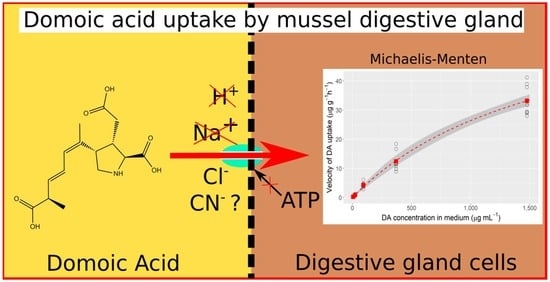
Characterization of the Domoic Acid Uptake Mechanism of the Mussel (Mytilus galloprovincialis) Digestive Gland
Abstract
:1. Introduction
2. Results
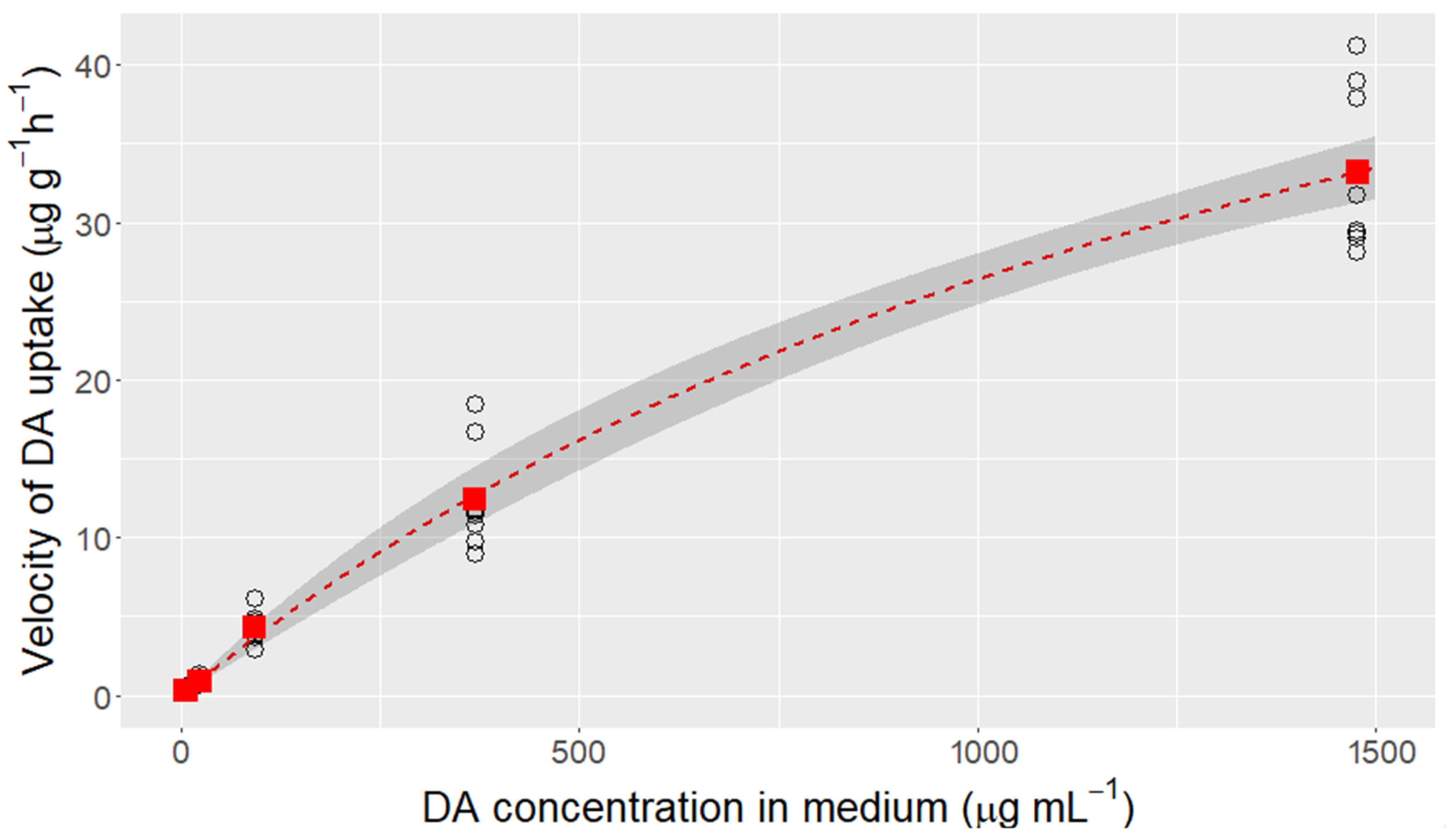
2.1. Domoic Acid Uptake Velocity and Saturation of the Transport
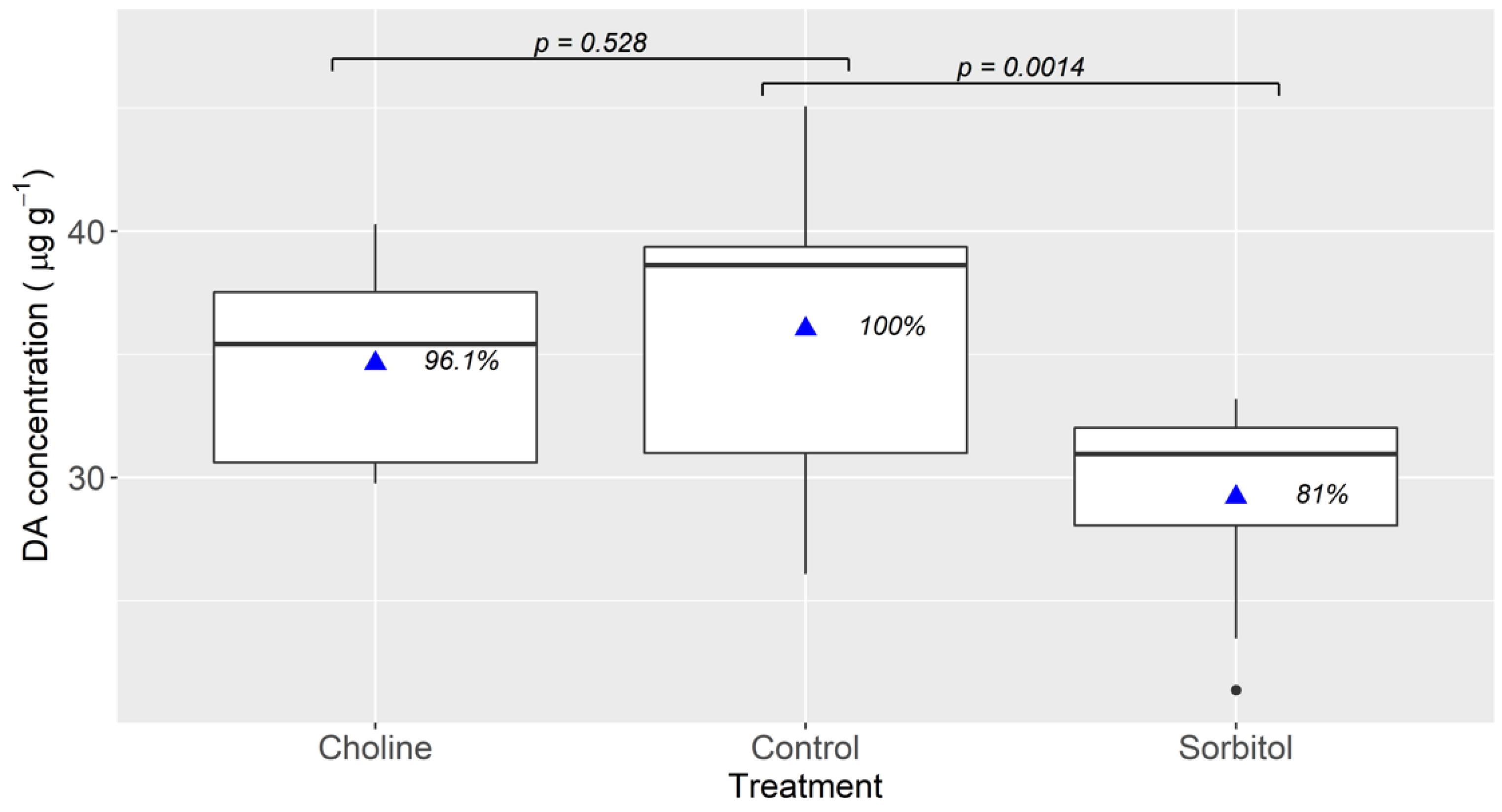
2.2. Effect of Environmental Sodium
2.3. Effects of Cyanide in a Chloride-Depleted Environment and pH
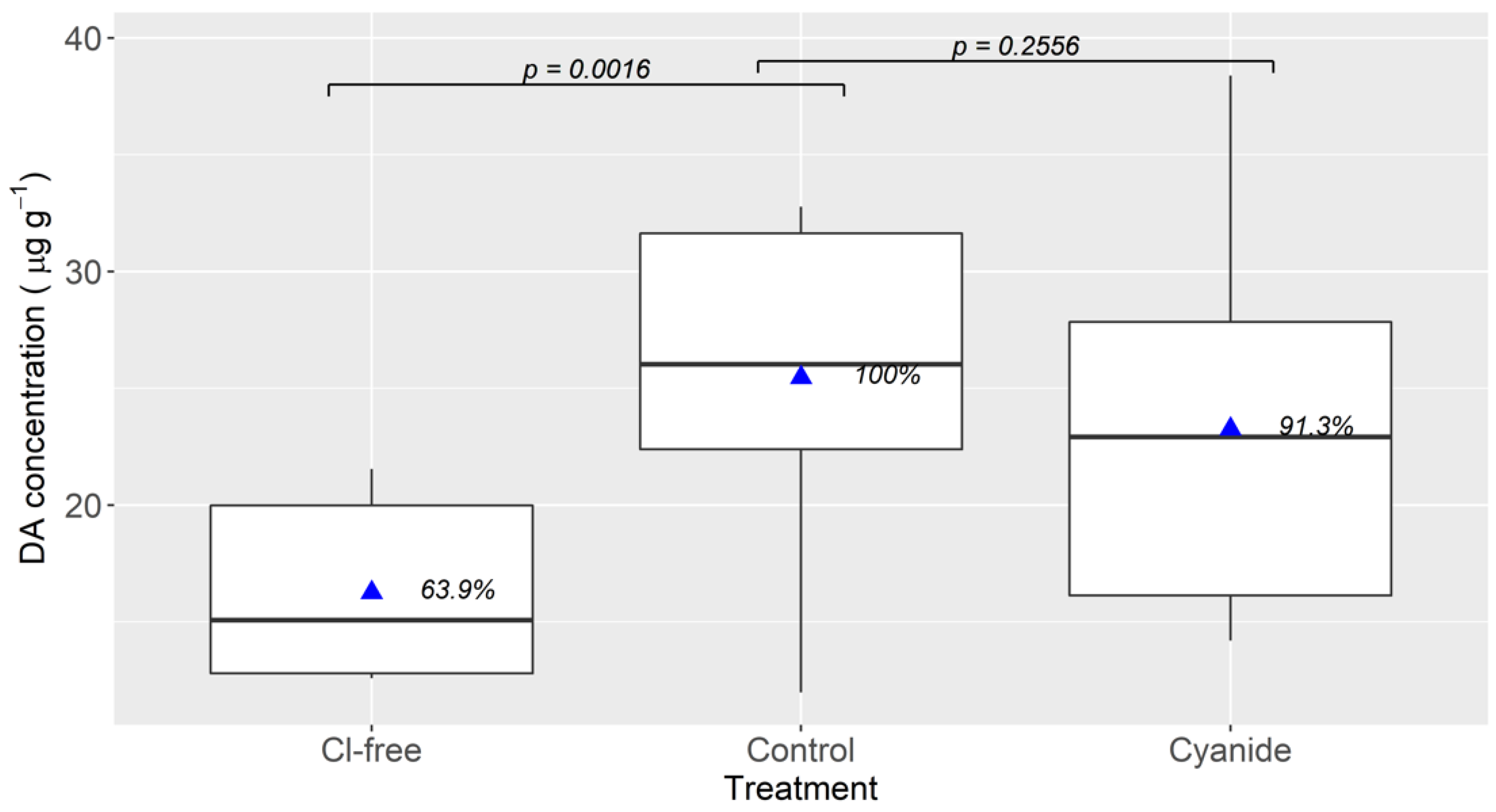
2.4. Effect of Environmental Chloride and Cyanide
3. Discussion
4. Materials and Methods
4.1. Biological Material and Sample Preparation
4.2. Domoic Acid Uptake Velocity and Saturation of the Transport
4.3. Effect of Environmental Sodium
4.4. Effects of Cyanide in a Chloride-Depleted Environment and pH
4.5. Effect of Environmental Chloride and Cyanide
4.6. LC-MS/MS Analysis
4.7. Statistical Analysis and Simulation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takemoto, T.; Daigo, K. Constituents of Chondria armata. Chem. Pharm. Bull. 1958, 6, 578–580. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.L.C.; Boyd, R.K.; Freitas, A.S.W.; Falk, M.; Foxall, R.A.; Jamieson, W.D.; Laycock, M.V.; McCulloch, A.W.; McInnes, A.G.; Odense, P. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from eastern Prince Edward Island. Can. J. Chem. 1989, 67, 481–490. [Google Scholar] [CrossRef]
- Perl, T.M.; Bedard, L.; Kosatsky, T.; Hockin, J.C.; Todd, E.C.; McNutt, L.A.; Remis, R.S. Amnesic shellfish poisoning: A new clinical syndrome due to domoic acid. Can. Dis. Wkly. Rep. 1990, 16, 7–8. [Google Scholar]
- Perl, T.M.; Bédard, L.; Kosatsky, T.; Hockin, J.C.; Todd, E.C.; Remis, R.S. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N. Engl. J. Med. 1990, 322, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Pulido, O.M. Domoic Acid Toxicologic Pathology: A Review. Mar. Drugs 2008, 6, 180–219. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.; Bird, C.; Defreitas, A.; Foxall, R.; Gilgan, M.; Hanic, L.; Johnson, G.; McCulloch, A.; Odense, P.; Pocklington, R.; et al. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Can. J. Fish. Aquat. Sci. 1989, 46, 1203–1215. [Google Scholar] [CrossRef]
