Association of Polygenic Risk Score and Bacterial Toxins at Screening Colonoscopy with Colorectal Cancer Progression: A Multicenter Case-Control Study
Abstract
:1. Introduction
2. Results
2.1. ADK vs. Controls Analysis
2.2. PA vs. Controls Analysis
2.3. HP vs. Controls Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
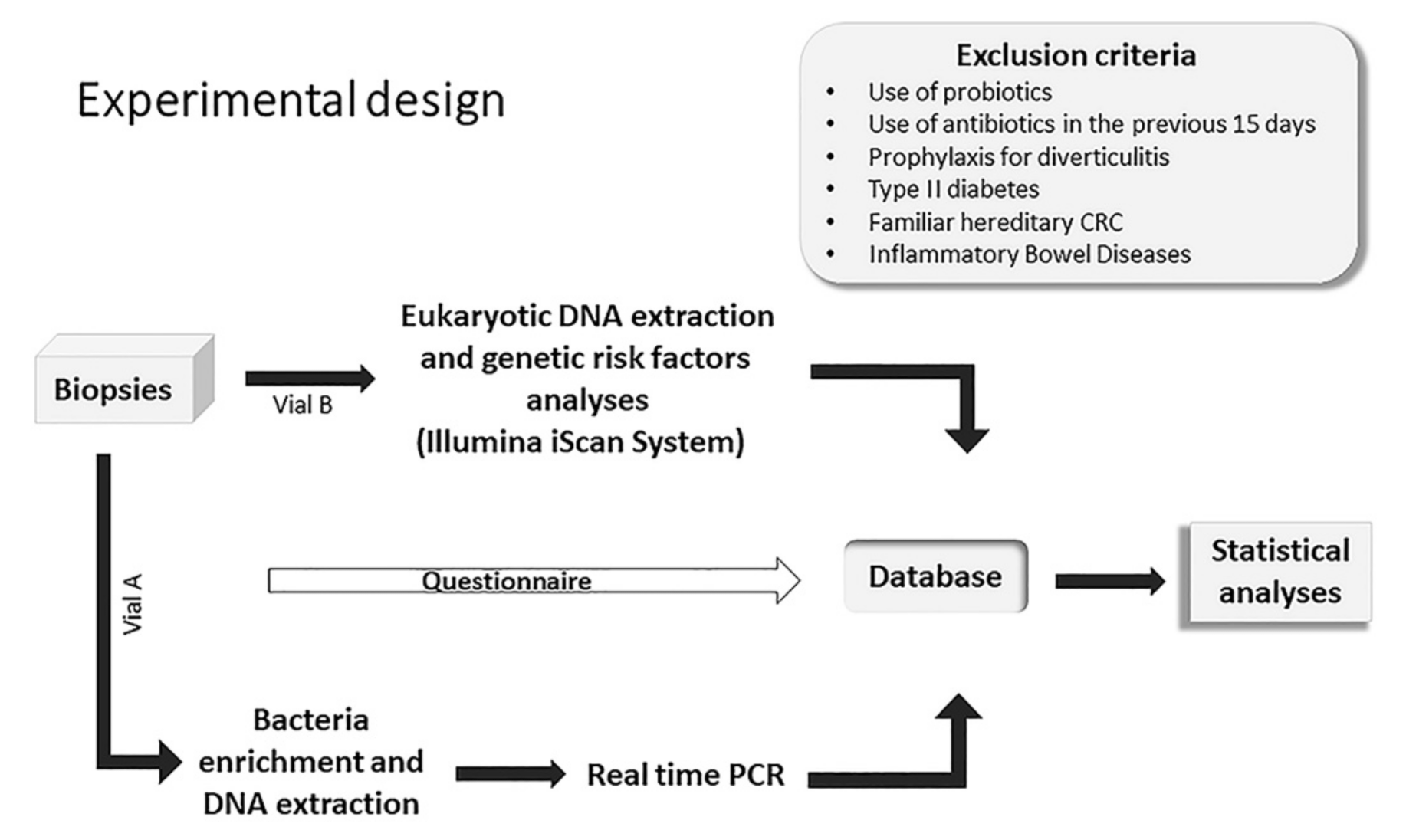
5.1. Study Design, Ethics Procedures and Adherence to STROBE Guidelines
5.2. Enrolment of Patients and Selection of Study Subjects
5.3. Biopsy Sample Collection and Storage
5.4. Bacteria Enrichment and DNA Extraction
5.5. Real-Time PCR Analysis
5.6. DNA Extraction and Genotyping of CRC Risk Loci
5.7. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GLOBOCAN Colorectal Cancer. Available online: https://gco.iarc.fr/today (accessed on 14 May 2021).
