A Biochemical and Pharmacological Characterization of Phospholipase A2 and Metalloproteinase Fractions from Eastern Russell’s Viper (Daboia siamensis) Venom: Two Major Components Associated with Acute Kidney Injury
Abstract
:1. Introduction
2. Results
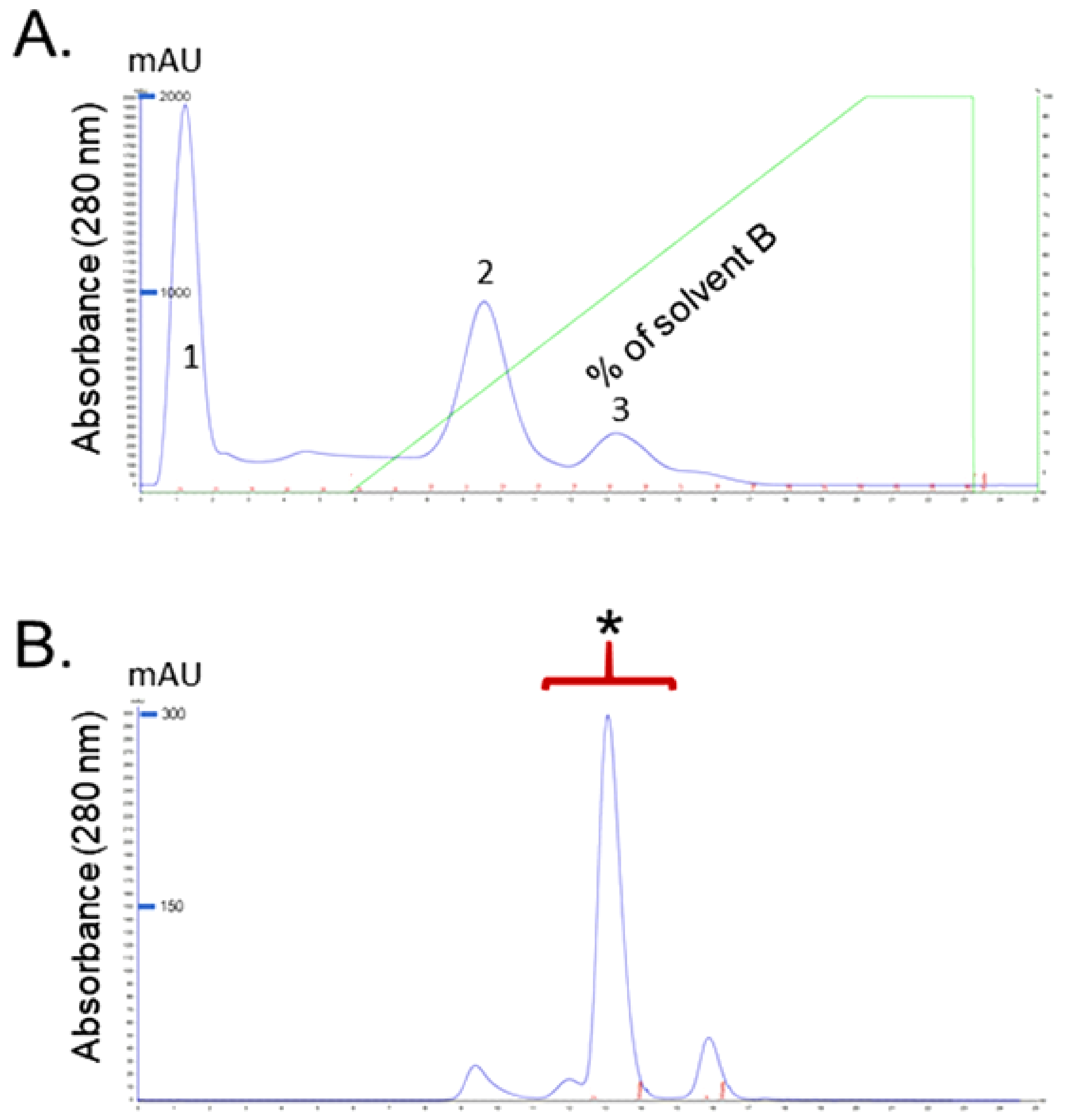
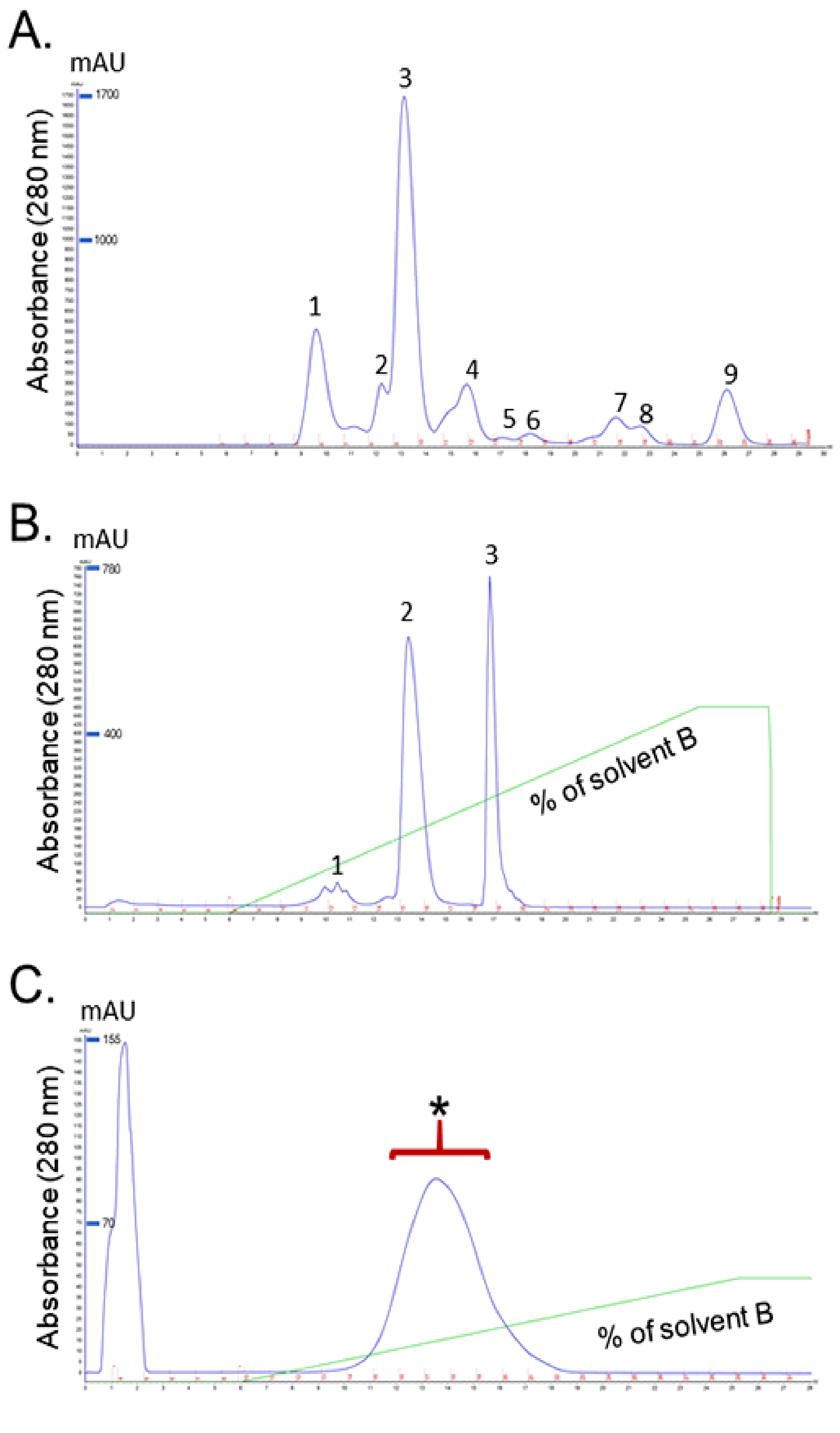
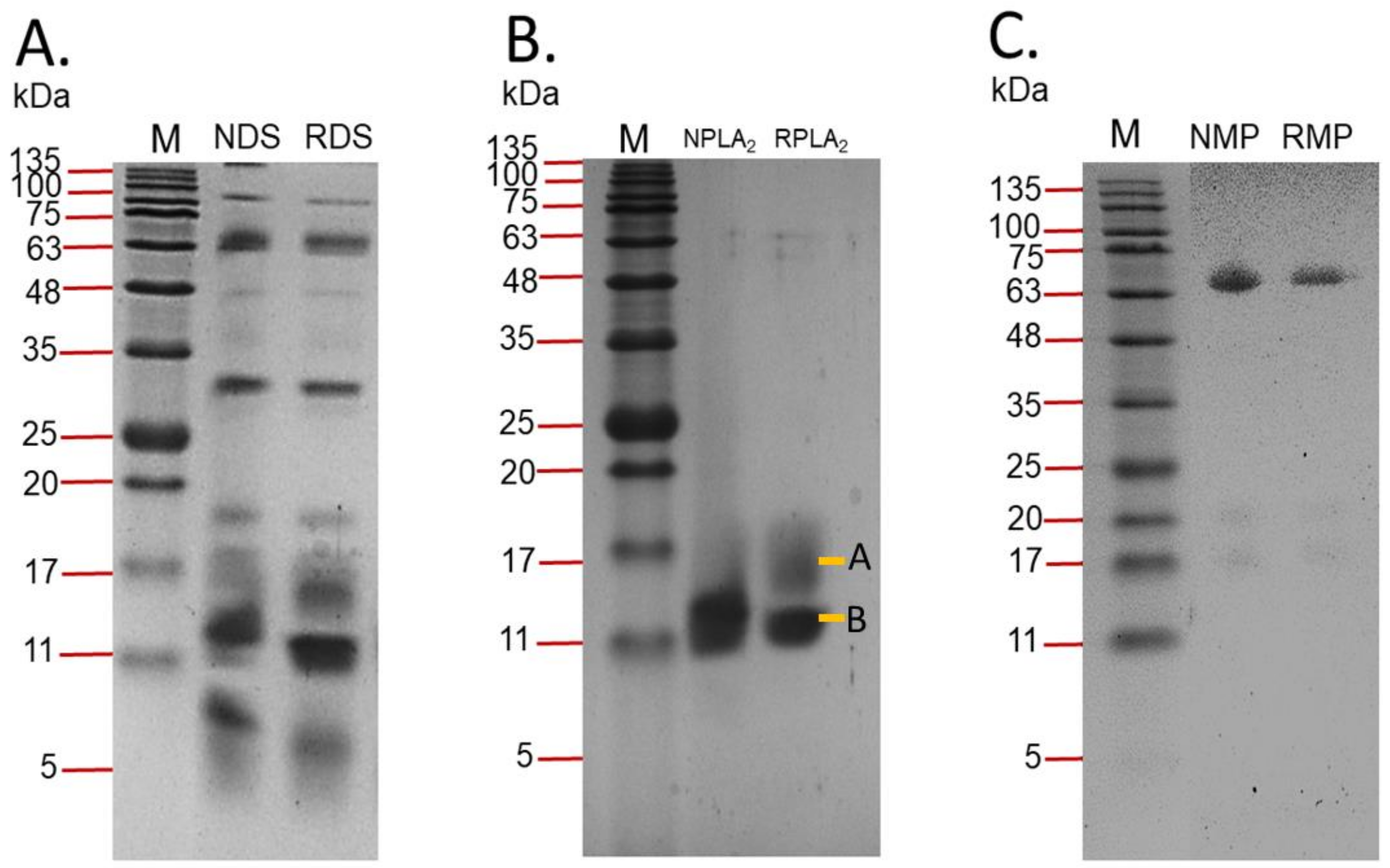
2.1. Identification of Purified Fractions
2.1.1. Isolation and Purification of RvPLA2 and RvMP
2.1.2. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.1.3. Phospholipase A2 Activity
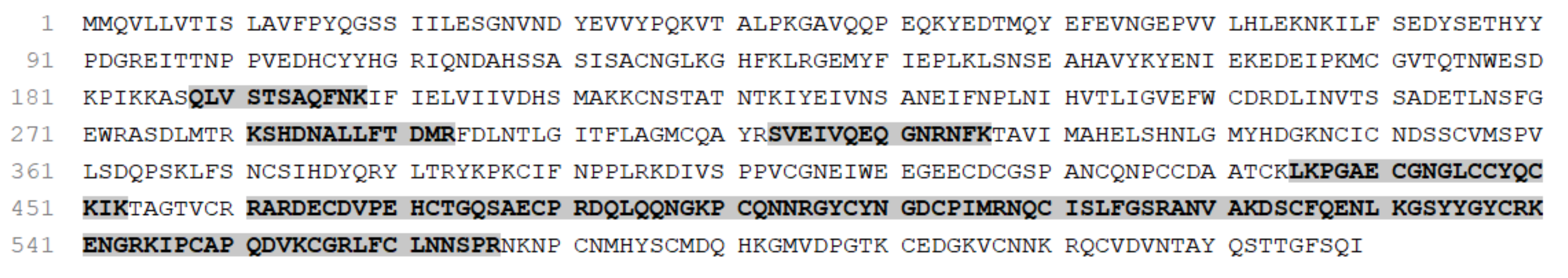
2.1.4. Protein Identification for RvMP
2.1.5. Protein Identification for RvPLA2
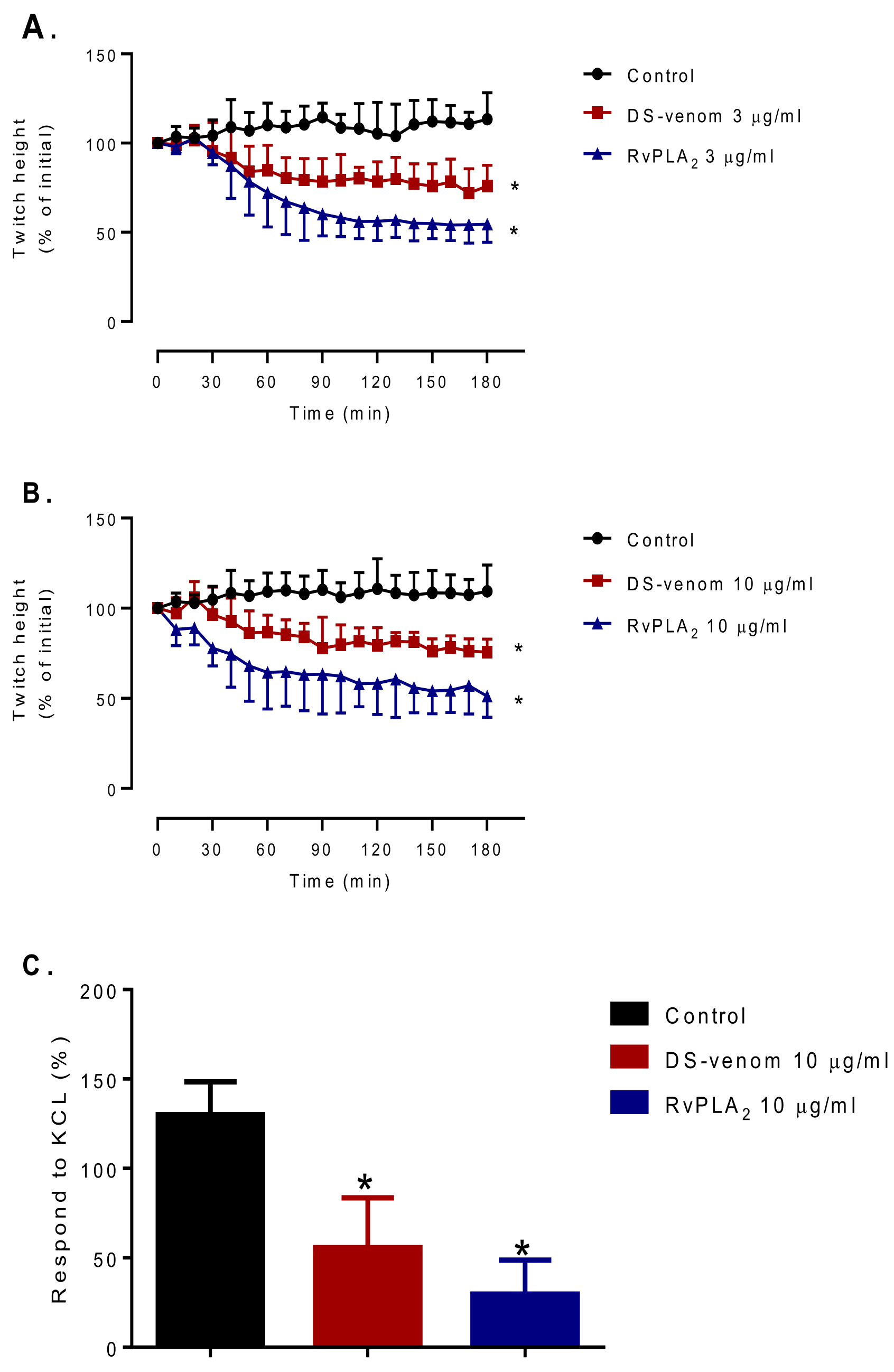
2.2. Myotoxicity Studies
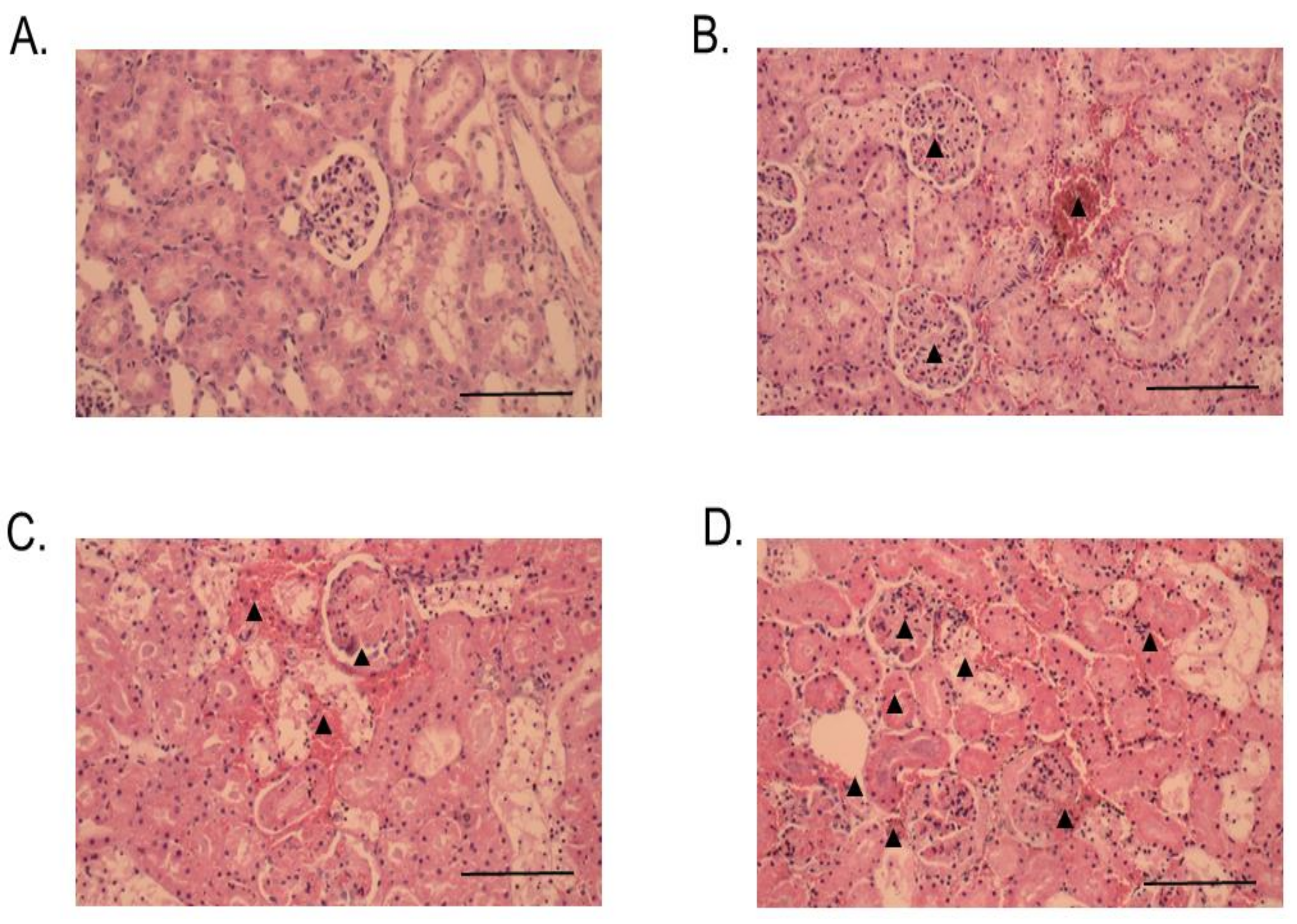
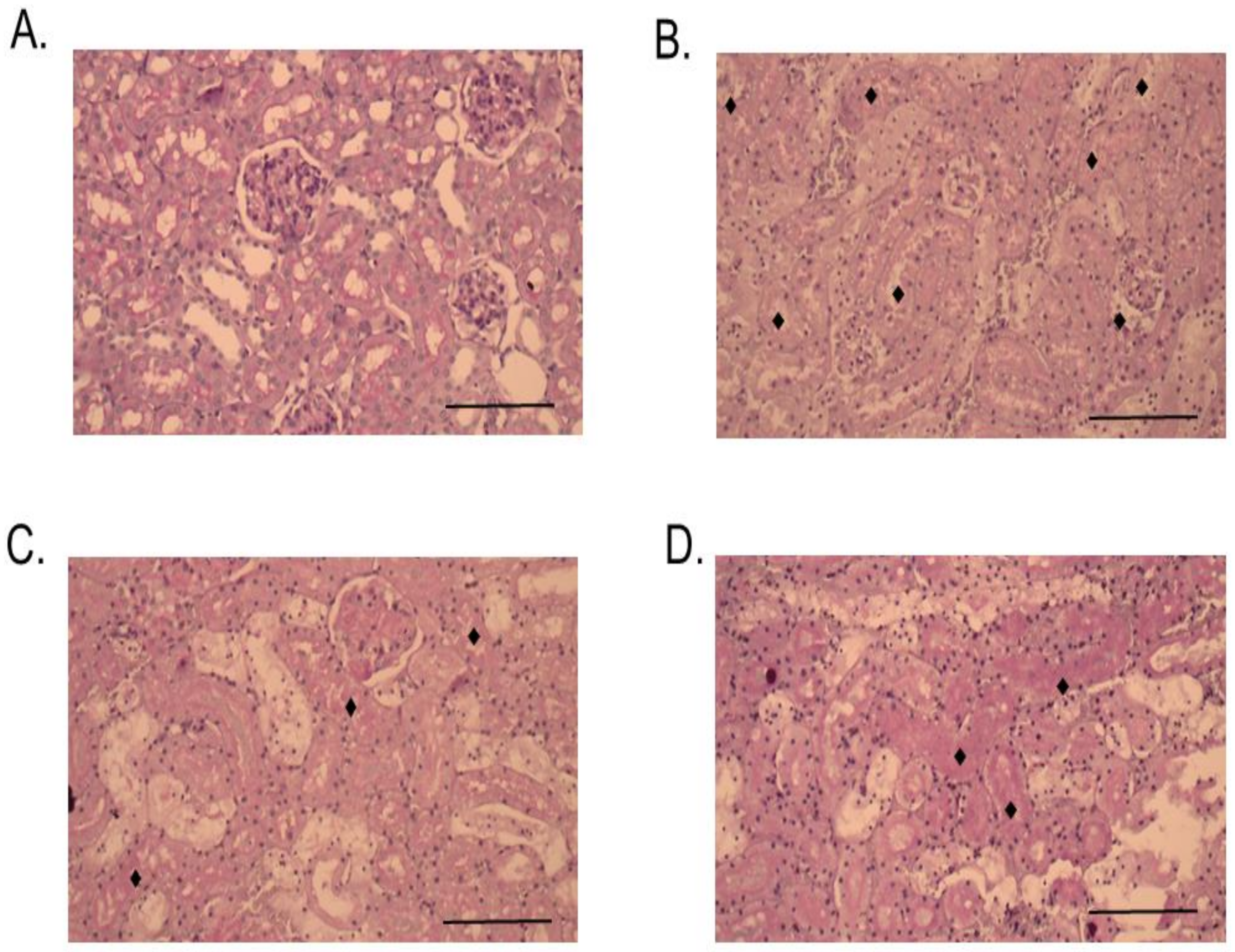
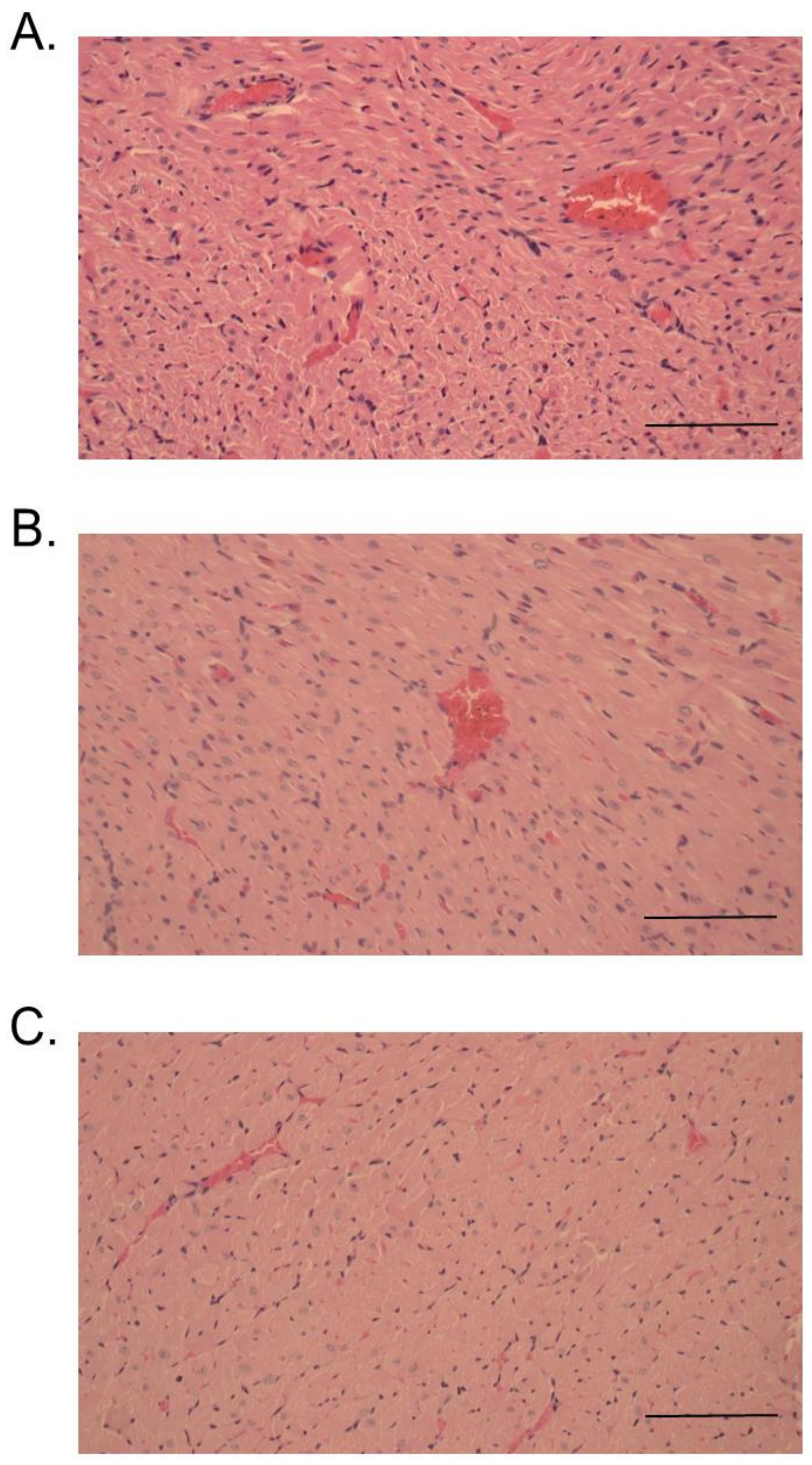
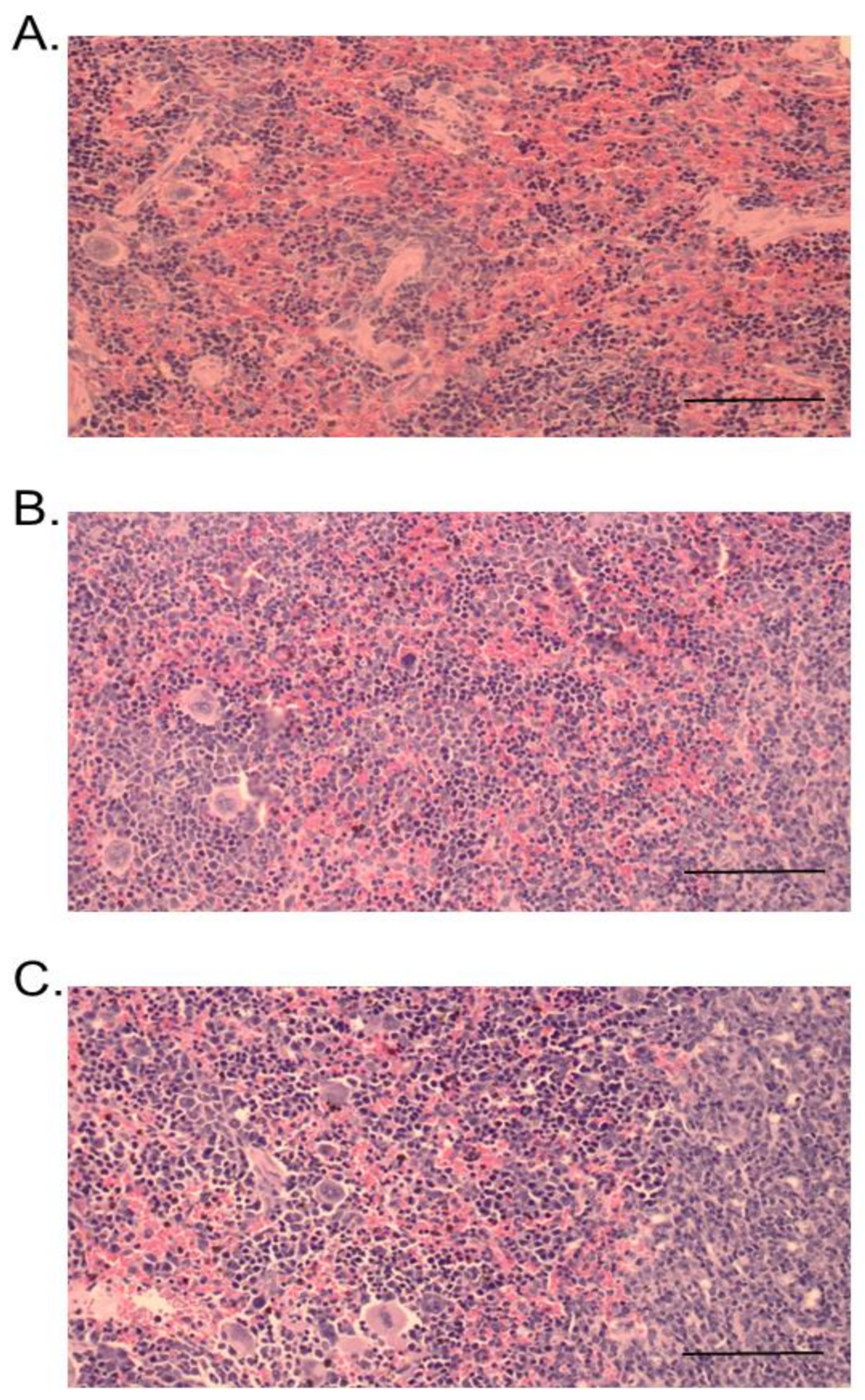
2.3. Histopathological Studies
3. Discussion
4. Materials and Methods
4.1. Snake Venoms
4.2. Protein Concentration
4.3. Fractionation of Venom
4.3.1. Purification RvPLA2
4.3.2. Purification of RvMP
4.4. Determination of PLA2 Activity
4.5. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.6. In-Solution Digestion of Protein
4.7. Nanoflow Liquid Chromatography-Ionization Coupled with Mass Spectrometry/Mass Spectrometry (ESI-LCMS/MS)
4.8. Main Venom Protein Identification
4.9. Animal Care and Ethics
4.10. Chick Biventer Cervicis Nerve-Muscle Preparation for Myotoxicity Determination
4.11. Histopathological Effects of RvPLA2 and RvMP on Rodent Tissues
4.11.1. Animal Treatments
4.11.2. Histopathological Studies