- Bates, S.S. Domoic-acid-producing diatoms: Another genus added. J. Phycol. 2000, 36, 978–983. [Google Scholar] [CrossRef]
- Bates, S.S. Amnesic shellfish poisoning: Domoic acid production by Pseudo-nitzschia diatoms. Aqua Info Aquac. Notes 2004, 16, 4. [Google Scholar]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef] [PubMed]
- Fehling, J.; Green, D.H.; Davidson, K.; Bolch, C.J.; Bates, S.S. Domoic acid production by Pseudo-nitzschia seriata (Bacillariophyceae) in Scottish waters. J. Phycol. 2004, 40, 622–630. [Google Scholar] [CrossRef]
- Gallacher, S.; Howard, G.; Hess, P.; MacDonald, E.; Kelly, M.C.; Bates, L.A.; Brown, N.; Mackenzie, M.; Gillibrand, P.; Turrell, W.R. The occurrence of Amnesic Shellfish poison in shellfish from Scottish waters. In Harmful Algal Blooms 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; IOC of UNESCO: Paris, French, 2001; pp. 30–33. [Google Scholar]
- Lelong, A.; Hegaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef] [Green Version]
- Sahraoui, I.; Bates, S.S.; Bouchouicha, D.; Mabrouk, H.H.; Hlaili, A.S. Toxicity of Pseudo-nitzschia populations from Bizerte Lagoon, Tunisia, southwest Mediterranean, and first report of domoic acid production by P. brasiliana. Diatom Res. 2011, 26, 293–303. [Google Scholar] [CrossRef]
- Tan, S.N.; Teng, S.T.; Lim, H.C.; Kotaki, Y.; Bates, S.S.; Leaw, C.P.; Lim, P.T. Diatom Nitzschia navis-varingica (Bacillariophyceae) and its domoic acid production from the mangrove environments of Malaysia. Harmful Algae 2016, 60, 139–149. [Google Scholar] [CrossRef]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef] [Green Version]
- Dao, H.V.; Takata, Y.; Sato, S.; Fukuyo, Y.; Kodama, M. Domoic acid in a bivalve Spondylus cruentus Nha Trang Bay Khanh Hoa Province, Vietnam. Coast. Mar. Sci. 2006, 30, 130–132. [Google Scholar]
