In Vivo Models and In Vitro Assays for the Assessment of Pertussis Toxin Activity
Abstract
1. Introduction
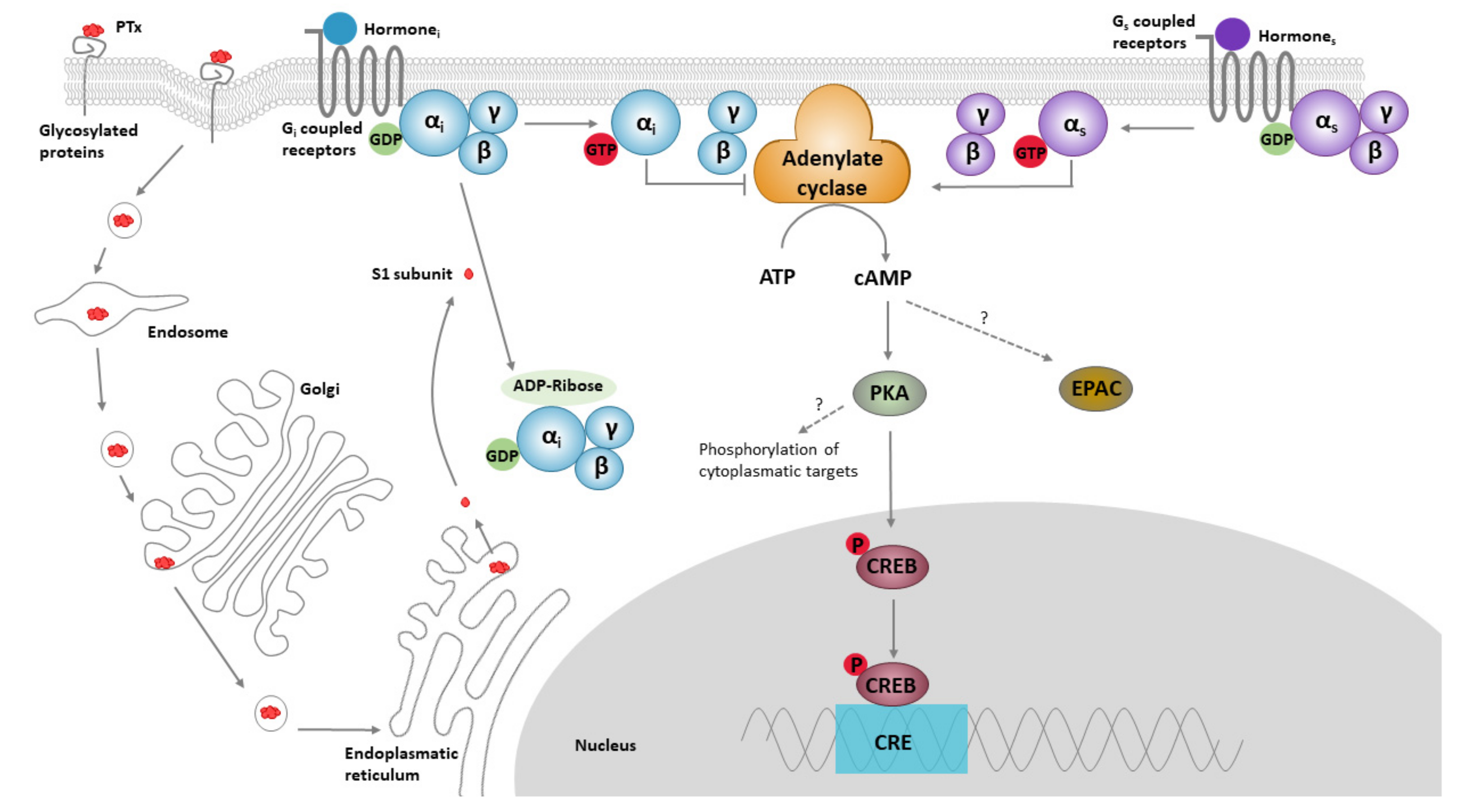
1.1. PTx Structure, Function and Biology
1.2. Role of PTx in Pathology and Toxicity
1.3. PTx Reference Preparations
2. In Vivo Models
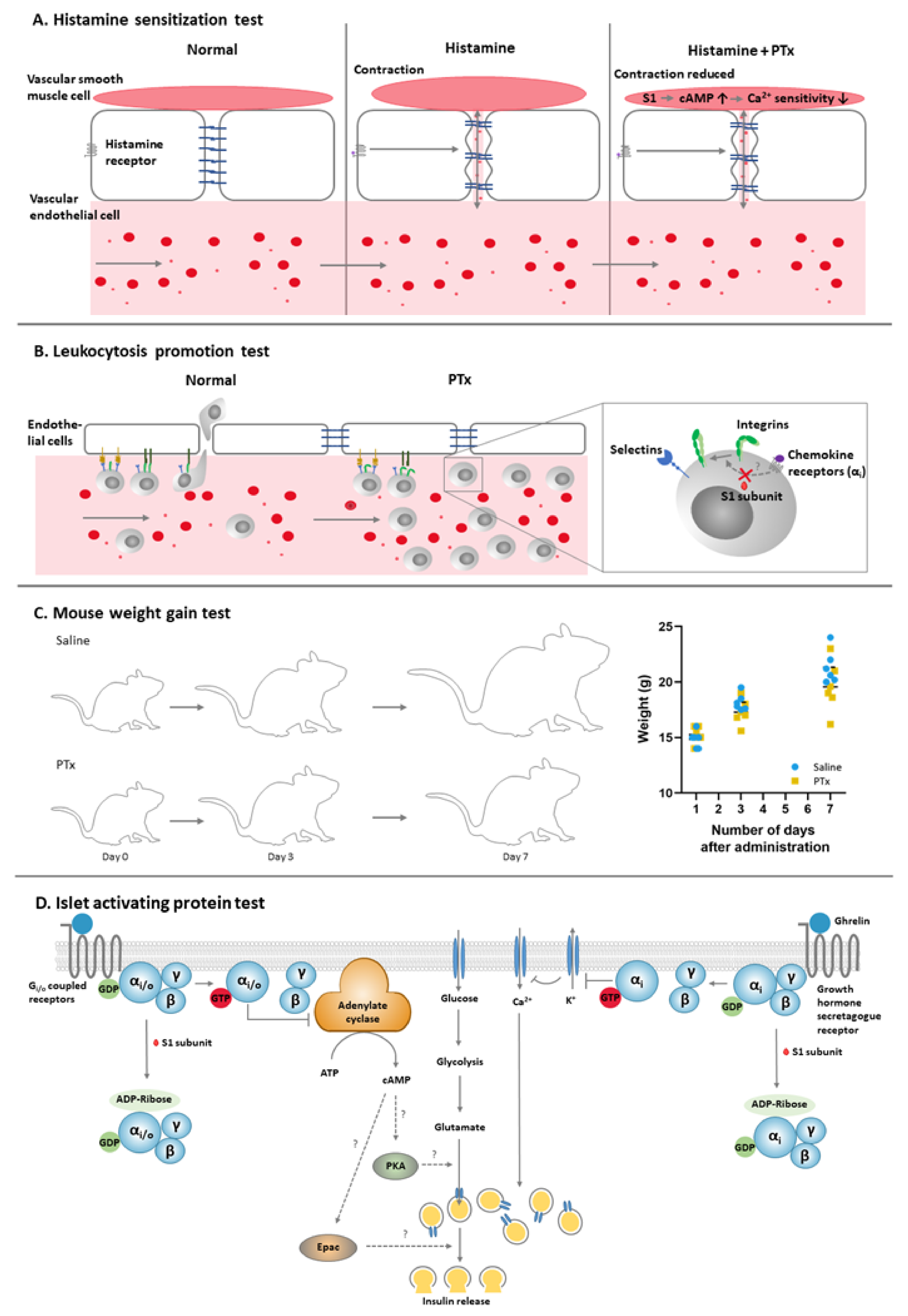
2.1. Histamine Sensitization Test (HIST)
2.2. Leukocytosis Promotion (LP) Test
2.3. Mouse Weight Gain (MWG) Test
2.4. Islet-Activating Protein (IAP) Test
2.5. Other In Vivo Models
2.6. Ex Vivo Methods
3. Biochemical Assays
3.1. Enzymatic-HPLC-Coupled Assay
3.2. Fetuin Binding Assay
4. Cellular Assays
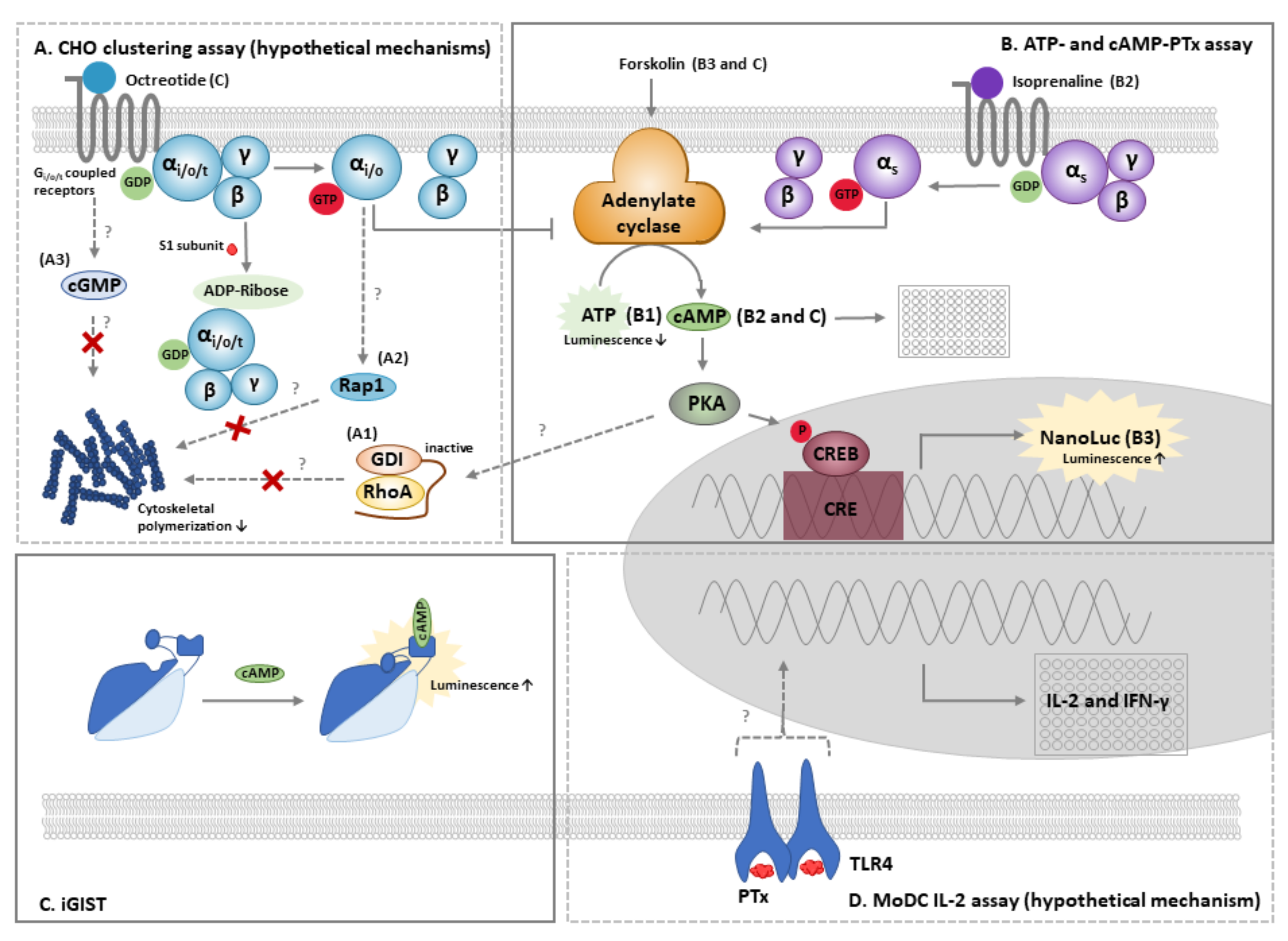
4.1. The CHO Cell Clustering Assay
4.2. The ATP and cAMP-PTx Assay
4.3. Other Cellular Methods
5. Regulatory Considerations of PTx Testing
6. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | adenylate cyclases |
| aP | acellular pertussis |
| BRP1 | Biological Reference Preparation 1 |
| CHO | Chinese hamster ovary |
| CRE | cAMP response elements |
| CREB | cAMP response element-binding protein |
| dPTx | genetically detoxified PTx |
| EAE | experimental autoimmune encephalomyelitis |
| ED | Effective Dose |
| EDQM | European Directorate for the Quality of Medicines & Healthcare |
| EPAC | ex-change protein directly activated by cAMP |
| FHA | filamentous hemagglutinin |
| HIST | histamine sensitization test |
| IAP | Islet-activating protein |
| IS | International Standard |
| IU | International Units |
| LP | Leukocytosis promotion |
| LPS | lipopolysaccharide |
| MoDC | monocyte derived dendritic cells |
| MS | multiple sclerosis |
| MWG | Mouse weight gain |
| NIBSC | National Institute for Biological Standards and Control |
| Ph. Eur. | European Pharmacopeia |
| PKA | protein kinase A |
| PTd | pertussis toxoid |
| PTx | Pertussis toxin |
| SSTR2 | somatostatin receptor 2 |
| WHO | World Health Organization |
| wP | whole-cell pertussis |
References
- Yeung, K.H.T.; Duclos, P.; Nelson, E.A.S.; Hutubessy, R.C.W. An update of the global burden of pertussis in children younger than 5 years: A modelling study. Lancet Infect. Dis. 2017, 17, 974–980. [Google Scholar] [CrossRef]
- Libster, R.; Edwards, K.M. Re-emergence of pertussis: What are the solutions? Expert Rev. Vaccines 2012, 11, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Ito, A.; Chiba, J.; Sato, Y. Monoclonal antibody against pertussis toxin: Effect on toxin activity and pertussis infections. Infect. Immun. 1984, 46, 422–428. [Google Scholar] [CrossRef]
- Black, W.J.; Munoz, J.J.; Peacock, M.G.; Schad, P.A.; Cowell, J.L.; Burchall, J.J.; Lim, M.; Kent, A.; Steinman, L.; Falkow, S. ADP-ribosyltransferase activity of pertussis toxin and immunomodulation by Bordetella pertussis. Science 1988, 240, 656–659. [Google Scholar] [CrossRef]
- Markey, K.; Asokanathan, C.; Feavers, I. Assays for Determining Pertussis Toxin Activity in Acellular Pertussis Vaccines. Toxins 2019, 11, 417. [Google Scholar] [CrossRef]
- Plaut, R.D.; Carbonetti, N.H. Retrograde transport of pertussis toxin in the mammalian cell. Cell. Microbiol. 2008, 10, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Katada, T.; Tamura, M.; Ui, M. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch. Biochem. Biophys. 1983, 224, 290–298. [Google Scholar] [CrossRef]
- Krueger, K.M.; Barbieri, J.T. The family of bacterial ADP-ribosylating exotoxins. Clin. Microbiol. Rev. 1995, 8, 34–47. [Google Scholar] [CrossRef]
- Carbonetti, N.H. Contribution of pertussis toxin to the pathogenesis of pertussis disease. Pathog. Dis. 2015, 73, ftv073. [Google Scholar] [CrossRef]
- de Gouw, D.; Diavatopoulos, D.A.; Bootsma, H.J.; Hermans, P.W.; Mooi, F.R. Pertussis: A matter of immune modulation. FEMS Microbiol. Rev. 2011, 35, 441–474. [Google Scholar] [CrossRef]
- Wu, E.H.; Wong, Y.H. Pertussis toxin-sensitive Gi/o proteins are involved in nerve growth factor-induced pro-survival Akt signaling cascade in PC12 cells. Cell. Signal. 2005, 17, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.H.; Wong, Y.H. Involvement of G i/o proteins in nerve growth factor-stimulated phosphorylation and degradation of tuberin in PC-12 cells and cortical neurons. Mol. Pharmacol. 2005, 67, 1195–1205. [Google Scholar] [CrossRef]
- Clark, M.J.; Harrison, C.; Zhong, H.; Neubig, R.R.; Traynor, J.R. Endogenous RGS protein action modulates mu-opioid signaling through Galphao. Effects on adenylyl cyclase, extracellular signal-regulated kinases, and intracellular calcium pathways. J. Biol. Chem. 2003, 278, 9418–9425. [Google Scholar] [CrossRef]
- Yung, L.Y.; Tso, P.H.; Wu, E.H.; Yu, J.C.; Ip, N.Y.; Wong, Y.H. Nerve growth factor-induced stimulation of p38 mitogen-activated protein kinase in PC12 cells is partially mediated via G(i/o) proteins. Cell. Signal. 2008, 20, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Strnad, C.F.; Carchman, R.A. Human T lymphocyte mitogenesis in response to the B oligomer of pertussis toxin is associated with an early elevation in cytosolic calcium concentrations. FEBS Lett. 1987, 225, 16–20. [Google Scholar] [CrossRef]
- Tamura, M.; Nogimori, K.; Yajima, M.; Ase, K.; Ui, M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J. Biol. Chem. 1983, 258, 6756–6761. [Google Scholar] [CrossRef]
- Toyota, T.; Kai, Y.; Kakizaki, M.; Sakai, A.; Goto, Y.; Yajima, M.; Ui, M. Effects of islet-activating protein (IAP) on blood glucose and plasma insulin in healthy volunteers (phase 1 studies). Tohoku J. Exp. Med. 1980, 130, 105–116. [Google Scholar] [CrossRef]
- Li, H.; Wong, W.S. Pertussis toxin activates tyrosine kinase signaling cascade in myelomonocytic cells: A mechanism for cell adhesion. Biochem. Biophys. Res. Commun. 2001, 283, 1077–1082. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Yang, D.; Chen, Q.; Leifer, C.A.; Segal, D.M.; Su, S.B.; Caspi, R.R.; Howard, Z.O.; Oppenheim, J.J. Induction of dendritic cell maturation by pertussis toxin and its B subunit differentially initiate Toll-like receptor 4-dependent signal transduction pathways. Exp. Hematol. 2006, 34, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Suda, R.; Nagamatsu, Y.; Tanabe, S.; Onohara, N.; Nakaya, M.; Kanaho, Y.; Shibata, T.; Uchida, K.; Sumimoto, H.; et al. Pertussis toxin up-regulates angiotensin type 1 receptors through Toll-like receptor 4-mediated Rac activation. J. Biol. Chem. 2010, 285, 15268–15277. [Google Scholar] [CrossRef]
