Paralytic and Amnesic Shellfish Toxins Impacts on Seabirds, Analyses and Management
Abstract
1. Introduction
2. Direct and Indirect Impacts from HABs on Marine Birds. Biotoxins and Other Bioactive Compounds
2.1. PbTXs
2.2. PSTs
2.3. ASTs
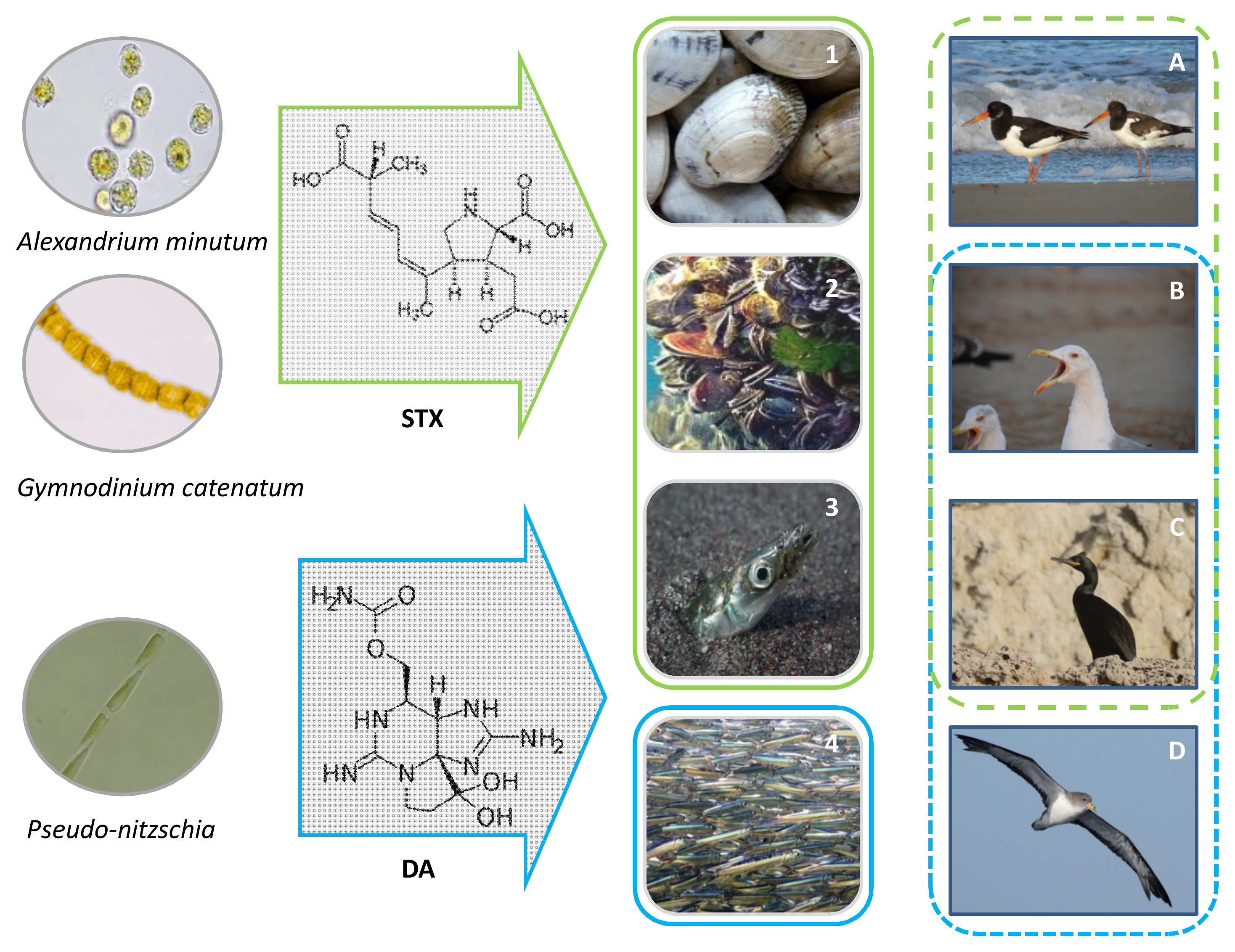
3. Vectors Involved in ASTs and PSTs Toxins Transmission to Seabirds
| Vectors | Affected Birds, Place and Dates | Phytoplankton Species | Observations | References |
|---|---|---|---|---|
| Clams, barnacles and other benthic mollusks | Common murres, pacific loons, gulls, white-winged scoters and others (Washington coast, USA); May 1942 | Gonyaulax catenella | Coincidence with PSP outbreak | [56] |
| Shellfish (e.g., mussels, clams) | Mostly shags but also: cormorants, terns, fulmars and others (Farne Islands, Northeastern England); May 1968 and spring 1975 | Gonyaulax tamarensis | Toxicity not determined in birds, only in shellfish samples collected | [44,45,57,88] |
| Filter-feeding bivalves (e.g., mussels and clams) | Black ducks, waterfowls, gulls and other shorebirds (from southern Maine to Cape Ann, USA); September 1972 | Gonyaulax | Toxicity not determined in birds, only in shellfish samples collected | [89,90,91] |
| Sand lances | Common terns, arctic terns, roseate terns, laughing gulls, herring gulls (Cape Cod, USA); June 1978 | Gonyaulax | PSTs only determined in sand lance | [46] |
| Mussels | Black oystercatchers, southern blackbacked gulls, Hartlaub’s gulls (South African coast); May 1979 | Gonyaulax catenella | Birds with internal lesions and empty stomachs, probably starved to death | [50,92,93] |
| Sand lances | Herring gulls (St. Lawrence Estuary, Canada); July 1996 | Alexandrium | PSTs in sand lance and in bird intestine and brain | [58] |
| Mollusks and planktivorous fish (e.g., sand lance and capelin) | 15 species, mostly larids especially Black-legged kittiwakes (St Lawrence Estuary, Canada); August 2008 | Alexandrium tamarense | PSTs in bird carcasses, mollusks, planktivorous fish, and plankton | [35] |