- Wassenaar, T.M. E. coli and colorectal cancer: A complex relationship that deserves a critical mindset. Crit. Rev. Microbiol. 2018, 44, 619–632. [Google Scholar] [CrossRef] [Green Version]
- Marley, A.R.; Nan, H. Epidemiology of colorectal cancer. Int. J. Mol. Epidemiol. Genet. 2016, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Fulbright, L.E.; Ellermann, M.; Arthur, J.C. The microbiome and the hallmarks of cancer. PLoS Pathog. 2017, 13, e1006480. [Google Scholar] [CrossRef]
- Jia, W.; Rajani, C.; Xu, H.; Zheng, X. Gut microbiota alterations are distinct for primary colorectal cancer and hepatocellular carcinoma. Protein Cell 2020, 12, 374–393. [Google Scholar] [CrossRef]
- Keku, T.O.; Dulal, S.; Deveaux, A.; Jovov, B.; Han, X. The gastrointestinal microbiota and colorectal cancer. Am. J. Physiol. Liver Physiol. 2015, 308, G351–G363. [Google Scholar] [CrossRef] [Green Version]
- Uronis, J.M.; Mühlbauer, M.; Herfarth, H.H.; Rubinas, T.C.; Jones, G.S.; Jobin, C. Modulation of the Intestinal Microbiota Alters Colitis-Associated Colorectal Cancer Susceptibility. PLoS ONE 2009, 4, e6026. [Google Scholar] [CrossRef] [Green Version]
- Sands, B.E. Inflammatory bowel disease: Past, present, and future. J. Gastroenterol. 2007, 42, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Elsland, D.; Neefjes, J. Bacterial infections and cancer. EMBO Rep. 2018, 19, e46632. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, C.; Carlini, F.; Germinario, E.A.P.; Maroccia, Z.; Travaglione, S.; Fabbri, A. Gut microbiota and colon cancer: A role for bacterial protein toxins? Int. J. Mol. Sci. 2020, 21, 6201. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M.; Higashi, H. Helicobacter pylori CagA: A new paradigm for bacterial carcinogenesis. Cancer Sci. 2005, 96, 835–843. [Google Scholar] [CrossRef]
- Purcell, R.V.; Pearson, J.; Aitchison, A.; Dixon, L.; Frizelle, F.A.; Keenan, J.I. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS ONE 2017, 12, e0171602. [Google Scholar] [CrossRef] [Green Version]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Huber, A.R.; van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature 2020, 580, 269–273. [Google Scholar] [CrossRef]
- Butterworth, A.S.; Higgins, J.P.T.; Pharoah, P. Relative and absolute risk of colorectal cancer for individuals with a family history: A meta-analysis. Eur. J. Cancer 2006, 42, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Broderick, P.; Carvajal-Carmona, L.; Pittman, A.M.; Webb, E.; Howarth, K.; Rowan, A.; Lubbe, S.; Spain, S.; Sullivan, K.; Fielding, S.; et al. A genome-wide association study shows that common alleles of SMAD7 influence colorectal cancer risk. Nat. Genet. 2007, 39, 1315–1317. [Google Scholar] [CrossRef]
- Huyghe, J.R.; Bien, S.A.; Harrison, T.A.; Kang, H.M.; Chen, S.; Schmit, S.L.; Conti, D.V.; Qu, C.; Jeon, J.; Edlund, C.K.; et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat. Genet. 2019, 51, 76–87. [Google Scholar] [CrossRef]
- Buc, E.; Dubois, D.; Sauvanet, P.; Raisch, J.; Delmas, J.; Darfeuille-Michaud, A.; Pezet, D.; Bonnet, R. High Prevalence of Mucosa-Associated E. coli Producing Cyclomodulin and Genotoxin in Colon Cancer. PLoS ONE 2013, 8, e56964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Yao, Q.; Li, S.; Ding, X.; Lu, Q.; Mao, H.; Liu, L.; Zheng, N.; Chen, S.; Shao, F. Glutamine deamidation and dysfunction of ubiquitin/NEDD8 induced by a bacterial effector family. Science 2010, 329, 1215–1218. [Google Scholar] [CrossRef] [Green Version]