- Xing, D.; Das, G.; Newland, P.; Corbel, M. Collaborative Study: Evaluation of Proposed International Standard of Pertussis Toxin Code JNIH-5; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Munoz, J. Permeability changes produced in mice by Bordetella pertussis. J. Immunol. 1961, 86, 618–626. [Google Scholar]
- van Meijeren, C.E.; Vleeming, W.; van de Kuil, T.; Manni, J.; Kegler, D.; Hendriksen, C.F.; de Wildt, D.J. In vivo pertussis toxin treatment reduces contraction of rat resistance arteries but not that of mouse trachea. Eur. J. Pharmacol. 2004, 488, 127–135. [Google Scholar] [CrossRef]
- Kappelle, I.V.S.-V.D.; Van Der Gun, J.W.; Marsman, F.R.; Hendriksen, C.F.; Van De Donk, H.J. Collaborative Study on Test Systems to Assess Toxicity of Whole Cell Pertussis Vaccine. Biologicals 1997, 25, 41–57. [Google Scholar] [CrossRef][Green Version]
- Ashworth, L.A.; Robinson, A.; Irons, L.I.; Morgan, C.P.; Isaacs, D. Antigens in whooping cough vaccine and antibody levels induced by vaccination of children. Lancet 1983, 2, 878–881. [Google Scholar] [CrossRef]
- Munoz, J.J.; Arai, H.; Bergman, R.K.; Sadowski, P.L. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect. Immun. 1981, 33, 820–826. [Google Scholar] [CrossRef]
- Xing, D.; Maes, A.; Behr-Gross, M.E.; Costanzo, A.; Daas, A.; Buchheit, K.H. Collaborative study for the standardisation of the histamine sensitizing test in mice and the CHO cell-based assay for the residual toxicity testing of acellular pertussis vaccines. Pharmeur. Bio. Sci. Notes 2010, 2010, 51–63. [Google Scholar]
- Bache, C.; Hoonakker, M.; Hendriksen, C.; Buchheit, K.-H.; Spreitzer, I.; Montag, T. Workshop on Animal free Detection of Pertussis Toxin in Vaccines—Alternatives to the Histamine Sensitisation Test. Biologicals 2012, 40, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Markey, K.; Asokanathan, C.; Tierney, S.; Hockley, J.; Douglas-Bardsley, A. Collaborative Study: Evaluation of Proposed Second International Standard for Pertussis Toxin Code: 15/126; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Wall, M.; Costanzo, A.; Le Tallec, D.; Isbrucker, R. Establishment of Pertussis toxin BRP batch 2 for CHO clustering assay. Pharmeur. Bio. Sci. Notes 2021, 2021, 69–87. [Google Scholar] [PubMed]
- Kind, L.S. The altered reactivity of mice after inoculation with Bordetella pertussis vaccine. Bacteriol. Rev. 1958, 22, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.K.; Munoz, J. Circulatory Collapse in Anaphylaxis and Histamine Toxicity in Mice. J. Immunol. 1965, 95, 1–8. [Google Scholar] [PubMed]
- Nencioni, L.; Pizza, M.G.; Volpini, G.; De Magistris, M.T.; Giovannoni, F.; Rappuoli, R. Properties of the B oligomer of pertussis toxin. Infect. Immun. 1991, 59, 4732–4734. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.; Bergman, R.K. Histamine-sensitizing factors from microbial agents, with special reference to Bordetella pertussis. Bacteriol. Rev. 1968, 32, 103–126. [Google Scholar] [CrossRef]
- Kost, C.K., Jr.; Herzer, W.A.; Li, P.J.; Jackson, E.K. Pertussis toxin-sensitive G-proteins and regulation of blood pressure in the spontaneously hypertensive rat. Clin. Exp. Pharmacol. Physiol. 1999, 26, 449–455. [Google Scholar] [CrossRef]
- Jackson, E.K. Pertussis toxin normalizes enhanced renovascular responses to angiotensin II in spontaneously hypertensive rats. Life Sci. 1994, 54, PL445–PL450. [Google Scholar] [CrossRef]
- Li, Y.; Anand-Srivastava, M.B. Inactivation of enhanced expression of G(i) proteins by pertussis toxin attenuates the development of high blood pressure in spontaneously hypertensive rats. Circ. Res. 2002, 91, 247–254. [Google Scholar] [CrossRef]
- Tabrizchi, R.; Triggle, C.R. Pressor actions of arginine vasopressin in pithed Sprague-Dawley, Wistar-Kyoto and spontaneously hypertensive rats before and after treatment with nifedipine or pertussis toxin. J. Hypertens. 1991, 9, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Hoonakker, M.; Arciniega, J.; Hendriksen, C. Safety testing of acellular pertussis vaccines: Use of animals and 3Rs alternatives. Hum. Vaccines Immunother. 2017, 13, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Redhead, K.; Seagroatt, V. The effects of purified components of Bordetella pertussis in the weight gain test for the toxicity testing of pertussis vaccines. J. Biol. Stand. 1986, 14, 57–65. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Takahashi, M.; Asada, S.; Ishida, S. Increased Levels of Active Pertussis Toxin May Aid a Pertussis Vaccine to Pass the Mouse Body Weight Gain Test. Biologicals 1994, 22, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Yajima, M.; Hosoda, K.; Kanbayashi, Y.; Nakamura, T.; Takahashi, I.; Ui, M. Biological Properties of Islets-Activating Protein (IAP) Purified from the Culture Medium of Bordetella pertussis. J. Biochem. 1978, 83, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Arciniega, J.L.; Corvettea, L.; Hsua, H.; Lynna, F.; Romania, T.; Dobbelaer, R. Target alternative vaccine safety testing strategies for pertussis toxin. Procedia Vaccinol. 2011, 5, 248–260. [Google Scholar] [CrossRef]
- Jensen, S.E.; Illigen, K.E.; Badsberg, J.H.; Haslov, K.R. Specificity and detection limit of a dermal temperature histamine sensitization test for absence of residual pertussis toxin in vaccines. J. Biol. Stand. 2012, 40, 36–40. [Google Scholar] [CrossRef]
- Gaines-Das, R.; Ochiai, M.; Douglas-Bardsley, A.; Asokanathan, C.; Horiuchi, Y.; Rigsby, P.; Corbel, M.J.; Xing, D.K. Transferability of dermal temperature histamine sensitization test for estimation of pertussis toxin activity in vaccines. Hum. Vaccine 2009, 5, 166–171. [Google Scholar] [CrossRef]