| Sand lance (birds died after eating them) | Nestlings of kittlitz murrelets (Alaska, USA); 2011 and 2012 | Alexandrium | STX detected in sand lances and 87% of nestling carcasses | [60] |
| Euphausiids and forage fish (e.g., sand lance, capelin, herring, juvenile pollock) | Common murres (Alaska, USA); 2015 and 2016 | Alexandrium catenella | PSTs detected in fish, invertebrates and in birds in which could be a secondary cause of death | [37] |
| Unknown | Northern fulmars, short-tailed shearwaters and murres, among others (Bering Sea and Chukchi Sea, Alaska, USA); June–September 2017 | Unknown | PSTs detected in carcasses. PSTs along with starvation probably caused bird die-off | [62] |
| Not reported | Common murres, surf scoters, white-winged scoters, Brandt’s cormorants, brown pelicans, double-crested cormorants, northern fulmars; several Washington and California counties, USA; September–October 2009, July 2015–March 2016, 2018 | Alexandrium sp. present in some areas | Low PSTs levels detected in carcasses | [64] |
4. Symptoms of PSP and ASP Intoxications in Seabirds/Birds
| Symptoms and Lesions | Symptoms and Lesions Details | Affected Birds | References |
|---|---|---|---|
| Neurological symptoms | Loss of equilibrium (inability to stand or even keep head up) | common murres, shags, terns, gulls, cormorants, eiders | [35,44,46] |
| Uncoordinated movements (ataxia) | |||
| Falling forward | |||
| Unable to take off | |||
| Convulsions | |||
| Mild to severe paralysis | |||
| Unable to move wings or legs | |||
| Paralysis in the oviduct | |||
| Eye symptoms | Pupil restriction | Shags | [44] |
| Gastrointestinal symptoms and lesions | Excess vomiting, food regurgitation | Gulls, white-winged scoters, shags, terns | [35,44,46,56] |
| Abnormal feces (i.e.: greenish, yellowish, brownish) | |||
| Excessive defecation | |||
| Protruding cloaca | |||
| Inflamed alimentary canal. Congestion of tracheal and oral mucosa | |||
| Intestinal inflammation and/or hemorrhage | |||
| Thickened duodenal or intestinal mucosa and pale mucoidal material | |||
| Circulatory and respiratory problems | Distended or dilated veins | Shags, terns | [35,44,46,57] |