- Marchès, O.; Ledger, T.N.; Boury, M.; Ohara, M.; Tu, X.; Goffaux, F.; Mainil, J.; Rosenshine, I.; Sugai, M.; De Rycke, J.; et al. Enteropathogenic and enterohaemorrhagic Escherichia coli deliver a novel effector called Cif, which blocks cell cycle G2/M transition. Mol. Microbiol. 2003, 50, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Taieb, F.; Nougayrède, J.P.; Watrin, C.; Samba-Louaka, A.; Oswald, E. Escherichia coli cyclomodulin Cif induces G2 arrest of the host cell cycle without activation of the DNA-damage checkpoint-signalling pathway. Cell. Microbiol. 2006, 8, 1910–1921. [Google Scholar] [CrossRef]
- Samba-louaka, A.; Nougayrède, J.P.; Watrin, C.; Jubelin, G.; Oswald, E.; Taieb, F. Bacterial cyclomodulin Cif blocks the host cell cycle by stabilizing the cyclin-dependent kinase inhibitors p21waf1 and p27kip1. Cell. Microbiol. 2008, 10, 2496–2508. [Google Scholar] [CrossRef] [PubMed]
- McCormack, R.M.; Lyapichev, K.; Olsson, M.L.; Podack, E.R.; Munson, G.P. Enteric pathogens deploy cell cycle inhibiting factors to block the bactericidal activity of Perforin-2. Elife 2015, 4, e06505. [Google Scholar] [CrossRef]
- Kumar, A.; Wu, H.; Collier-Hyams, L.S.; Hansen, J.M.; Li, T.; Yamoah, K.; Pan, Z.Q.; Jones, D.P.; Neish, A.S. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 2007, 26, 4457–4466. [Google Scholar] [CrossRef] [Green Version]
- Manicassamy, S.; Reizis, B.; Ravindran, R.; Nakaya, H.; Salazar-Gonzalez, R.M.; Wang, Y.C.; Pulendran, B. Activation of β-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 2010, 329, 849–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nougayrède, J.P.; Boury, M.; Tasca, C.; Marchès, O.; Milon, A.; Oswald, E.; De Rycke, J. Type III secretion-dependent cell cycle block caused in HeLa cells by enteropathogenic Escherichia coli O103. Infect. Immun. 2001, 69, 6785–6795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taieb, F.; Nougayrède, J.P.; Oswald, E. Cycle inhibiting factors (Cifs): Cyclomodulins that usurp the ubiquitin-dependent degradation pathway of host cells. Toxins 2011, 3, 356–368. [Google Scholar] [CrossRef] [Green Version]
- Lax, A.J. Opinion: Bacterial toxins and cancer--a case to answer? Nat. Rev. Microbiol. 2005, 3, 343–349. [Google Scholar] [CrossRef]
- Martin, H.M.; Campbell, B.J.; Hart, C.A.; Mpofu, C.; Nayar, M.; Singh, R.; Englyst, H.; Williams, H.F.; Rhodes, J.M. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology 2004, 127, 80–93. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, M.; Buc, E.; Sauvanet, P.; Darcha, C.; Dubois, D.; Pereira, B.; Dechelotte, P.; Bonnet, R.; Pezet, D.; Darfeuille-Michaud, A. Colonization of the human gut by E. coli and colorectal cancer risk. Clin. Cancer Res. 2014, 20, 859–867. [Google Scholar] [CrossRef] [Green Version]
- Iyadorai, T.; Mariappan, V.; Vellasamy, K.M.; Wanyiri, J.W.; Roslani, A.C.; Lee, G.K.; Sears, C.; Vadivelu, J. Prevalence and association of pks+ Escherichia coli with colorectal cancer in patients at the University Malaya Medical Centre, Malaysia. PLoS ONE 2020, 15, e0228217. [Google Scholar] [CrossRef]
- Nougayrède, J.-P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J.; Dobrindt, U.; Oswald, E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 2006, 313, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Bossuet-Greif, N.; Vignard, J.; Taieb, F.; Mirey, G.; Dubois, D.; Petit, C.; Oswald, E.; Nougayrède, J.-P. The Colibactin Genotoxin Generates DNA Interstrand Cross-Links in Infected Cells. MBio 2018, 9, e02393-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.R.; Jiang, Y.; Villalta, P.W.; Stornetta, A.; Boudreau, P.D.; Carrá, A.; Brennan, C.A.; Chun, E.; Ngo, L.; Samson, L.D.; et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science 2019, 363, eaar7785. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Kim, C.S.; Healy, A.R.; Wernke, K.M.; Wang, Z.; Frischling, M.C.; Shine, E.E.; Wang, W.; Herzon, S.B.; Crawford, J.M. Structure elucidation of colibactin and its DNA cross-links. Science 2019, 365, eaax2685. [Google Scholar] [CrossRef]
