ↘, TNF-α ↘ |
| Huangkui capsule | Gastric gavage, 0.75 g/kg, QD | Cai et al., 2017 [105] | Inhibition of the NADPH oxidase/ROS/ERK pathway | Animal | Adenine-induced CRF Rats | 18 | BUN ↘, Cr ↘ |
| Huangkui capsule | Gastric gavage, 0.675 g/kg, QD | Wang et al., 2019 [106] | Inhibition of the transformation of Trp to indole | Animal | 5/6 nephrectomy Rats | 21 | IS ↘ |
| Leonurine (LEO) | i.v., 50 mg/kg, QD | Xu et al., 2014 [107] | Inhibition of inflammatory and oxidative stress through downregulation of NF-kB | Animal | LPS-induced renal injury mice | 120 | BUN ↘, Cr ↘, IL-1 ↘, IL-6 ↘, IL-8 ↘, MDA ↘, TNF-α ↘ |
| Ligustrazine (LIG) | i.p., 80 mg/kg, once | Feng et al., 2011 [108] | Downregulation of oxidative stress and apoptosis, decrease in neutrophil infiltration | Animal | I/R-induced renal injury mice | 48 | MDA ↘, TNF-α ↘ |
| Notoginsenoside R1 (NR1) | i.p., 80 mg/kg, once | Liu et al., 2010 [109] | Blocking apoptosis and inflammatory response by suppressing p38 and NF-kB | Animal | I/R-induced renal injury rats | 24 | Cr ↘, TNF-α ↘ |
| Osthole | i.p., 40 mg/kg, once | Luo et al., 2016 [110] | Abrogating inflammation by suppressing JAK2/STAT3 signaling, activating PI3K/Akt signaling | Animal | I/R-induced renal injury rats | 70 | BUN ↘, Cr ↘, IL-6 ↘, TNF-α ↘ |
| Paeoniflorin (PF) | i.p., 30 mg/kg, once | Liu et al., 2015 [111] | Attenuation of inflammatory response by inhibiting CXCR3/CXCL | Animal | ConA-induced renal injury mice | 60 | BUN ↘, Cr ↘, IL-1β ↘ |
| Resveratrol | Gastric Gavage, 1 mg/kg, QD | Chen et al., 2016 [112] | Modulation of intestinal microbiota | Animal | ApoE(-/-) mice | 20 | TMAO ↘ |
| Tanshinone I | i.p., 120 mg/kg, QD | Feng et al., 2013 [113] | Enhancement of AAI metabolism by induction of CYP1A | Animal | AAI-induced renal injury mice | 40 | BUN ↘, Cr ↘ |
| Rhubarb | Enema, 0.5 g, QD | Lu et al., 2015 [114] | Antioxidation, anti-inflammation | Animal | 5/6 nephrectomy rats | 28 | Cr ↘, IS ↘ |
| Rhubarb | Enema, 2.12 g/kg, QD | Ji et al., 2020 [115] | Modulation of intestinal microbiota, improving the intestinal barrier, anti-inflammation | Animal | 5/6 nephrectomy rats | 30 | IL-1β ↘, IL-6 ↘ |
| SkQR1 | i.p., 400 nmol/kg, once | Plotnikov et al., 2011 [116] | Antioxidation | Animal | Glycerol-induced rhabdomyolysis rats | 36 | BUN ↘, MDA ↘ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.-H.; Yu, M.-C.; Wei, M.-J.; Kuo, K.-L. The Therapeutic Strategies for Uremic Toxins Control in Chronic Kidney Disease. Toxins 2021, 13, 573. https://doi.org/10.3390/toxins13080573
Lu P-H, Yu M-C, Wei M-J, Kuo K-L. The Therapeutic Strategies for Uremic Toxins Control in Chronic Kidney Disease. Toxins. 2021; 13(8):573. https://doi.org/10.3390/toxins13080573
Chicago/Turabian StyleLu, Ping-Hsun, Min-Chien Yu, Meng-Jiun Wei, and Ko-Lin Kuo. 2021. "The Therapeutic Strategies for Uremic Toxins Control in Chronic Kidney Disease" Toxins 13, no. 8: 573. https://doi.org/10.3390/toxins13080573
APA StyleLu, P.-H., Yu, M.-C., Wei, M.-J., & Kuo, K.-L. (2021). The Therapeutic Strategies for Uremic Toxins Control in Chronic Kidney Disease. Toxins, 13(8), 573. https://doi.org/10.3390/toxins13080573