| Hemorrhages at the base of the brain or elsewhere in the body | |||
| Failure of circulatory system. | |||
| Congestion of organs, including lungs | |||
| Frequent gasping | |||
| Starvation | Weight loss | Shags | [44] |
| Loss of subcutaneous fat | |||
| Other | Inability to lay eggs | Terns | [44] |
| Symptoms and Lesions | Symptoms and Lesions Details | Affected Birds | References |
|---|---|---|---|
| Neurological symptoms and lesions | Slow side-to-side head waving | Brown pelicans, Brandt’s cormorants, common murres, sooty shearwaters | [33,47,48,69,97] |
| Ventroflexed head | |||
| Torticollis | |||
| Wings partially extended | |||
| Motor tremors | |||
| Unable to take off | |||
| Inability to retract legs during flying | |||
| Clenching of toes | |||
| Scratching | |||
| Disorientation and loosing awareness of their surrounding | |||
| Loss of equilibrium (inability to stand or keep head up) | |||
| Uncoordinated movements (ataxia) | |||
| Falling on their back or side with feet paddling | |||
| Abnormal behavior (agitation or unusually docile, asocial behavior and irresponsiveness to handling) | |||
| Diffuse neural necrosis | |||
| Capillary endothelial cell hyperplasia | |||
| Myofiber necrosis in the right ventricular wall | |||
| Gastrointestinal symptoms | Vomiting, food regurgitation | Brown pelicans, Brandt’s cormorants, sooty shearwaters | [47,69] |
| Circulatory and respiratory problems | Focal hemorrhages at the adductor, sartorius, gracilis and vastus medialis muscles of the hind limb and the biceps brachii of the forelimb | brown pelicans, Brandt’s cormorants, | [47] |
| Vascular engorgement of the intestine | |||
| Starvation | Weight loss | Common murres | [98] |
| Loss of subcutaneous fat | |||
| Paralysis | Decreased mobility and responsiveness to stimulus | Common murres | [97] |
| Weakness and lethargy | |||
| Other | Focal muscle necrosis | Brown pelicans, ommon murres | [47,97] |
| Elevated serum creatinine kinasa, blood urea nitrogen and uric acid | |||
| Hypothermia | |||