- Grasso, F.; Frisan, T. Bacterial Genotoxins: Merging the DNA Damage Response into Infection Biology. Biomolecules 2015, 5, 1762–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbri, A.; Travaglione, S.; Rosadi, F.; Ballan, G.; Maroccia, Z.; Giambenedetti, M.; Guidotti, M.; Ødum, N.; Krejsgaard, T.; Fiorentini, C. The Escherichia coli protein toxin cytotoxic necrotizing factor 1 induces epithelial mesenchymal transition. Cell. Microbiol. 2019, 22, e13138. [Google Scholar] [CrossRef]
- Sears, C.L. Enterotoxigenic Bacteroides fragilis: A rogue among symbiotes. Clin. Microbiol. Rev. 2009, 22, 349–369. [Google Scholar] [CrossRef] [Green Version]
- Thiele Orberg, E.; Fan, H.; Tam, A.J.; Dejea, C.M.; Destefano Shields, C.E.; Wu, S.; Chung, L.; Finard, B.B.; Wu, X.; Fathi, P.; et al. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol. 2017, 10, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Thiele Orberg, E.; Geis, A.L.; Chan, J.L.; Fu, K.; DeStefano Shields, C.E.; Dejea, C.M.; Fathi, P.; Chen, J.; Finard, B.B.; et al. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe 2018, 23, 203–214.e5. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Morin, P.J.; Maouyo, D.; Sears, C.L. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology 2003, 124, 392–400. [Google Scholar] [CrossRef]
- Wu, S.; Rhee, K.J.; Zhang, M.; Franco, A.; Sears, C.L. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and γ-secretase-dependent E-cadherin cleavage. J. Cell Sci. 2007, 120, 1944–1952. [Google Scholar] [CrossRef] [Green Version]
- Rhee, K.J.; Wu, S.; Wu, X.; Huso, D.L.; Karim, B.; Franco, A.A.; Rabizadeh, S.; Golub, J.E.; Mathews, L.E.; Shin, J.; et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect. Immun. 2009, 77, 1708–1718. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef]
- Wick, E.C.; Rabizadeh, S.; Albesiano, E.; Wu, X.Q.; Wu, S.; Chan, J.; Rhee, K.J.; Ortega, G.; Huso, D.L.; Pardoll, D.; et al. Stat3 activation in murine colitis induced by enterotoxigenic bacteroides fragilis. Inflamm. Bowel Dis. 2014, 20, 821–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boleij, A.; Hechenbleikner, E.M.; Goodwin, A.C.; Badani, R.; Stein, E.M.; Lazarev, M.G.; Ellis, B.; Carroll, K.C.; Albesiano, E.; Wick, E.C.; et al. The bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 2015, 60, 208–215. [Google Scholar] [CrossRef]
- Haghi, F.; Goli, E.; Mirzaei, B.; Zeighami, H. The association between fecal enterotoxigenic B. fragilis with colorectal cancer. BMC Cancer 2019, 19, 879. [Google Scholar] [CrossRef] [Green Version]
- Zamani, S.; Taslimi, R.; Sarabi, A.; Jasemi, S.; Sechi, L.A.; Feizabadi, M.M. Enterotoxigenic Bacteroides fragilis: A Possible Etiological Candidate for Bacterially-Induced Colorectal Precancerous and Cancerous Lesions. Front. Cell. Infect. Microbiol. 2019, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A.; Kang, H.M.; Fuchsberger, C.; Danecek, P.; Sharp, K.; et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, P.R.; Danecek, P.; Palamara, P.F.; Fuchsberger, C.; Reshef, Y.A.; Finucane, H.K.; Schoenherr, S.; Forer, L.; McCarthy, S.; Abecasis, G.R.; et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat. Genet. 2016, 48, 1443–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durbin, R. Efficient haplotype matching and storage using the positional Burrows-Wheeler transform (PBWT). Bioinformatics 2014, 30, 1266–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.W.; O’Reilly, P.F. PRSice-2: Polygenic Risk Score software for biobank-scale data. Gigascience 2019, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- R Core Team; R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 7 June 2021).