- Álvarez, G.; Uribe, E.; Quijano-Scheggia, S.; López-Rivera, A.; Mariño, C.; Blanco, J. Domoic acid production by Pseudo-nitzschia australis and Pseudo-nitzschia calliantha isolated from North Chile. Harmful Algae 2009, 8, 938–945. [Google Scholar] [CrossRef]
- Blanco, J.; Bermúdez de la Puente, M.; Arévalo, F.; Salgado, C.; Moroño, A. Depuration of mussels (Mytilus galloprovincialis) contaminated with domoic acid. Aquat. Living Resour. 2002, 15, 53–60. [Google Scholar] [CrossRef]
- Novaczek, I.; Madhyastha, M.S.; Ablett, R.F.; Donald, A.; Johnson, G.; Nijjar, M.S.; Sims, D.E. Depuration of domoic acid from live blue mussels (Mytilus edulis). Can. J. Fish. Aquat. Sci. 1992, 49, 312–318. [Google Scholar] [CrossRef]
- Novaczek, I.; Madhyastha, M.S.; Ablett, R.F.; Johnson, G.; Nijjar, M.S.; Sims, D.E. Uptake, disposition and depuration of domoic acid by blue mussels (Mytilus edulis). Aquat. Toxicol. 1991, 21, 103–118. [Google Scholar] [CrossRef]
- Álvarez, G.; Uribe, E.; Regueiro, J.; Martin, H.; Gajardo, T.; Jara, L.; Blanco, J. Depuration and anatomical distribution of domoic acid in the surf clam Mesodesma donacium. Toxicon 2015, 102, 1–7. [Google Scholar] [CrossRef]
- Alvarez, G.; Rengel, J.; Araya, M.; Alvarez, F.; Pino, R.; Uribe, E.; Diaz, P.A.; Rossignoli, A.E.; Lopez-Rivera, A.; Blanco, J. Rapid Domoic Acid Depuration in the Scallop Argopecten purpuratus and Its Transfer from the Digestive Gland to Other Organs. Toxins 2020, 12, 698. [Google Scholar] [CrossRef]
- Drum, A.S.; Siebens, T.L.; Crecelius, E.A.; Elston, R.A. Domoic acid in the Pacific razor clam Siliqua patula (Dixon, 1789). J. Shellfish Res. 1993, 12, 443–450. [Google Scholar]
- Horner, R.A.; Kusske, M.B.; Moynihan, B.P.; Skinner, R.N.; Wekell, J.C. Retention of domoic acid by Pacific razor clams, Siliqua patula (Dixon, 1789): Preliminary study. J. Shellfish Res. 1993, 12, 451–456. [Google Scholar]
- Trainer, V.L.; Bill, B.D. Characterization of a domoic acid binding site from Pacific razor clam. Aquat. Toxicol. 2004, 69, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Acosta, C.P.; Bermúdez de la Puente, M.; Salgado, C. Depuration and anatomical distribution of the amnesic shellfish poisoning (ASP) toxin domoic acid in the king scallop Pecten maximus. Aquat. Toxicol. 2002, 60, 111–121. [Google Scholar] [CrossRef]