| Necrosis of pectoral muscles | |||
| Dark-brown urates |
5. Multifactorial Causes of Seabird’s MMEs
6. Determination of PSTs and ASTs Toxins in Seabirds
6.1. PSTs
6.1.1. Methods That Evaluate Total (or Partial) Sample PSP Toxicity
Mouse Bioassay (MBA)
Enzyme-Labeled Immunosorbent Assay (ELISA)
6.1.2. Methods That Allow the Detection and Quantification of Individual PSTs
Determination of PSTs in Seabird Samples by HPLC and LC-MS/MS Methods
6.1.3. Homogenization and Extraction Protocols: Adaption to Seabird Samples
6.1.4. Tissue Selection
6.2. ASTs
6.2.1. MBA
6.2.2. ELISA for DA
6.2.3. Instrumental Methods for DA
6.2.4. Homogenization, Extraction and Clean-Up Protocols: Adaption to Seabird Samples
6.2.5. Tissue Selection
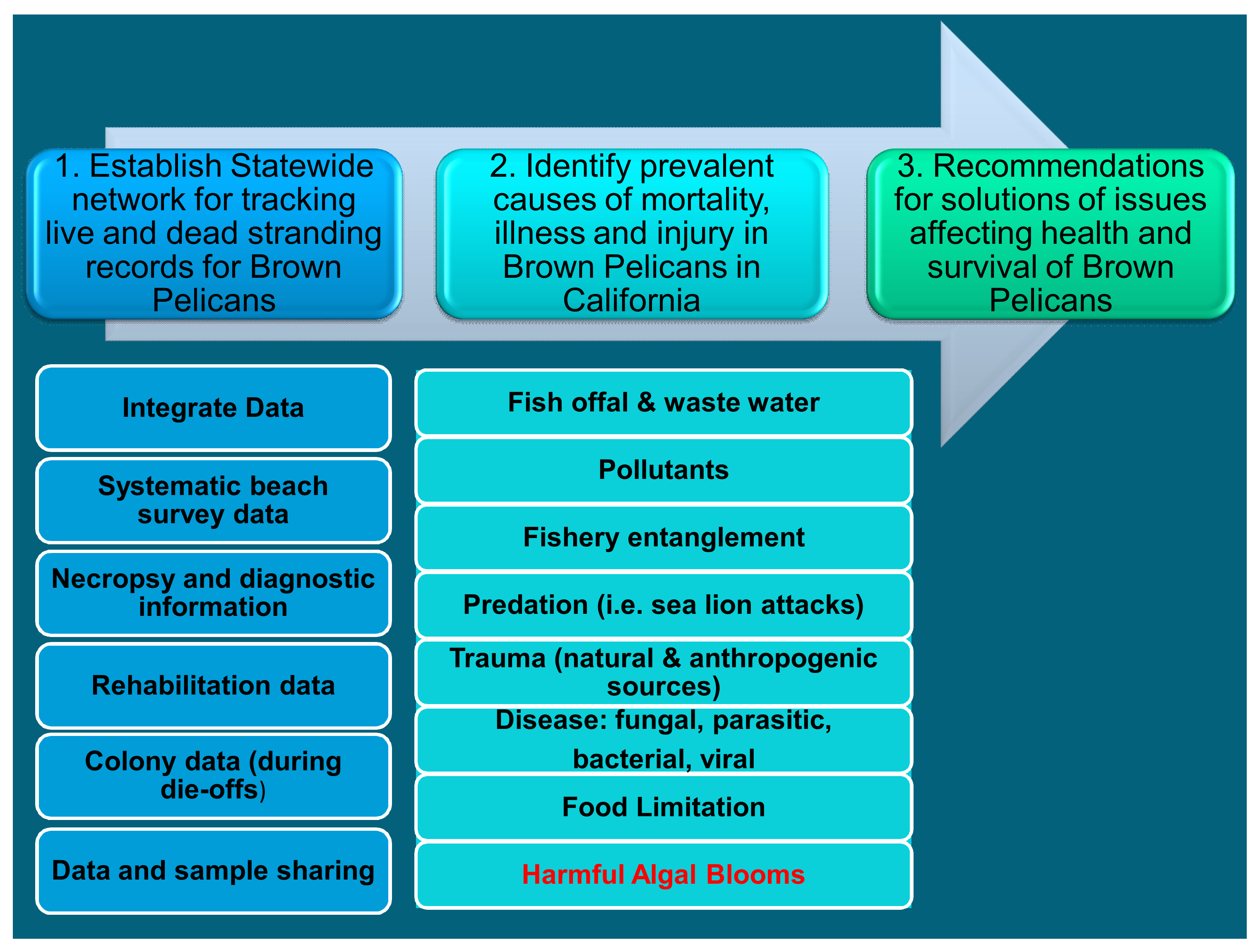
7. Management and Prevention
7.1. Entities Involved
7.1.1. Governmental Authorities
7.1.2. Environmental Non-Governmental Organizations (ENGOs)
7.1.3. Wildlife Rescue Hospitals
7.1.4. General Public
7.2. Prevention and Management Protocols
- Creating clear and easy ways to communicate the event. Communication channels are available to the general public and public agencies. There are several ways to communicate (phone numbers, email, online formulary, etc.).
- Training personnel involved in wildlife health and disease response. The training is given both online and locally by the USFWS Wildlife Health Office to the Department of the interior employees, and wildlife hospitals workers, among others. It includes information on personal protective training and equipment.
- Preparing first response kits that include, among others, personal protective equipment, important contacts list and material for collecting and packing the carcasses.
- Reporting the event. They provide a list with all the contacts in each area.
- Collecting basic information about the event: contact details of the person in the field, date of onset, exact location, etc. The response team records the exact number, species, sex and age of the carcasses, samples collected, preservation method and storage and symptoms shown in sick animals.