| Characteristic | Overall, N = 325 | Healthy Tissue (Controls), N = 162 | Hyperplastic Polyps (HP), N = 55 | Polyps/Adenoma (PA), N = 79 | Adenocarcinoma (ADK), N = 29 |
|---|---|---|---|---|---|
| Hospital, n (%) | |||||
| SU | 184 (57%) | 80 (49%) | 45 (82%) | 54 (68%) | 5 (17%) |
| RE | 141 (43%) | 82 (51%) | 10 (18%) | 25 (32%) | 24 (83%) |
| Sex, n (%) | |||||
| M | 162 (50%) | 66 (41%) | 34 (62%) | 46 (58%) | 16 (55%) |
| F | 163 (50%) | 96 (59%) | 21 (38%) | 33 (42%) | 13 (45%) |
| Age | |||||
| Mean (SD) | 60 (12) | 59 (12) | 60 (11) | 61 (12) | 64 (14) |
| Median (range) | 60 (22, 87) | 59 (22, 87) | 62 (29, 81) | 60 (36, 87) | 68 (34, 85) |
| BMI | |||||
| Mean (SD) | 25.4 (3.9) | 24.9 (4.1) | 26.1 (4.1) | 25.8 (3.4) | 25.9 (3.9) |
| Median (range) | 24.9 (13.8, 40.4) | 24.2 (13.8, 40.4) | 26.1 (16.4, 37.4) | 25.7 (19.7, 37.5) | 25.8 (19.4, 31.8) |
| Unknown | 6 | 5 | 0 | 0 | 1 |
| Alcohol consumption, n (%) | 76 (23%) | 35 (22%) | 20 (36%) | 16 (20%) | 5 (17%) |
| Wine consumption, n (%) | 165 (51%) | 75 (46%) | 31 (56%) | 45 (58%) | 14 (48%) |
| Unknown | 1 | 0 | 0 | 1 | 0 |
| Physical activity n (%) | 129 (40%) | 65 (40%) | 24 (44%) | 32 (41%) | 8 (29%) |
| Unknown | 2 | 1 | 0 | 0 | 1 |
| Non-drinker, n (%) | 52 (16%) | 30 (19%) | 3 (5.5%) | 10 (13%) | 9 (32%) |
| Unknown | 3 | 2 | 0 | 0 | 1 |
| Diet, n (%) | |||||
| Mediterranean | 293 (92%) | 148 (93%) | 45 (82%) | 73 (99%) | 27 (93%) |
| Vegetarian | 15 (4.7%) | 8 (5.0%) | 5 (9.1%) | 0 (0%) | 2 (6.9%) |
| Vegan | 1 (0.3%) | 1 (0.6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Other | 8 (2.5%) | 2 (1.3%) | 5 (9.1%) | 1 (1.4%) | 0 (0%) |
| Unknown | 8 | 3 | 0 | 5 | 0 |
| Smoking, n (%) | |||||
| No | 153 (47%) | 80 (49%) | 18 (33%) | 43 (54%) | 12 (41%) |
| Yes | 68 (21%) | 30 (19%) | 19 (35%) | 15 (19%) | 4 (14%) |
| Ex | 104 (32%) | 52 (32%) | 18 (33%) | 21 (27%) | 13 (45%) |
| Previous removal of polyps, n (%) | |||||
| Malignant polyp | 50 (15%) | 30 (19%) | 5 (9.1%) | 11 (14%) | 4 (14%) |