- Ochiai, M.; Yamamoto, A.; Kataoka, M.; Toyoizumi, H.; Arakawa, Y.; Horiuchi, Y. Highly sensitive histamine-sensitization test for residual activity of pertussis toxin in acellular pertussis vaccine. J. Biol. Stand. 2007, 35, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.D.; Corvette, L.J.; Ngundi, M.M.; Burns, D.L. Towards replacement of the acellular pertussis vaccine safety test: Comparison of in vitro cytotoxic activity and in vivo activity in mice. Vaccine 2017, 35, 7160–7165. [Google Scholar] [CrossRef]
- Morse, S.I.; Morse, J.H. Isolation and properties of the leukocytosis- and lymphocytosis-promoting factor of Bordetella pertussis. J. Exp. Med. 1976, 143, 1483–1502. [Google Scholar] [CrossRef]
- Morse, S.I. Lymphocytosis-promoting factor of Bordetella pertussis: Isolation, characterization, and biological activity. J. Infect. Dis. 1977, 136, S234–S238. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Saxena, S.N.; Sharma, S.B.; Ahuja, S. The effects of purified pertussis components and lipopolysaccharide on the results of the mouse weight gain test. J. Biol. Stand. 1988, 16, 321–331. [Google Scholar] [CrossRef]
- Sakuma, S.; Imagawa, Y.; Tokunaga, E.; Ohtomo, N. Increase in intradermal vascular permeability caused by pertussis toxin from Bordetella pertussis. Microbiol. Immunol. 1987, 31, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Brito, G.A.; Souza, M.H.; Melo-Filho, A.A.; Hewlett, E.L.; Lima, A.A.; Flores, C.A.; Ribeiro, R.A. Role of pertussis toxin A subunit in neutrophil migration and vascular permeability. Infect. Immun. 1997, 65, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Carbonetti, N.H. Pertussis leukocytosis: Mechanisms, clinical relevance and treatment. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef]
- Pierce, C.; Klein, N.; Peters, M. Is leukocytosis a predictor of mortality in severe pertussis infection? Intensive Care Med. 2000, 26, 1512–1514. [Google Scholar] [CrossRef]
- Heininger, U.; Stehr, K.; Schmitt-Grohe, S.; Lorenz, C.; Rost, R.; Christenson, P.D.; Uberall, M.; Cherry, J.D. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatric Infect. Dis. J. 1994, 13, 306–309. [Google Scholar] [CrossRef]
- Bouchez, V.; Brun, D.; Cantinelli, T.; Dore, G.; Njamkepo, E.; Guiso, N. First report and detailed characterization of B. pertussis isolates not expressing Pertussis Toxin or Pertactin. Vaccine 2009, 27, 6034–6041. [Google Scholar] [CrossRef]
- Nguyen, A.W.; Wagner, E.K.; Laber, J.R.; Goodfield, L.L.; Smallridge, W.E.; Harvill, E.T.; Papin, J.F.; Wolf, R.F.; Padlan, E.A.; Bristol, A.; et al. A cocktail of humanized anti-pertussis toxin antibodies limits disease in murine and baboon models of whooping cough. Sci. Transl. Med. 2015, 7, 316ra195. [Google Scholar] [CrossRef]
- Samore, M.H.; Siber, G.R. Effect of pertussis toxin on susceptibility of infant rats to Haemophilus influenzae type b. J. Infect. Dis. 1992, 165, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Elahi, S.; Brownlie, R.; Korzeniowski, J.; Buchanan, R.; O’Connor, B.; Peppler, M.S.; Halperin, S.A.; Lee, S.F.; Babiuk, L.A.; Gerdts, V. Infection of newborn piglets with Bordetella pertussis: A new model for pertussis. Infect. Immun. 2005, 73, 3636–3645. [Google Scholar] [CrossRef] [PubMed]
- Hinds, P.W., 2nd; Yin, C.; Salvato, M.S.; Pauza, C.D. Pertussis toxin induces lymphocytosis in rhesus macaques. J. Med. Primatol. 1996, 25, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Pauza, C.D.; Hinds, P.W., 2nd; Yin, C.; McKechnie, T.S.; Hinds, S.B.; Salvato, M.S. The lymphocytosis-promoting agent pertussis toxin affects virus burden and lymphocyte distribution in the SIV-infected rhesus macaque. AIDS research and human retroviruses 1997, 13, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Warnock, R.A.; Askari, S.; Butcher, E.C.; von Andrian, U.H. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J. Exp. Med. 1998, 187, 205–216. [Google Scholar] [CrossRef]
- Pham, T.H.; Baluk, P.; Xu, Y.; Grigorova, I.; Bankovich, A.J.; Pappu, R.; Coughlin, S.R.; McDonald, D.M.; Schwab, S.R.; Cyster, J.G. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 2010, 207, 17–27. [Google Scholar] [CrossRef]
- Nencioni, L.; Pizza, M.; Bugnoli, M.; De Magistris, T.; Di Tommaso, A.; Giovannoni, F.; Manetti, R.; Marsili, I.; Matteucci, G.; Nucci, D.; et al. Characterization of genetically inactivated pertussis toxin mutants: Candidates for a new vaccine against whooping cough. Infect. Immun. 1990, 58, 1308–1315. [Google Scholar] [CrossRef]
- WHO. Manual for Quality Control of Diphtheria, Tetanus and Pertussis Vaccines. Available online: http://apps.who.int/iris/bitstream/10665/80681/1/WHO_IVB_11.11_eng.pdf (accessed on 9 August 2021).
- WHO. Annex 4 Recommendations to Assure the Quality, Safety and Efficacy of Acellular Pertussis Vaccines; Replacement of Annex 2 of WHO Technical Report Series; WHO: Geneva, Switzerland, 2013; Volume 878. [Google Scholar]
- Pittman, M.; Cox, C.B. Pertussis Vaccine Testing for Freedom-from-Toxicity. Appl. Microbiol. 1965, 13, 447–456. [Google Scholar] [CrossRef]
- Butler, N.R.; Voyce, M.A.; Burland, W.L.; Hilton, M.L. Advantages of aluminium hydroxide adsorbed combined diphtheria, tetanus, and pertussis vaccines for the immunization of infants. BMJ 1969, 1, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; van Ramshorst, J.D.; Drion, E.F. Relation between toxicity tests in mice and reactions in children using four lots of quadruple vaccine (DPT-polio). Sym. Series Immunobiol. Stand. 1969, 10, 53–62. [Google Scholar]
- Hilton, M.L.; Burland, W.L. Pertussis-containing vaccines: The relationship between laboratory toxicity tests and reactions in children. Sym. Ser. Immunobiol. Stand. 1970, 13, 150–156. [Google Scholar]