- Blanco, J.; Acosta, C.P.; Mariño, C.; Muñíz, S.; Martín, H.; Moroño, A.; Correa, J.; Arévalo, F.; Salgado, C. Depuration of domoic acid from different body compartments of the King Scallop Pecten maximus grown in raft culture and natural bed. Aquat. Living Resour. 2006, 19, 257–265. [Google Scholar] [CrossRef]
- Bogan, Y.M.; Kennedy, D.; Harkin, A.L.; Gillespie, J.; Hess, P.; Slater, J.W. Comparison of domoic acid concentration in king scallops, Pecten maximus from seabed and suspended culture systems. J. Shellfish Res. 2006, 25, 129–135. [Google Scholar] [CrossRef]
- Mafra, L.L.; Bricelj, V.M.; Ouellette, C.; Bates, S.S. Feeding mechanics as the basis for differential uptake of the neurotoxin domoic acid by oysters, Crassostrea virginica, and mussels, Mytilus edulis. Aquat. Toxicol. 2010, 97, 160–171. [Google Scholar] [CrossRef]
- Mafra, L., Jr.; Bricelj, V.; Ward, J. Mechanisms contributing to low domoic acid uptake by oysters feeding on Pseudo-nitzschia cells. II. Selective rejection. Aquat. Biol. 2009, 6, 213–226. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Marine biotoxins in shellfish–Summary on regulated marine biotoxins. EFSA J. 2009, 7. [Google Scholar] [CrossRef]
- Arévalo, F.; Bermúdez de la Puente, M.; Salgado, C. Seguimiento de biotoxinas marinas en las Rías Gallegas: Control y evolución durante los años 1995–1996. In Proceedings of V Reunión Ibérica de Fitoplancton Tóxico y Biotoxinas, Actas de la Reunión; Vieites, J.M., Leira, F., Eds.; Anfaco-Cecopesca: Vigo, Spain, 1997; pp. 90–101. [Google Scholar]
- Madhyastha, M.S.; Novaczek, I.; Ablett, R.F.; Johnson, G.; Nijjar, M.S.; Sims, D.E. In vitro study of domoic acid uptake by digestive gland tissue of blue mussel (Mytilus edulis L.). Aquat. Toxicol. 1991, 20, 73–82. [Google Scholar] [CrossRef]
- Manahan, D.T. The uptake and metabolism of dissolved amino acids by bivalve larvae. Biol. Bull. 1983, 164, 236–250. [Google Scholar] [CrossRef]