| Benign polyp | 80 (25%) | 29 (18%) | 28 (51%) | 21 (27%) | 2 (7.1%) |
| No removal | 191 (59%) | 101 (63%) | 22 (40%) | 46 (59%) | 22 (79%) |
| Unknown | 4 | 2 | 0 | 1 | 1 |
| Biopsy site, n (%) | |||||
| Caecum | 25 (7.7%) | 5 (3.1%) | 4 (7.3%) | 14 (18%) | 2 (6.9%) |
| Ascending | 54 (17%) | 27 (17%) | 9 (16%) | 17 (22%) | 1 (3.4%) |
| Hepatic flexure | 5 (1.5%) | 0 (0%) | 1 (1.8%) | 4 (5.1%) | 0 (0%) |
| Transverse | 13 (4.0%) | 1 (0.6%) | 6 (11%) | 6 (7.6%) | 0 (0%) |
| Descending | 15 (4.6%) | 0 (0%) | 2 (3.6%) | 11 (14%) | 2 (6.9%) |
| Sigma | 174 (54%) | 120 (75%) | 22 (40%) | 21 (27%) | 11 (38%) |
| Colon nas | 1 (0.3%) | 1 (0.6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Rectum | 37 (11%) | 7 (4.3%) | 11 (20%) | 6 (7.6%) | 13 (45%) |
| Unknown | 1 | 1 | 0 | 0 | 0 |
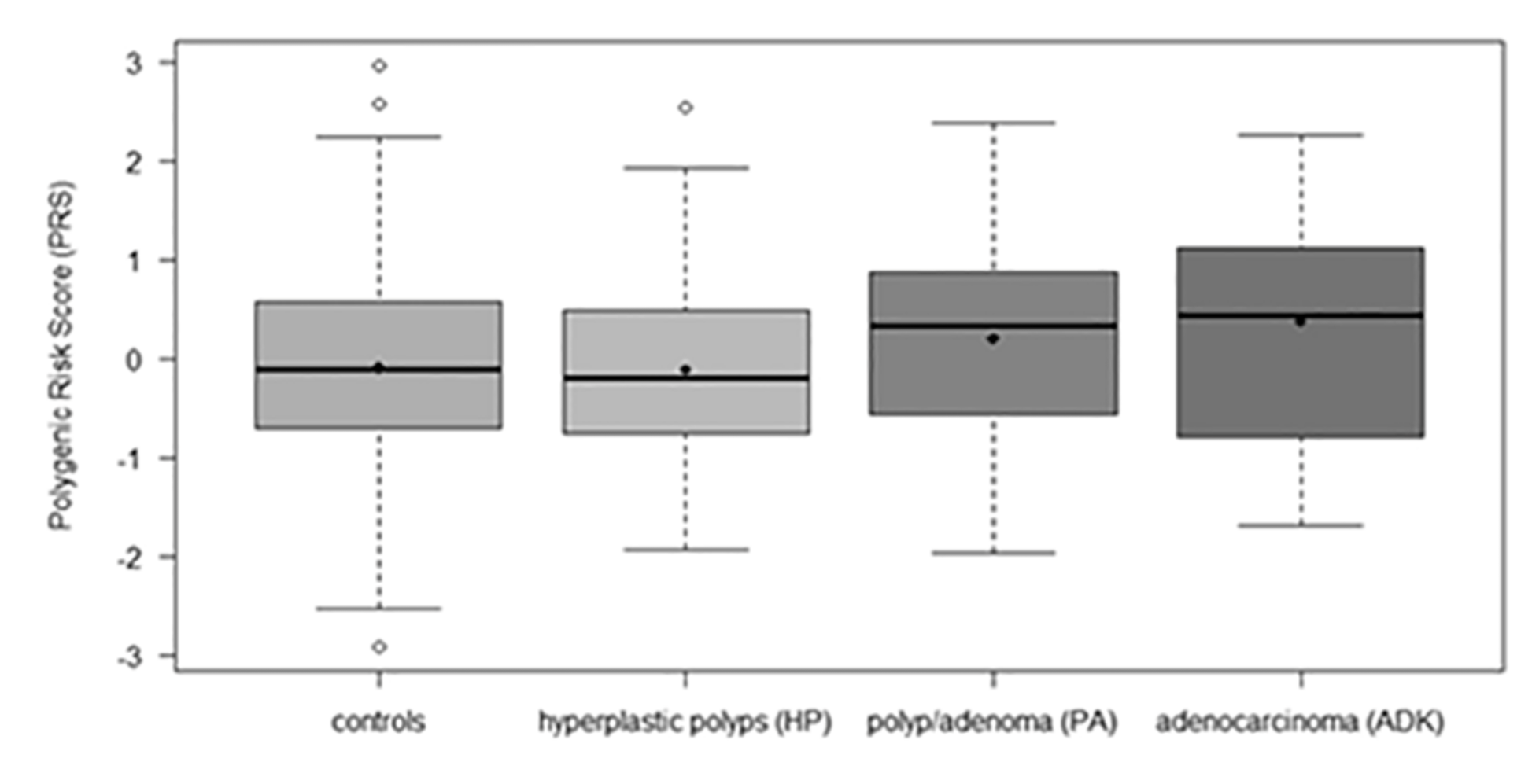
| Polygenic Risk Score (PRS) | |||||
| Mean (SD) | 0.01 (1.00) | −0.10 (1.01) | −0.11 (0.95) | 0.20 (0.94) | 0.36 (1.15) |
| Median (range) | 0.03 (−2.92, 2.96) | −0.11 (−2.92, 2.96) | −0.20 (−1.94, 2.53) | 0.33 (−1.98, 2.37) | 0.43 (−1.70, 2.26) |
| Unknown | 52 | 17 | 11 | 17 | 7 |
| Characteristic | Overall, N = 325 | Healthy Tissue (Controls), N = 162 | Hyperplastic Polyps (HP), N = 55 | Polyps/Adenoma (PA), N = 79 | Adenocarcinoma (ADK), N = 29 |
|---|---|---|---|---|---|
| cif, n (%) | 32 (10%) | 11 (7.1%) | 7 (13%) | 12 (16%) | 2 (7.4%) |
| Unknown | 17 | 7 | 3 | 5 | 2 |
| cdt, n (%) | 40 (13%) | 18 (12%) | 2 (3.8%) | 15 (20%) | 5 (19%) |
| Unknown | 17 | 7 | 3 | 5 | 2 |
| cnf1, n (%) | 89 (29%) | 42 (27%) | 16 (31%) | 20 (27%) | 11 (41%) |
| Unknown | 17 | 7 | 3 | 5 | 2 |
| pks+, n (%) | 117 (38%) | 54 (35%) | 22 (42%) | 27 (36%) | 14 (52%) |
| Unknown | 17 | 7 | 3 | 5 | 2 |
| bft, n (%) | 43 (13%) | 23 (14%) | 6 (11%) | 10 (13%) | 4 (14%) |
| Unknown | 6 | 3 | 1 | 1 | 1 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Characteristic | OR 1 | 95%CI 1 | p-Value 1 | OR 1 | 95%CI 1 | p-Value 1 |