- Perkins, F.T.; Sheffield, F.; Miller, C.L.; Skegg, J.L. The comparison of toxicity of pertussis vaccines in children and mice. Sym. Series Immunobiol. Stand. 1970, 13, 41–49. [Google Scholar]
- Komatsu, M.; McDermott, A.M.; Gillison, S.L.; Sharp, G.W. Time course of action of pertussis toxin to block the inhibition of stimulated insulin release by norepinephrine. Endocrinology 1995, 136, 1857–1863. [Google Scholar] [CrossRef]
- Katada, T.; Ui, M. Slow interaction of islet-activating protein with pancreatic islets during primary culture to cause reversal of alpha-adrenergic inhibition of insulin secretion. J. Biol. Chem. 1980, 255, 9580–9588. [Google Scholar] [CrossRef]
- Katada, T.; Ui, M. Islet-activating protein. A modifier of receptor-mediated regulation of rat islet adenylate cyclase. J. Biol. Chem. 1981, 256, 8310–8317. [Google Scholar] [CrossRef]
- Malaisse, W.J.; Svoboda, M.; Dufrane, S.P.; Malaisse-Lagae, F.; Christophe, J. Effect of Bordetella pertussis toxin on ADP-ribosylation of membrane proteins, adenylate cyclase activity and insulin release in rat pancreatic islets. Biochem. Biophys. Res. Commun. 1984, 124, 190–196. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Monograph 0161. Pertussis Vaccine (Whole Cell, Adsorbed), 7th Ed. ed; European Department for the Quality of Medicines within the Council of Europe: Strasbourg, France, 2011. [Google Scholar]
- Oddy, J.G.; Evans, D.G. The effects produced by toxic and non-toxic extracts of H. pertussis and Br. bronchiseptica on the blood sugar of rabbits. J. Path. Bact. 1940, 50, 11–16. [Google Scholar] [CrossRef]
- Pittman, M. Mouse breeds and the toxicity test for pertussis vaccine. Dev. Biol. Stand. 1980, 45, 129–135. [Google Scholar]
- Gulbenkian, A.; Schobert, L.; Nixon, C.; Tabachnick, I.I. Metabolic effects of pertussis sensitization in mice and rats. Endocrinology 1968, 83, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Kreeftenberg, J.G.; van Straaten-van de Kappelle, I.; de Wildt, D.J.; Terligen, J.B.; Peters, W.J.; Walvoort, H.C. A biphasic serum glucose response in mice to inoculation with pertussis vaccine. J. Biol. Stand. 1984, 12, 151–157. [Google Scholar] [CrossRef]
- Katada, T.; Ui, M. Islet-activating protein. Enhanced insulin secretion and cyclic AMP accumulation in pancreatic islets due to activation of native calcium ionophores. J. Biol. Chem. 1979, 254, 469–479. [Google Scholar] [CrossRef]
- Vega, S.C.; Leiss, V.; Piekorz, R.; Calaminus, C.; Pexa, K.; Vuozzo, M.; Schmid, A.M.; Devanathan, V.; Kesenheimer, C.; Pichler, B.J.; et al. Selective protection of murine cerebral Gi/o-proteins from inactivation by parenterally injected pertussis toxin. J. Mol. Med. 2020, 98, 97–110. [Google Scholar] [CrossRef]
- Neer, E.J.; Lok, J.M.; Wolf, L.G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J. Biol. Chem. 1984, 259, 14222–14229. [Google Scholar] [CrossRef]
- Gheni, G.; Ogura, M.; Iwasaki, M.; Yokoi, N.; Minami, K.; Nakayama, Y.; Harada, K.; Hastoy, B.; Wu, X.; Takahashi, H.; et al. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell. Rep. 2014, 9, 661–673. [Google Scholar] [CrossRef]
- Sato, H.; Sato, Y.; Ito, A.; Ohishi, I. Effect of monoclonal antibody to pertussis toxin on toxin activity. Infect. Immun. 1987, 55, 909–915. [Google Scholar] [CrossRef]
- Kerfoot, S.M.; Long, E.M.; Hickey, M.J.; Andonegui, G.; Lapointe, B.M.; Zanardo, R.C.; Bonder, C.; James, W.G.; Robbins, S.M.; Kubes, P. TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J. Immunol. 2004, 173, 7070–7077. [Google Scholar] [CrossRef]
- Carbonetti, N.H.; Artamonova, G.V.; Van Rooijen, N.; Ayala, V.I. Pertussis toxin targets airway macrophages to promote Bordetella pertussis infection of the respiratory tract. Infect. Immun. 2007, 75, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Wu, Y.; Sun, S.; Shi, M.; Sun, Y.; Yang, C.; Pei, G.; Gu, Y.; Zhong, C.; Sun, B. Pertussis toxin enhances Th1 responses by stimulation of dendritic cells. J. Immunol. 2003, 170, 1728–1736. [Google Scholar] [CrossRef]
- Meade, B.D.; Kind, P.D.; Manclark, C.R. Altered mononuclear phagocyte function in mice treated with the lymphocytosis promoting factor of Bordetella pertussis. Dev. Biol. Stand. 1985, 61, 63–74. [Google Scholar] [PubMed]
- Lummen, G.; Sperling, H.; Eisenhardt, A.; vom Dorp, F.; Otto, T.; Rubben, H. Influence of pertussis toxin on superficial bladder carcinoma in rats. Urol. Res. 2002, 30, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Lummen, G.; Virchow, S.; Rumenapp, U.; Schmidt, M.; Wieland, T.; Otto, T.; Rubben, H.; Jakobs, K.H. Identification of G protein-coupled receptors potently stimulating migration of human transitional-cell carcinoma cells. NSAPCC 1997, 356, 769–776. [Google Scholar] [CrossRef]
- Gupta, R.K.; Saxena, S.N.; Sharma, S.B.; Ahuja, S. Hemagglutination activities of purified pertussis toxin and filamentous hemagglutinin against erythrocytes from various animals. Microbiol. Immunol. 1990, 34, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, H.H.; Shive, C.L.; Forsthuber, T.G. Pertussis toxin modulates the immune response to neuroantigens injected in incomplete Freund’s adjuvant: Induction of Th1 cells and experimental autoimmune encephalomyelitis in the presence of high frequencies of Th2 cells. J. Immunol. 2002, 169, 117–125. [Google Scholar] [CrossRef]
- Chen, X.; Howard, O.M.; Oppenheim, J.J. Pertussis toxin by inducing IL-6 promotes the generation of IL-17-producing CD4 cells. J. Immunol. 2007, 178, 6123–6129. [Google Scholar] [CrossRef]
- Hofstetter, H.H.; Grau, C.; Buttmann, M.; Forsthuber, T.G.; Gaupp, S.; Toyka, K.V.; Gold, R. The PLPp-specific T-cell population promoted by pertussis toxin is characterized by high frequencies of IL-17-producing cells. Cytokine 2007, 40, 35–43. [Google Scholar] [CrossRef]
- Murphey, C.; Chang, S.; Zhang, X.; Arulanandam, B.; Forsthuber, T.G. Induction of polyclonal CD8+ T cell activation and effector function by Pertussis toxin. Cell. Immunol. 2011, 267, 50–55. [Google Scholar] [CrossRef]