- Collection, packaging and shipping the carcasses. Contacting the laboratory to assist in samples collection, packaging and storage. Collecting the freshest dead specimens that should be representative of all the affected species. Discarding carcasses appropriately to prevent scavenging.
- Communicating the results in a direct an efficient way involving general public, national agencies, residents, wildlife hospitals staff, social media, etc.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Moore, S.K.; Tomlinson, M.C.; Silke, J.; Cusack, C.K. Living with harmful algal blooms in a changing world: Strategies for modeling and mitigating their effects in coastal marine ecosystems. In Coastal and Marine Hazards, Risks, and Disasters; John, F., Shroder, J.T., Ellis, D.J.S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 495–561. ISBN 9780123965387. [Google Scholar]
- Kudela, R.; Berdalet, E.; Enevoldsen, H.; Pitcher, G.; Raine, R.; Urban, E. GEOHAB–The Global Ecology and Oceanography of Harmful Algal Blooms Program: Motivation, goals, and legacy. Oceanography 2017, 30, 12–21. [Google Scholar] [CrossRef]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 1016. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Berdalet, E.; Kudela, R.; Urban, E.; Enevoldsen, H.; Banas, N.; Bresnan, E.; Burford, M.; Davidson, K.; Gobler, C.; Karlson, B.; et al. GlobalHAB: A new program to promote international research, observations, and modeling of harmful algal blooms in aquatic systems. Oceanography 2017, 30, 70–81. [Google Scholar] [CrossRef]
- Glibert, P.M. Eutrophication, harmful algae and biodiversity—Challenging paradigms in a world of complex nutrient changes. Mar. Pollut. Bull. 2017, 124, 591–606. [Google Scholar] [CrossRef]
- Gobler, C.J.; Doherty, O.M.; Hattenrath-Lehmann, T.K.; Griffith, A.W.; Kang, Y.; Litaker, R.W. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl. Acad. Sci. USA 2017, 114, 4975–4980. [Google Scholar] [CrossRef]
- Lambert, J. Heat wave blamed for seabird die-off. Sci. News 2020, 1, 11. [Google Scholar]
- Piatt, J.F.; Parrish, J.K.; Renner, H.M.; Schoen, S.K.; Jones, T.T.; Arimitsu, M.L.; Kuletz, K.J.; Bodenstein, B.; García-Reyes, M.; Duerr, R.S.; et al. Extreme mortality and reproductive failure of Common Murres resulting from the northeast Pacific marine heatwave of 2014–2016. PLoS ONE 2020, 15, e0226087. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.-Y.D.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Otero, P.; Rodríguez, P.; Botana, A.M.; Alfonso, A.; Botana, L.M. Analysis of natural toxins. In Liquid Chromatography: Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 411–430. ISBN 9780124158061. [Google Scholar]
- Ben-Gigirey, B.; Turner, A.D.; Gago-Martínez, A. Instrumental methods for paralytic shellfish toxins. In Marine and Freshwater Toxins, Springer; Gopalakrishnakone, P., Haddad, V., Jr., Tubaro, A., Kim, E., Kem, W.R., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 43–69. ISBN 9789400766501. [Google Scholar]
- Gagez, A.L.; Bonnet, A.; Pineau, P.; Graber, M. Identification and quantification of domoic acid by UHPLC/QTOF tandem mass spectrometry, with simultaneous identification of non-target photodegradation products. Int. J. Environ. Anal. Chem. 2017, 97, 1192–1205. [Google Scholar] [CrossRef][Green Version]