| cif, yes vs. no | 1.05 | 0.16, 4.21 | 0.95 | |||
| cdt, yes vs. no | 1.73 | 0.53, 4.86 | 0.32 | |||
| cnf1, yes vs. no | 1.85 | 0.78, 4.28 | 0.15 | |||
| pks+, yes vs. no | 2.01 | 0.88, 4.64 | 0.10 | |||
| bft, yes vs. no | 0.99 | 0.27, 2.85 | 0.98 | |||
| Sum of aerobic toxins | 1.60 | 1.02, 2.54 | 0.042 | |||
| Sum of all toxins | 1.47 | 0.96, 2.24 | 0.073 | |||
| Polygenic Risk Score (PRS) | 1.55 | 1.00, 2.45 | 0.055 | 1.55 | 1.00, 2.45 | 0.055 |
| Age | 1.04 | 1.01, 1.08 | 0.027 | |||
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Characteristic | OR 1 | 95%CI 1 | p-Value 1 | OR 1 | 95%CI 1 | p-Value 1 |
| cif, yes vs. no | 2.53 | 1.06, 6.14 | 0.036 | 2.57 | 1.06, 6.33 | 0.037 |
| cdt, yes vs. no | 1.94 | 0.90, 4.10 | 0.085 | |||
| cnf1, yes vs. no | 1.20 | 0.59, 2.35 | 0.61 | |||
| pks+, yes vs. no | 1.37 | 0.72, 2.60 | 0.33 | |||
| bft, yes vs. no | 0.74 | 0.26, 1.82 | 0.54 | |||
| Polygenic Risk Score (PRS) | 1.36 | 1.00, 1.87 | 0.051 | |||
| Sex, F vs. M | 0.49 | 0.28, 0.85 | 0.011 | 0.48 | 0.27, 0.84 | 0.011 |
| BMI | 1.07 | 1.00, 1.15 | 0.068 | |||
| Wine consumption, yes vs. no | 1.58 | 0.92, 2.74 | 0.10 | |||
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Characteristic | OR 1 | 95%CI 1 | p-Value 1 | OR1 | 95%CI 1 | p-Value 1 |
| cif, yes vs. no | 2.04 | 0.71, 5.49 | 0.17 | |||
| cdt, yes vs. no | 0.30 | 0.05, 1.11 | 0.12 | |||
| cnf1, yes vs. no | 1.20 | 0.59, 2.35 | 0.61 | |||
| pks+, yes vs. no | 1.37 | 0.72, 2.60 | 0.33 | |||
| bft, yes vs. no | 0.74 | 0.26, 1.82 | 0.54 | |||
| Sex, F vs. M | 0.42 | 0.22, 0.79 | 0.007 | |||
| BMI | 1.08 | 1.00, 1.16 | 0.047 | 1.10 | 1.01, 1.19 | 0.026 |
| Alcohol consumption, yes vs. no | 2.07 | 1.06, 4.02 | 0.032 | |||
| Non-drinker, yes vs. no | 0.25 | 0.06, 0.74 | 0.027 | 0.23 | 0.05, 0.73 | 0.026 |
| Smoking | ||||||
| Yes vs. no | 2.81 | 1.31, 6.13 | 0.008 | |||
| Ex vs. no | 1.54 | 0.73, 3.24 | 0.25 | |||
| Smoking, yes vs. no | 2.01 | 1.07, 3.88 | 0.034 | |||