4.12. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Venomous snakes of the South-East Asia Region, their venoms and pathophysiology of human envenoming. In Guidelines for the Management of Snake-Bites, 2nd ed.; WHO: Geneva, Switzerland, 2016; Volume 2. [Google Scholar]
- Wuster, W. The genus Daboia (Serpentes: Viperidae): Russell’s viper. Hamadryad 1998, 23, 33–40. [Google Scholar]
- O’ Shea, M. Venomous Snake of the World; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Hung, D.Z.; Yu, Y.J.; Hsu, C.L.; Lin, T.J. Antivenom treatment and renal dysfunction in Russell’s viper snakebite in Taiwan: A case series. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Myint, L.; Warrell, D.A.; Phillips, R.E.; Tin Nu, S.; Tun, P.; Maung Maung, L. Bites by Russell’s viper (Vipera russelli siamensis) in Burma: Haemostatic, vascular, and renal disturbances and response to treatment. Lancet 1985, 2, 1259–1264. [Google Scholar] [CrossRef]
- Sitprija, V. Snakebite nephropathy. Nephrology 2006, 11, 442–448. [Google Scholar] [CrossRef]
- Maduwage, K.; Isbister, G.K. Current treatment for venom-induced consumption coagulopathy resulting from snakebite. PLoS Negl. Trop. Dis. 2014, 8, 3220. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.; Samarasinghe, R.; Pilapitiya, S.; Dahanayake, N.; Siribaddana, S. Viper bites complicate chronic agrochemical nephropathy in rural Sri Lanka. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Su, H.Y.; Huang, S.W.; Mao, Y.C.; Liu, M.W.; Lee, K.H.; Lai, P.F.; Tsai, M.J. Clinical and laboratory features distinguishing between Deinagkistrodon acutus and Daboia siamensis envenomation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 43. [Google Scholar] [CrossRef] [PubMed]