- Beck, T.C.; Gomes, A.C.; Cyster, J.G.; Pereira, J.P. CXCR4 and a cell-extrinsic mechanism control immature B lymphocyte egress from bone marrow. J. Exp. Med. 2014, 211, 2567–2581. [Google Scholar] [CrossRef]
- Sindt, K.A.; Hewlett, E.L.; Redpath, G.T.; Rappuoli, R.; Gray, L.S.; Vandenberg, S.R. Pertussis toxin activates platelets through an interaction with platelet glycoprotein Ib. Infect. Immun. 1994, 62, 3108–3114. [Google Scholar] [CrossRef]
- Banga, H.S.; Walker, R.K.; Winberry, L.K.; Rittenhouse, S.E. Pertussis toxin can activate human platelets. Comparative effects of holotoxin and its ADP-ribosylating S1 subunit. J. Biol. Chem. 1987, 262, 14871–14874. [Google Scholar] [CrossRef]
- Endoh, M.; Soga, M.; Nakase, Y. In vitro assay for histamine-sensitizing factor of Bordetella pertussis. Microbiol. Immunol. 1980, 24, 887–890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weber, M.S.; Benkhoucha, M.; Lehmann-Horn, K.; Hertzenberg, D.; Sellner, J.; Santiago-Raber, M.L.; Chofflon, M.; Hemmer, B.; Zamvil, S.S.; Lalive, P.H. Repetitive pertussis toxin promotes development of regulatory T cells and prevents central nervous system autoimmune disease. PLoS ONE 2010, 5, e16009. [Google Scholar] [CrossRef]
- Yin, J.X.; Tu, J.L.; Lin, H.J.; Shi, F.D.; Liu, R.L.; Zhao, C.B.; Coons, S.W.; Kuniyoshi, S.; Shi, J. Centrally administered pertussis toxin inhibits microglia migration to the spinal cord and prevents dissemination of disease in an EAE mouse model. PLoS ONE 2010, 5, e12400. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Morales, M.; Sanchez-Garcia, F.J.; Guevara-Salazar, P.; Arrieta, O.; Hernandez-Pedro, N.Y.; Sanchez-Garcia, A.; Perez-Madrigal, R.; Rangel-Lopez, E.; Pineda, B.; Sotelo, J. Adjuvant immunotherapy of C6 glioma in rats with pertussis toxin. J. Cancer Res. Clin. Oncol. 2012, 138, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Magana-Maldonado, R.; Manoutcharian, K.; Hernandez-Pedro, N.Y.; Rangel-Lopez, E.; Perez-De la Cruz, V.; Rodriguez-Balderas, C.; Sotelo, J.; Pineda, B. Concomitant treatment with pertussis toxin plus temozolomide increases the survival of rats bearing intracerebral RG2 glioma. J. Cancer Res. Clin. Oncol. 2014, 140, 291–301. [Google Scholar] [CrossRef]
- Otto, T.; Lummen, G.; Kalble, T.; Recker, F.; Krege, S.; Bex, A.; Noll, F.; Rubben, H. Intravesical therapy with pertussis toxin before radical cystectomy in patients with bladder cancer: A Phase I study. Urology 1999, 54, 458–460. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Schmidt, W. Inhibition of pertussis toxin binding to model receptors by antipeptide antibodies directed at an antigenic domain of the S2 subunit. Infect. Immun. 1989, 57, 3828–3833. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Seitz, U.; Burk, U. Characterization of monoclonal antibodies directed against domains of pertussis toxin involved in receptor recognition. FEMS Microbiol. Immunol. 1991, 3, 269–278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nencioni, L.; Volpini, G.; Peppoloni, S.; Bugnoli, M.; De Magistris, T.; Marsili, I.; Rappuoli, R. Properties of pertussis toxin mutant PT-9K/129G after formaldehyde treatment. Infect. Immun. 1991, 59, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Burnette, W.N.; Arciniega, J.L.; Mar, V.L.; Burns, D.L. Properties of pertussis toxin B oligomer assembled in vitro from recombinant polypeptides produced by Escherichia coli. Infect. Immun. 1992, 60, 2252–2256. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.A.; Issekutz, T.B.; Kasina, A. Modulation of Bordetella pertussis infection with monoclonal antibodies to pertussis toxin. J. Infect. Dis. 1991, 163, 355–361. [Google Scholar] [CrossRef]
- Garcia-Sainz, J.A. Decreased sensitivity to alpha 2 adrenergic amines, adenosine and prostaglandins in white fat cells from hamsters treated with pertussis vaccine. FEBS Lett. 1981, 126, 306–308. [Google Scholar] [CrossRef]
- Olansky, L.; Myers, G.A.; Pohl, S.L.; Hewlett, E.L. Promotion of lipolysis in rat adipocytes by pertussis toxin: Reversal of endogenous inhibition. Proc. Natl. Acad. Sci. USA 1983, 80, 6547–6551. [Google Scholar] [CrossRef]
- Martinez-Olmedo, M.A.; Garcia-Sainz, J.A. Effect of pertussis toxin on the hormonal regulation of cyclic AMP levels in hamster fat cells. BBA 1983, 760, 215–220. [Google Scholar] [CrossRef]
- Garcia-Sainz, J.A.; Romero-Avila, M.T.; Ruiz-Arriaga, A.; Ruiz-Puente, J.; Agundis, C.; Ortiz, V.; Isibasi, A. Characterization and detoxification of an easily prepared acellular pertussis vaccine. Antigenic role of the A protomer of pertussis toxin. Vaccine 1992, 10, 341–344. [Google Scholar] [CrossRef]
- Nicosia, A.; Perugini, M.; Franzini, C.; Casagli, M.C.; Borri, M.G.; Antoni, G.; Almoni, M.; Neri, P.; Ratti, G.; Rappuoli, R. Cloning and sequencing of the pertussis toxin genes: Operon structure and gene duplication. Proc. Natl. Acad. Sci. USA 1986, 83, 4631–4635. [Google Scholar] [CrossRef]
- Oh, H.; Kim, B.G.; Nam, K.T.; Hong, S.H.; Ahn, D.H.; Choi, G.S.; Kim, H.; Hong, J.T.; Ahn, B.Y. Characterization of the carbohydrate binding and ADP-ribosyltransferase activities of chemically detoxified pertussis toxins. Vaccine 2013, 31, 2988–2993. [Google Scholar] [CrossRef]
- Metz, B.; Kersten, G.F.; Hoogerhout, P.; Brugghe, H.F.; Timmermans, H.A.; de Jong, A.; Meiring, H.; ten Hove, J.; Hennink, W.E.; Crommelin, D.J.; et al. Identification of formaldehyde-induced modifications in proteins: Reactions with model peptides. J. Biol. Chem. 2004, 279, 6235–6243. [Google Scholar] [CrossRef]
- Milligan, G. Techniques used in the identification and analysis of function of pertussis toxin-sensitive guanine nucleotide binding proteins. Biochem. J. 1988, 255, 1–13. [Google Scholar] [CrossRef]
- Scheuring, J.; Berti, P.J.; Schramm, V.L. Transition-state structure for the ADP-ribosylation of recombinant Gialpha1 subunits by pertussis toxin. Biochemistry 1998, 37, 2748–2758. [Google Scholar] [CrossRef]