- Jørgensen, N.O.G. Uptake of L-valine and other amino acids by the polychaete Nereis virens. Mar. Biol. 1979, 52, 45–52. [Google Scholar] [CrossRef]
- Jørgensen, N.; Kristensen, E. Uptake of Amino Acids by Three Species of Nereis (Annelida: Polychaeta). I. Transport Kinetics and Net Uptake from Natural Concentrations. Mar. Ecol. Prog. Ser. 1980, 3, 329–340. [Google Scholar] [CrossRef]
- Blewett, T.A.; Goss, G.G. A novel pathway of nutrient absorption in crustaceans: Branchial amino acid uptake in the green shore crab (Carcinus maenas). Proc. Biol. Sci. 2017, 284. [Google Scholar] [CrossRef] [Green Version]
- Rice, M.A.; Stephens, G.C. Uptake and internal distribution of exogenously supplied amino acids in the Pacific oyster, Crassostrea gigas (Thunberg). Aquaculture 1987, 66, 19–31. [Google Scholar] [CrossRef]
- Cusack, C.K.; Bates, S.S.; Quilliam, M.A.; Patching, J.W.; Raine, R. Confirmation of domoic acid production by Pseudo-nitzschia australis (Bacillariophyceae) isolated from Irish waters. J. Phycol. 2002, 38, 1106–1112. [Google Scholar] [CrossRef]
- Walz, P.M.; Garrison, D.L.; Graham, W.M.; Cattey, M.A.; Tjeerdema, R.S.; Silver, M.W. Domoic acid-producing diatom blooms in Monterey Bay, California: 1991–1993. Nat. Toxins 1994, 2, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Bricelj, V.M.; Shumway, S.E. Paralytic Shellfish Toxins in Bivalve Molluscs: Occurrence, Transfer Kinetics, and Biotransformation. Rev. Fish. Sci. 1998, 6, 315–383. [Google Scholar] [CrossRef]
- Seal, R.P.; Amara, S.G. EXCITATORY AMINO ACID TRANSPORTERS: A Family in Flux. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 431–456. [Google Scholar] [CrossRef]
- Kristensen, A.S.; Andersen, J.; Jørgensen, T.N.; Sørensen, L.; Eriksen, J.; Loland, C.J.; Strømgaard, K.; Gether, U. SLC6 Neurotransmitter Transporters: Structure, Function, and Regulation. Pharmacol. Rev. 2011, 63, 585–640. [Google Scholar] [CrossRef] [PubMed]