- Alfred, S.; Bates, D.; White, J.; Mahmood, M.A.; Warrell, D.A.; Thwin, K.T.; Thein, M.M.; Sint San, S.S.; Myint, Y.L.; Swe, H.K.; et al. Acute Kidney Injury Following Eastern Russell’s Viper (Daboia siamensis) Snakebite in Myanmar. Kidney Int. Rep. 2019, 4, 1337–1341. [Google Scholar] [CrossRef] [Green Version]
- Kularatne, S.A.; Silva, A.; Weerakoon, K.; Maduwage, K.; Walathara, C.; Paranagama, R.; Mendis, S. Revisiting Russell’s viper (Daboia russelii) bite in Sri Lanka: Is abdominal pain an early feature of systemic envenoming? PLoS ONE 2014, 9, 90198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.; Kuruppu, S.; Othman, I.; Goode, R.J.; Hodgson, W.C.; Isbister, G.K. Neurotoxicity in Sri Lankan Russell’s Viper (Daboia russelii) Envenoming is Primarily due to U1-viperitoxin-Dr1a, a Pre-Synaptic Neurotoxin. Neurotox. Res. 2017, 31, 11–19. [Google Scholar] [CrossRef]
- Phillips, R.E.; Theakston, R.D.; Warrell, D.A.; Galigedara, Y.; Abeysekera, D.T.; Dissanayaka, P.; Hutton, R.A.; Aloysius, D.J. Paralysis, rhabdomyolysis and haemolysis caused by bites of Russell’s viper (Vipera russelli pulchella) in Sri Lanka: Failure of Indian (Haffkine) antivenom. Q. J. Med. 1988, 68, 691–715. [Google Scholar] [PubMed]
- Tan, K.Y.; Tan, N.H.; Tan, C.H. Venom proteomics and antivenom neutralization for the Chinese eastern Russell’s viper, Daboia siamensis from Guangxi and Taiwan. Sci. Rep. 2018, 8, 8545. [Google Scholar] [CrossRef] [PubMed]
- Saikia, D.; Majumdar, S.; Mukherjee, A.K. Mechanism of in vivo anticoagulant and haemolytic activity by a neutral phospholipase A(2) purified from Daboia russelii russelii venom: Correlation with clinical manifestations in Russell’s Viper envenomed patients. Toxicon 2013, 76, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.K.; Hall, R.H.; Ghose, A.C. Purification and characterization of a potent hemolytic toxin with phospholipase A2 activity from the venom of Indian Russell’s viper. Mol. Cell Biochem. 