- Graf, R.; Codina, J.; Birnbaumer, L. Peptide inhibitors of ADP-ribosylation by pertussis toxin are substrates with affinities comparable to those of the trimeric GTP-binding proteins. Mol. Pharmacol. 1992, 42, 760–764. [Google Scholar]
- Cyr, T.; Menzies, A.J.; Calver, J.; Whitehouse, L.W. A quantitative analysis for the ADP-ribosylation activity of pertussis toxin: An enzymatic-HPLC coupled assay applicable to formulated whole cell and acellular pertussis vaccine products. J. Biol. Stand. 2001, 29, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.T.; Canthaboo, C.; Menzies, J.A.; Cyr, T.; Whitehouse, L.W.; Jones, C.; Corbel, M.J.; Xing, D. Detection of residual pertussis toxin in vaccines using a modified ribosylation assay. Vaccine 2002, 21, 44–52. [Google Scholar] [CrossRef]
- Isbrucker, R.; Arciniega, J.; McFarland, R.; Chapsal, J.M.; Xing, D.; Bache, C.; Nelson, S.; Costanzo, A.; Hoonakker, M.; Castiaux, A.; et al. Report on the international workshop on alternatives to the murine histamine sensitization test (HIST) for acellular pertussis vaccines: State of the science and the path forward. J. Biol. Stand. 2014, 42, 114–122. [Google Scholar] [CrossRef]
- Xing, D.; Yuen, C.T.; Asokanathan, C.; Rigsby, P.; Horiuchi, Y. Evaluation of an in vitro assay system as a potential alternative to current histamine sensitization test for acellular pertussis vaccines. J. Biol. Stand. 2012, 40, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Isbrucker, R.; Wagner, L.; Daas, A.; Costanzo, A. Transferability study of CHO cell clustering assays for monitoring of pertussis toxin activity in acellular pertussis vaccines. Pharmeur. Bio. Sci. Notes 2016, 2016-05, 97–114. [Google Scholar]
- Gomez, S.R.; Xing, D.K.; Corbel, M.J.; Coote, J.; Parton, R.; Yuen, C.T. Development of a carbohydrate binding assay for the B-oligomer of pertussis toxin and toxoid. Anal. Biochem. 2006, 356, 244–253. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Bliu, A.; Prior, F. Modified binding assay for improved sensitivity and specificity in the detection of residual pertussis toxin in vaccine preparations. Vaccine 2010, 28, 2687–2692. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.R.; Yuen, C.T.; Asokanathan, C.; Douglas-Bardsley, A.; Corbel, M.J.; Coote, J.G.; Parton, R.; Xing, D.K. ADP-ribosylation activity in pertussis vaccines and its relationship to the in vivo histamine-sensitisation test. Vaccine 2007, 25, 3311–3318. [Google Scholar] [CrossRef]
- Douglas-Bardsley, A.; Asokanathan, C.; Tierney, S.; Hockley, J.; Markey, K. Collaborative study for the calibration of the replacement International Standard for pertussis toxin for use in histamine sensitisation and CHO cell clustering assays. J. Biol. Stand. 2019, 62, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hoonakker, M.E.; Verhagen, L.M.; van der Maas, L.; Sloots, A.; Hendriksen, C.F. Reporter cell lines for detection of pertussis toxin in acellular pertussis vaccines as a functional animal-free alternative to the in vivo histamine sensitization test. Vaccine 2017, 35, 1152–1160. [Google Scholar] [CrossRef]
- Paramonov, V.M.; Sahlgren, C.; Rivero-Muller, A.; Pulliainen, A.T. iGIST-A Kinetic Bioassay for Pertussis Toxin Based on Its Effect on Inhibitory GPCR Signaling. ACS Sens. 2020, 5, 3438–3448. [Google Scholar] [CrossRef]
- Bernardo, L.; Corallo, L.; Caterini, J.; Su, J.; Gisonni-Lex, L.; Gajewska, B. Application of xCELLigence real-time cell analysis to the microplate assay for pertussis toxin induced clustering in CHO cells. PLoS ONE 2021, 16, e0248491. [Google Scholar] [CrossRef]
- Vaessen, S.F.; Verkoeijen, S.; Vandebriel, R.J.; Bruysters, M.W.; Pennings, J.L.; Bos, R.; Krul, C.A.; Akkermans, A.M. Identification of biomarkers to detect residual pertussis toxin using microarray analysis of dendritic cells. Vaccine 2013, 31, 5223–5231. [Google Scholar] [CrossRef] [PubMed]
- Oishi, A.; Makita, N.; Sato, J.; Iiri, T. Regulation of RhoA signaling by the cAMP-dependent phosphorylation of RhoGDIalpha. J. Biol. Chem. 2012, 287, 38705–38715. [Google Scholar] [CrossRef] [PubMed]
- Locht, C.; Coutte, L.; Mielcarek, N. The ins and outs of pertussis toxin. FEBS J. 2011, 278, 4668–4682. [Google Scholar] [CrossRef]
- Millen, S.H.; Lewallen, D.M.; Herr, A.B.; Iyer, S.S.; Weiss, A.A. Identification and characterization of the carbohydrate ligands recognized by pertussis toxin via a glycan microarray and surface plasmon resonance. Biochemistry 2010, 49, 5954–5967. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, E.L.; Sauer, K.T.; Myers, G.A.; Cowell, J.L.; Guerrant, R.L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect. Immun. 1983, 40, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.G.; McNamara, U.; Carbonetti, N.H. Expression, activity and cytotoxicity of pertussis toxin S1 subunit in transfected mammalian cells. Cell. Microbiol. 2001, 3, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bartley, T.D.; Whiteley, D.W.; Mar, V.L.; Burns, D.L.; Burnette, W.N. Pertussis holotoxoid formed in vitro with a genetically deactivated S1 subunit. Proc. Natl. Acad. Sci. USA 1989, 86, 8353–8357. [Google Scholar] [CrossRef]
- Krueger, K.M.; Barbieri, J.T. Assignment of functional domains involved in ADP-ribosylation and B-oligomer binding within the carboxyl terminus of the S1 subunit of pertussis toxin. Infect. Immun. 1994, 62, 2071–2078. [Google Scholar] [CrossRef]
- Zamith, H.; Godinho, R.O.; da Costa Junior, V.L.; Corrado, A.P. The quantitative analysis of the mechanism involved in pertussis toxin-mediated cell clustering and its implications in the in vitro quality control of diphtheria tetanus and whole cell pertussis vaccines. Toxicol. In Vitro 2021, 70, 105029. [Google Scholar] [CrossRef]
- Burns, D.L.; Kenimer, J.G.; Manclark, C.R. Role of the A subunit of pertussis toxin in alteration of Chinese hamster ovary cell morphology. Infect. Immun. 1987, 55, 24–28. [Google Scholar] [CrossRef]
- Hall, A. Rho GTPases and the actin cytoskeleton. Science 1998, 279, 509–514. [Google Scholar] [CrossRef]