- Gokul, E.A.; Raitsos, D.E.; Gittings, J.A.; Alkawri, A.; Hoteit, I. Remotely sensing harmful algal blooms in the Red Sea. PLoS ONE 2019, 14, e0215463. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; Fong, S.Y.T.; Hungerford, J.; McNabb, P.S.; Boundy, M.J.; Harwood, D.T. Ultrahigh-performance hydrophilic interaction liquid chromatography with tandem mass spectrometry method for the determination of paralytic shellfish toxins and tetrodotoxin in mussels, oysters, clams, cockles, and scallops: Collaborative study. J. AOAC Int. 2020, 103, 533–562. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Lefebvre, K.A.; Flewelling, L.J. Effects of toxic microalgae on marine organisms. In Toxins and Biologically Active Compounds from Microalgae; Rossini, G.P., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; Volume 2, pp. 379–449. ISBN 9781482231472. [Google Scholar]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- He, Y.; Fekete, A.; Chen, G.; Harir, M.; Zhang, L.; Tong, P.; Schmitt-Kopplin, P. Analytical approaches for an important shellfish poisoning agent: Domoic acid. J. Agric. Food Chem. 2010, 58, 11525–11533. [Google Scholar] [CrossRef]
- Turner, J.T.; Tester, P.A. Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnol. Oceanogr. 1997, 42, 1203–1214. [Google Scholar] [CrossRef]
- Deeds, J.R.; Landsberg, J.H.; Etheridge, S.M.; Pitcher, G.C.; Watt Longan, S. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Rossignoli, A.E.; Riobó, P.; Rodríguez, F. First report of paralytic shellfish toxins in marine invertebrates and fish in Spain. Toxins 2020, 12, 723. [Google Scholar] [CrossRef]
- Dean, K.J.; Hatfield, R.G.; Lee, V.; Alexander, R.P.; Lewis, A.M.; Maskrey, B.H.; Alves, M.T.; Hatton, B.; Coates, L.N.; Capuzzo, E.; et al. Multiple new paralytic shellfish toxin vectors in offshore north sea benthos, a deep secret exposed. Mar. Drugs 2020, 18, 400. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Marine Biotoxins. Paralytic Shellfish Poisoning; FAO: Rome, Italy, 2004; pp. 5–52. [Google Scholar]
- Martínez, A.; Garrido-Maestu, A.; Ben-Gigirey, B.; Chapela, M.J.; González, V.; Vieites, J.M.; Cabado, A.G. Marine Biotoxins. In Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 869–904. [Google Scholar]
- Visciano, P.; Schirone, M.; Berti, M.; Milandri, A.; Tofalo, R.; Suzzi, G. Marine biotoxins: Occurrence, toxicity, regulatory limits and reference methods. Front. Microbiol. 2016, 7, 1051. [Google Scholar] [CrossRef]
- Murk, A.J.; Nicolas, J.; Smulders, F.J.M.; Bürk, C.; Gerssen, A. Marine biotoxins: Types of poisoning, underlying mechanisms of action and risk management programmes. In Chemical Hazards in Foods of Animal Origin. Food Safety Assurance and Veterinary Public Health; Frans, J.M., Smulders, I.M.C.M., Rietjens, M.D.R., Eds.; Wageningen Academic Publishers: Wageningen, The Neteherlands, 2019; pp. 207–239. ISBN 9789086868773. [Google Scholar]
- Camphuysen, C.J.; Wright, P.J.; Leopold, M.; Huppop, O.; Reid, J.B. A review of the causes, and consequences at the population level, of mass mortalities of seabirds. ICES Coop. Res. Rep. 1999, 232, 51–66. [Google Scholar]
- Shumway, S.E.; Allen, S.M.; Boersma, P.D. Marine birds and harmful algal blooms: Sporadic victims or under-reported events? Harmful Algae 2003, 2, 1–17. [Google Scholar] [CrossRef]
- Van Deventer, M. Brevetoxins in Marine Birds: Evidence of Trophic Transfer and the Role of Prey Fish as Toxin Vector. Master’s Thesis, College of Marine Science, University of South Florida, Tampa, FL, USA, 2007. [Google Scholar]
- Lewitus, A.J.; Horner, R.A.; Caron, D.A.; Garcia-Mendoza, E.; Hickey, B.M.; Hunter, M.; Huppert, D.D.; Kudela, R.M.; Langlois, G.W.; Largier, J.L.; et al. Harmful algal blooms along the North American West Coast region: History, trends, causes, and impacts. Harmful Algae 2012, 19, 133–159. [Google Scholar] [CrossRef]
- Gibble, C.M.; Hoover, B.A. Interactions between seabirds and harmful algal blooms. In Harmful Algal Blooms: A Compendium Desk Reference; Shumway, S.E., Burkholder, J.M., Morton, S.L., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 223–242. [Google Scholar]
- Friend, M.; Franson, J.C. Field Manual of Wildlife Diseases. General Field Procedures and Diseases of Birds; Ciganovich, E.A., Ed.; U.S. Geological Survey, Biological Resources Division: Madison, WI, USA, 1999; ISBN 0607880961.