2002, 237, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Risch, M.; Georgieva, D.; von Bergen, M.; Jehmlich, N.; Genov, N.; Arni, R.K.; Betzel, C. Snake venomics of the Siamese Russell’s viper (Daboia russelli siamensis)—Relation to pharmacological activities. J. Proteom. 2009, 72, 256–269. [Google Scholar] [CrossRef]
- Mitrmoonpitak, C.; Chulasugandha, P.; Khow, O.; Noiprom, J.; Chaiyabutr, N.; Sitprija, V. Effects of phospholipase A2 and metalloprotease fractions of Russell’s viper venom on cytokines and renal hemodynamics in dogs. Toxicon 2013, 61, 47–53. [Google Scholar] [CrossRef]
- Chen, H.S.; Tsai, H.Y.; Wang, Y.M.; Tsai, I.H. P-III hemorrhagic metalloproteinases from Russell’s viper venom: Cloning, characterization, phylogenetic and functional site analyses. Biochimie 2008, 90, 1486–1498. [Google Scholar] [CrossRef]
- Silva, A.; Johnston, C.; Kuruppu, S.; Kneisz, D.; Maduwage, K.; Kleifeld, O.; Smith, A.I.; Siribaddana, S.; Buckley, N.A.; Hodgson, W.C.; et al. Clinical and Pharmacological Investigation of Myotoxicity in Sri Lankan Russell’s Viper (Daboia russelii) Envenoming. PLoS Negl. Trop. Dis. 2016, 10, 0005172. [Google Scholar] [CrossRef]
- Chaiyabutr, N.; Chanhome, L.; Vasaruchapong, T.; Laoungbua, P.; Khow, O.; Rungsipipat, A.; Sitprija, V. The pathophysiological effects of Russell’s viper (Daboia siamensis) venom and its fractions in the isolated perfused rabbit kidney model: A potential role for platelet activating factor. Toxicon X 2020, 7, 100046. [Google Scholar] [CrossRef]