- Boudko, D.Y. Ancestry and progeny of nutrient amino acid transporters. Proc. Natl. Acad. Sci. USA 2005, 102, 1360–1365. [Google Scholar] [CrossRef] [Green Version]
- Hyde, R.; Taylor, P.M.; Hundal, H.S. Amino acid transporters: Roles in amino acid sensing and signalling in animal cells. Biochem. J. 2003, 373, 1. [Google Scholar] [CrossRef] [Green Version]
- Preston, R.L. Transport of amino acids by marine invertebrates. J. Exp. Zool. 1993, 265, 410–421. [Google Scholar] [CrossRef]
- Bröer, S. Amino Acid Transport Across Mammalian Intestinal and Renal Epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef]
- Bröer, S. The SLC6 orphans are forming a family of amino acid transporters. Neurochem. Int. 2006, 48, 559–567. [Google Scholar] [CrossRef]
- Chen, N.-H.; Reith, M.E.A.; Quick, M.W. Synaptic uptake and beyond: The sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflügers Arch. Eur. J. Physiol. 2004, 447, 519–531. [Google Scholar] [CrossRef]
- Hatanaka, T.; Haramura, M.; Fei, Y.-J.; Miyauchi, S.; Bridges, C.C.; Ganapathy, P.S.; Smith, S.B.; Ganapathy, V.; Ganapathy, M.E. Transport of Amino Acid-Based Prodrugs by the Na+-and Cl−-Coupled Amino Acid Transporter ATB 0,+ and Expression of the Transporter in Tissues Amenable for Drug Delivery. J. Pharmacol. Exp. Ther. 2004, 308, 1138–1147. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, P.L.; Goehring, A.; Shankaranarayanan, A.; Gouaux, E. Structure and mechanism of a Na+-independent amino acid transporter. Science 2009, 325, 1010–1014. [Google Scholar] [CrossRef] [Green Version]
- Koyama, Y.; Ishibashi, T.; Baba, A. Increase in Chloride-Dependent l-Glutamate Transport Activity in Synaptic Membrane After In Vitro Ischemic Treatment. J. Neurochem. 2002, 65, 1798–1804. [Google Scholar] [CrossRef]
- Miyaji, T.; Echigo, N.; Hiasa, M.; Senoh, S.; Omote, H.; Moriyama, Y. Identification of a vesicular aspartate transporter. Proc. Natl. Acad. Sci. USA 2008, 105, 11720–11724. [Google Scholar] [CrossRef] [Green Version]