| Previous removal of polyps | ||||||
| Yes benign vs. yes malignant | 5.79 | 2.10, 18.9 | 0.001 | 5.45 | 1.90, 18.3 | 0.003 |
| No vs. yes malignant | 1.31 | 0.49, 4.16 | 0.62 | 1.17 | 0.42, 3.81 | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piciocchi, A.; Germinario, E.A.P.; Garcia Etxebarria, K.; Rossi, S.; Sanchez-Mete, L.; Porowska, B.; Stigliano, V.; Trentino, P.; Oddi, A.; Accarpio, F.; et al. Association of Polygenic Risk Score and Bacterial Toxins at Screening Colonoscopy with Colorectal Cancer Progression: A Multicenter Case-Control Study. Toxins 2021, 13, 569. https://doi.org/10.3390/toxins13080569
Piciocchi A, Germinario EAP, Garcia Etxebarria K, Rossi S, Sanchez-Mete L, Porowska B, Stigliano V, Trentino P, Oddi A, Accarpio F, et al. Association of Polygenic Risk Score and Bacterial Toxins at Screening Colonoscopy with Colorectal Cancer Progression: A Multicenter Case-Control Study. Toxins. 2021; 13(8):569. https://doi.org/10.3390/toxins13080569
Chicago/Turabian StylePiciocchi, Alfonso, Elena Angela Pia Germinario, Koldo Garcia Etxebarria, Silvia Rossi, Lupe Sanchez-Mete, Barbara Porowska, Vittoria Stigliano, Paolo Trentino, Andrea Oddi, Fabio Accarpio, and et al. 2021. "Association of Polygenic Risk Score and Bacterial Toxins at Screening Colonoscopy with Colorectal Cancer Progression: A Multicenter Case-Control Study" Toxins 13, no. 8: 569. https://doi.org/10.3390/toxins13080569
APA StylePiciocchi, A., Germinario, E. A. P., Garcia Etxebarria, K., Rossi, S., Sanchez-Mete, L., Porowska, B., Stigliano, V., Trentino, P., Oddi, A., Accarpio, F., Grazi, G. L., Bruno, G., Bonucci, M., Giambenedetti, M., Spigaglia, P., Barbanti, F., Owczarek, S., Luzzi, I., Delibato, E., ... Fabbri, A. (2021). Association of Polygenic Risk Score and Bacterial Toxins at Screening Colonoscopy with Colorectal Cancer Progression: A Multicenter Case-Control Study. Toxins, 13(8), 569. https://doi.org/10.3390/toxins13080569