- Jordan, J.D.; He, J.C.; Eungdamrong, N.J.; Gomes, I.; Ali, W.; Nguyen, T.; Bivona, T.G.; Philips, M.R.; Devi, L.A.; Iyengar, R. Cannabinoid receptor-induced neurite outgrowth is mediated by Rap1 activation through G(alpha)o/i-triggered proteasomal degradation of Rap1GAPII. J. Biol. Chem. 2005, 280, 11413–11421. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Siber, G.R. Need for a reference preparation of pertussis antitoxin for Chinese hamster ovary cell assay. J. Biol. Stand. 1995, 23, 71–73. [Google Scholar] [CrossRef]
- Xing, D.; Das, R.G.; Newland, P.; Corbel, M. Comparison of the bioactivity of reference preparations for assaying Bordetella pertussis toxin activity in vaccines by the histamine sensitisation and Chinese hamster ovary-cell tests: Assessment of validity of expression of activity in terms of protein concentration. Vaccine 2002, 20, 3535–3542. [Google Scholar]
- Markey, K.; Douglas-Bardsley, A.; Hockley, J.; Le Tallec, D.; Costanzo, A. Calibration of pertussis toxin BRP batch 1 in a standardised CHO cell-based clustering assay. Pharmeur. Bio. Sci. Notes 2018, 2018, 112–123. [Google Scholar]
- Fujiwara, H.; Iwasa, S. The quantitative assay of the clustering activity of the lymphocytosis-promoting factor (pertussis toxin) of Bordetella pertussis on Chinese hamster ovary (CHO) cells. J. Biol. Stand. 1989, 17, 53–64. [Google Scholar] [CrossRef]
- Gillenius, P.; Jaatmaa, E.; Askelof, P.; Granstrom, M.; Tiru, M. The standardization of an assay for pertussis toxin and antitoxin in microplate culture of Chinese hamster ovary cells. J. Biol. Stand. 1985, 13, 61–66. [Google Scholar] [CrossRef]
- Hoonakker, M.E.; Ruiterkamp, N.; Hendriksen, C.F. The cAMP assay: A functional in vitro alternative to the in vivo Histamine Sensitization test. Vaccine 2010, 28, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.A.; Burns, D.L.; Kanaho, Y.; Liu, T.Y.; Hewlett, E.L.; Moss, J. ADP-ribosylation of transducin by pertussis toxin. J. Biol. Chem. 1985, 260, 13478–13482. [Google Scholar] [CrossRef]
- van der Heden van Noort, G.J. Chemical Tools to Study Protein ADP-Ribosylation. ACS Omega 2020, 5, 1743–1751. [Google Scholar] [CrossRef]
- Yan, K.; Gao, L.N.; Cui, Y.L.; Zhang, Y.; Zhou, X. The cyclic AMP signaling pathway: Exploring targets for successful drug discovery (Review). Mol. Med. Rep. 2016, 13, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, J.U.; Nikolaev, V.O. Biophysical techniques for detection of cAMP and cGMP in living cells. Int. J. Mol. Sci. 2013, 14, 25–8025. [Google Scholar] [CrossRef]
- Paramonov, V.M.; Desai, D.; Kettiger, H.; Mamaeva, V.; Rosenholm, J.M.; Sahlgren, C.; Rivero-Muller, A. Targeting Somatostatin Receptors By Functionalized Mesoporous Silica Nanoparticles—Are We Striking Home? Nanotheranostics 2018, 2, 320–346. [Google Scholar] [CrossRef]
- Mangmool, S.; Kurose, H. G(i/o) protein-dependent and -independent actions of Pertussis Toxin (PTX). Toxins 2011, 3, 884–899. [Google Scholar] [CrossRef] [PubMed]
- Momose, H.; Mizukami, T.; Ochiai, M.; Hamaguchi, I.; Yamaguchi, K. A new method for the evaluation of vaccine safety based on comprehensive gene expression analysis. J. Biomed. Biotechnol. 2010, 2010, 361841. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, I.; Imai, J.; Momose, H.; Kawamura, M.; Mizukami, T.; Naito, S.; Maeyama, J.; Masumi, A.; Kuramitsu, M.; Takizawa, K.; et al. Application of quantitative gene expression analysis for pertussis vaccine safety control. Vaccine 2008, 26, 4686–4696. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Fric, J.; Wong, A.Y.; Ricciardi-Castagnoli, P. Interleukin-2 production by dendritic cells and its immuno-regulatory functions. Front. Immunol. 2012, 3, 161. [Google Scholar] [CrossRef] [PubMed]
- Boyman, O.; Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012, 12, 180–190. [Google Scholar] [CrossRef]
- Vremec, D.; O’Keeffe, M.; Hochrein, H.; Fuchsberger, M.; Caminschi, I.; Lahoud, M.; Shortman, K. Production of interferons by dendritic cells, plasmacytoid cells, natural killer cells, and interferon-producing killer dendritic cells. Blood 2007, 109, 1165–1173. [Google Scholar] [CrossRef]
- Nasso, M.; Fedele, G.; Spensieri, F.; Palazzo, R.; Costantino, P.; Rappuoli, R.; Ausiello, C.M. Genetically detoxified pertussis toxin induces Th1/Th17 immune response through MAPKs and IL-10-dependent mechanisms. J. Immunol. 2009, 183, 1892–1899. [Google Scholar] [CrossRef]
- Ausiello, C.M.; Fedele, G.; Urbani, F.; Lande, R.; Di Carlo, B.; Cassone, A. Native and genetically inactivated pertussis toxins induce human dendritic cell maturation and synergize with lipopolysaccharide in promoting T helper type 1 responses. J. Infect. Dis. 2002, 186, 351–360. [Google Scholar] [CrossRef]
- Tonon, S.; Goriely, S.; Aksoy, E.; Pradier, O.; Del Giudice, G.; Trannoy, E.; Willems, F.; Goldman, M.; De Wit, D. Bordetella pertussis toxin induces the release of inflammatory cytokines and dendritic cell activation in whole blood: Impaired responses in human newborns. Eur. J. Immunol. 2002, 32, 3118–3125. [Google Scholar] [CrossRef]
- Bagley, K.C.; Abdelwahab, S.F.; Tuskan, R.G.; Fouts, T.R.; Lewis, G.K. Pertussis toxin and the adenylate cyclase toxin from Bordetella pertussis activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cAMP-dependent pathway. J. Leukoc. Biol. 2002, 72, 962–969. [Google Scholar]
- Bache, C.; Spreitzer, I.; Becker, B.; Loeschner, B.; Rosskopf, U.; Hanschmann, K.M.; Schwanig, M.; Schneider, C.K.; Lieb, B.; Montag, T. Bordetella Pertussis Toxin does not induce the release of pro-inflammatory cytokines in human whole blood. Med. Microbiol. Immunol. 2012, 201, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, H.; Dewit, J.; Van der Wegen, A.; De Braekeleer, J.; Van Heule, G.; Dekegel, D.; De Baetselier, P. The lymphocytosis promoting action of pertussis toxin can be mimicked in vitro. Holotoxin but not the B subunit inhibits invasion of human T lymphoma cells through fibroblast monolayers. J. Immunol. Methods 1991, 144, 231–240. [Google Scholar] [CrossRef]