- Khunsap, S.; Pakmanee, N.; Khow, O.; Chanhome, L.; Sitprija, V.; Suntravat, M.; Lucena, S.E.; Perez, J.C.; Sanchez, E.E. Purification of a phospholipase A(2) from Daboia russelii siamensis venom with anticancer effects. J. Venom Res. 2011, 2, 42–51. [Google Scholar]
- Tsai, I.H.; Tsai, H.Y.; Wang, Y.M.; Tun, P.; Warrell, D.A. Venom phospholipases of Russell’s vipers from Myanmar and eastern India--cloning, characterization and phylogeographic analysis. Biochim. Biophys. 2007, 1774, 1020–1028. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M. Insights into and speculations about snake venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 2008, 275, 3016–3030. [Google Scholar] [CrossRef] [PubMed]
- Bernardoni, J.L.; Sousa, L.F.; Wermelinger, L.S.; Lopes, A.S.; Prezoto, B.C.; Serrano, S.M.; Zingali, R.B.; Moura-da-Silva, A.M. Functional variability of snake venom metalloproteinases: Adaptive advantages in targeting different prey and implications for human envenomation. PLoS ONE 2014, 9, 109651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowda, D.C.; Jackson, C.M.; Hensley, P.; Davidson, E.A. Factor X-activating glycoprotein of Russell’s viper venom. Polypeptide composition and characterization of the carbohydrate moieties. J. Biol. Chem. 1994, 269, 10644–10650. [Google Scholar] [CrossRef]
- Chaisakul, J.; Alsolaiss, J.; Charoenpitakchai, M.; Wiwatwarayos, K.; Sookprasert, N.; Harrison, R.A.; Chaiyabutr, N.; Chanhome, L.; Tan, C.H.; Casewell, N.R. Evaluation of the geographical utility of Eastern Russell’s viper (Daboia siamensis) antivenom from Thailand and an assessment of its protective effects against venom-induced nephrotoxicity. PLoS Negl. Trop. Dis. 2019, 13, 0007338. [Google Scholar] [CrossRef] [Green Version]