- Fauquier, D.A.; Flewelling, L.J.; Maucher, J.M.; Keller, M.; Kinse, M.J.; Johnson, C.K.; Henry, M.; Gannon, J.G.; Ramsdell, J.S.; Landsberg, J.H. Brevetoxicosis in seabirds naturally exposed to Karenia brevis blooms along the central West Coast of Florida. J. Wildl. Dis. 2013, 49, 246–260. [Google Scholar] [CrossRef]
- Starr, M.; Lair, S.; Michaud, S.; Scarratt, M.; Quilliam, M.; Lefaivre, D.; Robert, M.; Wotherspoon, A.; Michaud, R.; Ménard, N.; et al. Multispecies mass mortality of marine fauna linked to a toxic dinoflagellate bloom. PLoS ONE 2017, 12, e0176299. [Google Scholar] [CrossRef]
- Soliño, L.; Ferrer-Obiol, J.; Navarro-Herrero, L.; González-Solís, J.; Costa, P.R. Are pelagic seabirds exposed to amnesic shellfish poisoning toxins? Harmful Algae 2019, 84, 172–180. [Google Scholar] [CrossRef]
- Van Hemert, C.; Schoen, S.K.; Litaker, R.W.; Smith, M.M.; Arimitsu, M.L.; Piatt, J.F.; Holland, W.C.; Ransom Hardison, D.; Pearce, J.M. Algal toxins in Alaskan seabirds: Evaluating the role of saxitoxin and domoic acid in a large-scale die-off of Common Murres. Harmful Algae 2020, 92, 101730. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Casero, M.V.; Mazuet, C.; Riobó, P.; Rodriguez, F. Paretic syndrome in gulls from Southern Portugal: Searching for the causative agent. In Proceedings of the 1st International Electronic Conference on Toxins (IECT2021), Online. 16–31 January 2021. [Google Scholar]
- White, A.E.; Watkins-Brandt, K.S.; McKibben, S.M.; Wood, A.M.; Hunter, M.; Forster, Z.; Du, X.; Peterson, W.T. Large-scale bloom of Akashiwo sanguinea in the Northern California current system in 2009. Harmful Algae 2014, 37, 38–46. [Google Scholar] [CrossRef]
- Du, X.; Peterson, W.; McCulloch, A.; Liu, G. An unusual bloom of the dinoflagellate Akashiwo sanguinea off the central Oregon, USA, coast in autumn 2009. Harmful Algae 2011, 10, 784–793. [Google Scholar] [CrossRef]
- Phillips, E.M.; Zamon, J.E.; Nevins, H.M.; Gibble, C.M.; Duerr, R.S.; Kerr, L.H. Summary of birds killed by a harmful algal bloom along the south Washington and north Oregon coasts during October 2009. Northwest. Nat. 2011, 92, 120–126. [Google Scholar] [CrossRef]
- Jessup, D.A.; Miller, M.A.; Ryan, J.P.; Nevins, H.M.; Kerkering, H.A.; Mekebri, A.; Crane, D.B.; Johnson, T.A.; Kudela, R.M. Mass stranding of marine birds caused by a surfactant-producing red tide. PLoS ONE 2009, 4, e4550. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, A.; Farías, L.; Tapia, F.; Varela, D.; Vásquez, M. Comisión Marea Roja. Informe Final; Universidad de los Lagos: Osorno, Chile, 2016; pp. 1–64. [Google Scholar]
- Coulson, J.C.; Potts, G.R.; Deans, I.R.; Fraser, S.M. Exceptional mortality of shags and other sea birds caused by paralytic shellfish poison. Br. Birds 1968, 61, 381–406. [Google Scholar]
- Armstrong, H.; Coulson, J.C.; Hawkey, P.; Hudson, M.J. Further mass seabird deaths from paralytic shellfish poisoning. Br. Birds 1978, 71, 58–68. [Google Scholar]
- Nisbet, I.C.T. Paralytic shellfish poisoning: Effects on breeding terns. Condor 1983, 85, 338–345. [Google Scholar] [CrossRef]