- Spangrude, G.J.; Sacchi, F.; Hill, H.R.; Van Epps, D.E.; Daynes, R.A. Inhibition of lymphocyte and neutrophil chemotaxis by pertussis toxin. J. Immunol. 1985, 135, 4135–4143. [Google Scholar] [PubMed]
- Kugler, S.; Bocker, K.; Heusipp, G.; Greune, L.; Kim, K.S.; Schmidt, M.A. Pertussis toxin transiently affects barrier integrity, organelle organization and transmigration of monocytes in a human brain microvascular endothelial cell barrier model. Cell. Microbiol. 2007, 9, 619–632. [Google Scholar] [CrossRef]
- Gilder, A.S.; Wang, L.; Natali, L.; Karimi-Mostowfi, N.; Brifault, C.; Gonias, S.L. Pertussis Toxin Is a Robust and Selective Inhibitor of High Grade Glioma Cell Migration and Invasion. PLoS ONE 2016, 11, e0168418. [Google Scholar] [CrossRef]
| PTx Dose | PTx Source | Response | Reference | |
|---|---|---|---|---|
| In adults | 1.0 μg/kg | B. pertussis Tohama | no adverse effects | [17] |
| In children | 260 to 300 ng | PTx in two wP vaccines | considered safe for vaccination | [24,25] |
| In mice | 200 ng | purified from B. pertussis 3779 | no deaths | [26] |
| In mice (HIST) * | 12 IU ** | BRP1 (HIST) | ED50 | [27] |
| In mice (HIST) * | 1–2 IU *** | BRP1 (HIST) | ED5 | [28] |
| Name | Provider | µg/Vial | IU/Vial HIST | IU/Vial CHO |
|---|---|---|---|---|
| JNIH-5 | WHO | 62.5 * | 10,000 | 10,000 |
| 2nd IS | WHO | 20 | 1881 | 680 |
| BRP1 | EDQM | 50 | 7500 | 1360 |
| BRP2 | EDQM | n.d. | n.d. | 130 |
| LIST Biological | LIST Biological | 50 | n.d. | n.d. |
| Model | PTx Source and Detected Range | PTx Range Detected in Vaccines | Coverage of PTx Properties and/or Mechanism | Main Area of Application * | Ref. | ||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||
| HIST lethal pass/fail | 2–12 IU (HIST) BRP1 ** | 2–15 ng LIST Biological | 5–125 ng U.S. PT control preparation | 5–74 ng/mL *** (wP vaccine), 2–15 ng LIST Biological | Binding, internalisation, and ADP ribosylation | Release testing and research | [24,27,33,43,47] |
| HIST temperature pass/fail | 1.5–7.5 IU (HIST) BRP1 | Binding, internalisation, and ADP ribosylation | Release testing and research | [44] | |||
| HIST temperature quantitative | 1–4 HSU/mL of Japanese ref. aP preparation | 0.58–5.25 IU (NIBSC 90/518) | 0.01–1 IU § (aP vaccine) | Binding, internalisation, and ADP ribosylation | Release testing and research | [45,46] | |
| LP | 20–4000 ng (NIH 114 (3779B)) §§ | 188–1500 ng (JNIH-5) | Binding, internalisation, and ADP ribosylation | Release testing and research | [48,49,50] | ||
| MWG | 113–450 ng (W28) | 4000 ng §§ (strain n.s.) | 375–1500 ng (JNIH-5) | 65–370 ng *** (wP vaccine) | Unknown | Release testing of wP vaccines and research | [24,40,41,50] |
| IAP | 8–2000 ng (Tohama) §§ | Binding, internalisation, and ADP ribosylation | Research | [42] | |||
| Vascular permeability | 1–100,000 ng (Tohama) §§ | B oligomer-dependent | Research | [51,52] | |||
| Assay | Source of PTx and Detected Range | Detected Range in aP Vaccines | Coverage of PTx Properties | Compatible with | Main Area of Application * | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||
| Fetuin ELISA | 4–250 ng/well (NIBSC 90/518) | 1.5–196 ng/mL (Sanofi Pasteur) | 0.36–3.63 IU (CHO)/mL BRP1 | Binding | Purified PTx and desorbed aP vaccine preparations | Research | [123,126,127] | |
| HPLC | 62.5–4000 ng/mL (NIBSC 90/518) a | 15.6–500 ng/mL (NIBSC 90/518) b | 10–100 ng (NIBSC 90/518) c | 0.5–2.25 µg/mL (NIBSC 90/518) | Enzymatic activity | Purified PTx and complete aP and wP vaccine preparations ** | Research | [121,122,123,128] |
| CHO cell clustering (visual reading) | 1.27–1813 mIU (CHO)/mL BRP1 | 1.14–8 mIU/mL JNIH-5 | 0.1–30 ng/mL LIST Biological | 181–725 mIU (CHO)/mL BRP1 *** | Binding, internalisation, enzymatic activity | Purified PTx and pellet fraction aP vaccines | Bulk testing of aP vaccines and research | [47,125,129,130] |
| CHO cell clustering (confluence analysis) | 1–1000 ng/mL LIST Biological | Binding, internalisation, enzymatic activity | Purified PTx | Research | [131] | |||
| CHO cell clustering (impedance) | 23–5803 mIU (CHO)/mL BRP1 | 0.4–49 ng/mL (PTx Sanofi Pasteur) | 453 and 1813 mIU (CHO)/mL BRP1 § | Binding, internalisation, enzymatic activity | Purified PTx, PTd | Research | [132] | |
| CHO cell clustering (3N method) | 3–725 mIU (CHO)/mL BRP1 | 0.005–4 ng/mL LIST Biological | 45–181 mIU (CHO)/mL BRP1 | Binding, internalisation, enzymatic activity | Purified PTx and pellet fraction aP vaccines | Research | Hoonakker et al. submitted | |
| cAMP-PTx reporter | 23–136 mIU (CHO)/mL BRP1 (linear range) | 25–1600 mIU/mL JNIH-5 | 5-160 ng/mL WHO 2nd IS 15/126 | 68–363 mIU (CHO)/mL BRP1 (linear range) | Binding, internalisation, enzymatic activity | Purified PTx and pellet fraction aP vaccines | Research | [130] Hoonakker et al. in preparation |
| iGIST | 1–1000 ng/mL LIST Biological | 1–1000 ng/mL Invitrogen | 100 ng/mL LIST Biological | Binding, internalisation, enzymatic activity | Purified PTx and complete aP vaccines | Research | [131] | |
| MoDC IL-2 | 12.5–50 IU/mL JNIH-5 | 100 and 250 ng/mL GSK | Unknown | Purified PTx | Research | [133] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoonakker, M.E. In Vivo Models and In Vitro Assays for the Assessment of Pertussis Toxin Activity. Toxins 2021, 13, 565. https://doi.org/10.3390/toxins13080565
Hoonakker ME. In Vivo Models and In Vitro Assays for the Assessment of Pertussis Toxin Activity. Toxins. 2021; 13(8):565. https://doi.org/10.3390/toxins13080565
Chicago/Turabian StyleHoonakker, Marieke Esther. 2021. "In Vivo Models and In Vitro Assays for the Assessment of Pertussis Toxin Activity" Toxins 13, no. 8: 565. https://doi.org/10.3390/toxins13080565
APA StyleHoonakker, M. E. (2021). In Vivo Models and In Vitro Assays for the Assessment of Pertussis Toxin Activity. Toxins, 13(8), 565. https://doi.org/10.3390/toxins13080565