- Charoenpitakchai, M.; Wiwatwarayos, K.; Jaisupa, N.; Rusmili, M.R.A.; Mangmool, S.; Hodgson, W.C.; Ruangpratheep, C.; Chanhome, L.; Chaisakul, J. Non-neurotoxic activity of Malayan krait (Bungarus candidus) venom from Thailand. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 9. [Google Scholar] [CrossRef] [Green Version]
- Honda, Z.; Ishii, S.; Shimizu, T. Platelet-activating factor receptor. J. Biochem. 2002, 131, 773–779. [Google Scholar] [CrossRef]
- Gopalakrishnan, N. Snake Envenoming horizontal line An Underreported Cause of Acute Kidney Injury. Kidney Int. Rep. 2019, 4, 643–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitprija, V.; Sitprija, S. Renal effects and injury induced by animal toxins. Toxicon 2012, 60, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Chugh, K.S. Snake-bite-induced acute renal failure in India. Kidney Int. 1989, 35, 891–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakthirajan, R.; Dhanapriya, J.; Varghese, A.; Saravanakumar, K.; Dineshkumar, T.; Balasubramaniyan, T.; Gopalakrishnan, N.; Abraham Kurien, A. Clinical profile and outcome of pigment-induced nephropathy. Clin. Kidney J. 2018, 11, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Trinh, K.X.; Khac, Q.L.; Trinh, L.X.; Warrell, D.A. Hyponatraemia, rhabdomyolysis, alterations in blood pressure and persistent mydriasis in patients envenomed by Malayan kraits (Bungarus candidus) in southern Viet Nam. Toxicon 2010, 56, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.J.; Hodgson, W.C.; O’Leary, M.; Isbister, G.K. Pharmacokinetics and pharmacodynamics of the myotoxic venom of Pseudechis australis (mulga snake) in the anesthetised rat. Clin. Toxicol 2014, 52, 604–610. [Google Scholar] [CrossRef]