- Pazos, A.J.; Ventoso, P.; Martínez-Escauriaza, R.; Pérez-Paralle, M.L.; Blanco, J.; Trivino, J.C.; Sanchez, J.L. Transcriptional response after exposure to domoic acid-producing Pseudo-nitzschia in the digestive gland of the mussel Mytilus galloprovincialis. Toxicon 2017, 140, 60–71. [Google Scholar] [CrossRef]
- Wolkoff, A.W.; Samuelson, A.C.; Johansen, K.L.; Nakata, R.; Withers, D.M.; Sosiak, A. Influence of Cl− on organic anion transport in short-term cultured rat hepatocytes and isolated perfused rat liver. J. Clin. Investig. 1987, 79, 1259–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwer, M.S.; Wolkoff, A.W. Basolateral Plasma Membrane Organic Anion Transporters. In The Liver, 1st ed.; Arias, I.M., Alter, H.J., Boyer, J.L., Cohen, D.E., Shafritz, D.A., Thorgeirsson, S.S., Wolkoff, A.W., Eds.; Wiley: New York, NY, USA, 2020; pp. 327–336. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin, Germany, 2016. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soetaert, K.; Petzoldt, T.; Setzer, R.W. Solving Differential Equations in R: Package deSolve. J. Stat. Softw. 2010, 33. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, J.; Mariño, C.; Martín, H.; Álvarez, G.; Rossignoli, A.E. Characterization of the Domoic Acid Uptake Mechanism of the Mussel (Mytilus galloprovincialis) Digestive Gland. Toxins 2021, 13, 458. https://doi.org/10.3390/toxins13070458
Blanco J, Mariño C, Martín H, Álvarez G, Rossignoli AE. Characterization of the Domoic Acid Uptake Mechanism of the Mussel (Mytilus galloprovincialis) Digestive Gland. Toxins. 2021; 13(7):458. https://doi.org/10.3390/toxins13070458
Chicago/Turabian StyleBlanco, Juan, Carmen Mariño, Helena Martín, Gonzalo Álvarez, and Araceli E. Rossignoli. 2021. "Characterization of the Domoic Acid Uptake Mechanism of the Mussel (Mytilus galloprovincialis) Digestive Gland" Toxins 13, no. 7: 458. https://doi.org/10.3390/toxins13070458
APA StyleBlanco, J., Mariño, C., Martín, H., Álvarez, G., & Rossignoli, A. E. (2021). Characterization of the Domoic Acid Uptake Mechanism of the Mussel (Mytilus galloprovincialis) Digestive Gland. Toxins, 13(7), 458. https://doi.org/10.3390/toxins13070458