- Lomonte, B.; Gutierrez, J.M. A new muscle damaging toxin, myotoxin II, from the venom of the snake Bothrops asper (terciopelo). Toxicon 1989, 27, 725–733. [Google Scholar] [CrossRef]
- Harvey, A.L.; Barfaraz, A.; Thomson, E.; Faiz, A.; Preston, S.; Harris, J.B. Screening of snake venoms for neurotoxic and myotoxic effects using simple in vitro preparations from rodents and chicks. Toxicon 1994, 32, 257–265. [Google Scholar] [CrossRef]
- Chaisakul, J.; Rusmili, M.R.A.; Alsolaiss, J.; Albulescu, L.O.; Harrison, R.A.; Othman, I.; Casewell, N.R. In Vitro Immunological Cross-Reactivity of Thai Polyvalent and Monovalent Antivenoms with Asian Viper Venoms. Toxins 2020, 12, 766. [Google Scholar] [CrossRef]
- Silva, A.; Pilapitiya, S.; Siribaddana, S. Acute myocardial infarction following a possible direct intravenous bite of Russell’s viper (Daboia russelli). BMC Res. Notes 2012, 5, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Silva, N.L.; Gooneratne, L.; Wijewickrama, E. Acute myocardial infarction associated with thrombotic microangiopathy following a hump-nosed viper bite: A case report. J. Med. Case Rep. 2017, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Mackessy, S.P. An aqueous endpoint assay of snake venom phospholipase A2. Toxicon 1996, 34, 1149–1155. [Google Scholar] [CrossRef]
- Anson, M.L. The Estimation of Pepsin, Trypsin, Papain, and Cathepsin with Hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaisakul, J.; Khow, O.; Wiwatwarayos, K.; Rusmili, M.R.A.; Prasert, W.; Othman, I.; Abidin, S.A.Z.; Charoenpitakchai, M.; Hodgson, W.C.; Chanhome, L.; et al. A Biochemical and Pharmacological Characterization of Phospholipase A2 and Metalloproteinase Fractions from Eastern Russell’s Viper (Daboia siamensis) Venom: Two Major Components Associated with Acute Kidney Injury. Toxins 2021, 13, 521. https://doi.org/10.3390/toxins13080521
Chaisakul J, Khow O, Wiwatwarayos K, Rusmili MRA, Prasert W, Othman I, Abidin SAZ, Charoenpitakchai M, Hodgson WC, Chanhome L, et al. A Biochemical and Pharmacological Characterization of Phospholipase A2 and Metalloproteinase Fractions from Eastern Russell’s Viper (Daboia siamensis) Venom: Two Major Components Associated with Acute Kidney Injury. Toxins. 2021; 13(8):521. https://doi.org/10.3390/toxins13080521
Chicago/Turabian StyleChaisakul, Janeyuth, Orawan Khow, Kulachet Wiwatwarayos, Muhamad Rusdi Ahmad Rusmili, Watcharamon Prasert, Iekhsan Othman, Syafiq Asnawi Zainal Abidin, Mongkon Charoenpitakchai, Wayne C. Hodgson, Lawan Chanhome, and et al. 2021. "A Biochemical and Pharmacological Characterization of Phospholipase A2 and Metalloproteinase Fractions from Eastern Russell’s Viper (Daboia siamensis) Venom: Two Major Components Associated with Acute Kidney Injury" Toxins 13, no. 8: 521. https://doi.org/10.3390/toxins13080521
APA StyleChaisakul, J., Khow, O., Wiwatwarayos, K., Rusmili, M. R. A., Prasert, W., Othman, I., Abidin, S. A. Z., Charoenpitakchai, M., Hodgson, W. C., Chanhome, L., & Chaiyabutr, N. (2021). A Biochemical and Pharmacological Characterization of Phospholipase A2 and Metalloproteinase Fractions from Eastern Russell’s Viper (Daboia siamensis) Venom: Two Major Components Associated with Acute Kidney Injury. Toxins, 13(8), 521. https://doi.org/10.3390/toxins13080521