- Work, T.M.; Barr, B.; Beale, A.M.; Fritz, L.; Michael, A.; Wright, J.L.C.; Url, S. Epidemiology of domoic acid poisoning in Brown Pelicans (Pelecanus occidentalis) and Brandt’s Cormorants (Phalacrocorax penicillatus) in California. J. Zoo Wildl. Med. 1993, 24, 54–62. [Google Scholar]
- Sierra-Beltrán, A.; Palafox-Uribe, M.; Grajales-Montiel, J.; Cruz-Villacorta, A.; Ochoa, J.L. Sea bird mortality at Cabo San Lucas, Mexico: Evidence that toxic diatom blooms are spreading. Toxicon 1997, 35, 447–453. [Google Scholar] [CrossRef]
- Kreuder, C.; Mazet, J.A.K.; Bossart, G.D.; Carpenter, T.E.; Holyoak, M.; Elie, M.S.; Wright, S.D. Clinicopathologic features of suspected brevetoxicosis in double-crested cormorants (Phalacrocorax auritus) along the Florida Gulf Coast. J. Zoo Wildl. Med. 2002, 33, 8–15. [Google Scholar] [CrossRef]
- Stephen, V.C.; Hockey, P.A.R. Evidence for an increasing incidence and severity of harmful algal blooms in the Southern Benguela region. S. Afr. J. Sci. 2007, 103, 223–231. [Google Scholar]
- Kvitek, R.G. Sequestered Paralytic Shellfish Poisoning toxins mediate glaucous-winged gull predation on bivalve prey. Auk 1991, 108, 381–392. [Google Scholar]
- Kvitek, R.; Bretz, C. Shorebird foraging behavior, diet, and abundance vary with harmful algal bloom toxin concentrations in invertebrate prey. Mar. Ecol. Prog. Ser. 2005, 293, 303–309. [Google Scholar] [CrossRef]
- Suryan, R.M.; Irons, D.B.; Benson, J. Prey switching and variable foraging strategies of Black-legged Kittiwakes and the effect on reproductive success. Condor 2000, 102, 374–384. [Google Scholar] [CrossRef]
- Lira, C.L.; Bermúdez, R.J.; Torres, G.; Borbor-Córdova, M.J. Monitoring toxins in bivalves and humans during algal blooms. In Proceedings of the Seventeenth International Conference on Harmful Algae, Florianópolis, Brazil, 9–14 October 2016; p. 101. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on Marine Biotoxins in Shellfish- Saxitoxin Group. EFSA J. 2009, 1019, 1–76. [Google Scholar]
- McKernan, D.L.; Scheffer, V.B. Unusual numbers of dead birds on the Washington Coast. Condor 1942, 44, 264–266. [Google Scholar] [CrossRef]
- Coulson, J.C.; Potts, G.R.; Deans, I.R.; Fraser, S.M. Mortality of shags and other seabirds caused by paralytic shellfish poison. Nature 1968, 220, 23–24. [Google Scholar] [CrossRef]
- Levasseur, M.; Michaud, S.; Bonneau, E.; Cantin, G.; Auger, F.; Gagne, A.; Claveau, R. Overview of the August 1996 red tide event in the St. Lawrence: Effects of a storm surge. In Canadian Technical Report of Fisheries and Aquatic Sciences No. 2138, Proceedings of the Fifth Canadian Workshop on Harmful Marine Algae, St. John’s, NF, USA, 11–13 September 1996; Penney, R.W., Ed.; Minister of Public Works and Government Services Canada: New Brunswick, Canada, 1996; p. 76. [Google Scholar]
- Landsberg, J.H.; Vargo, G.A.; Flewelling, L.J.; Wiley, F.E. Algal biotoxins. In Infectious Diseases of Wild Birds; Thomas, N., Hunter, B., Atkinson, C., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 431–455. ISBN 9780813828121. [Google Scholar]
- Shearn-Bochsler, V.; Lance, E.W.; Corcoran, R.; Piatt, J.; Bodenstein, B.; Frame, E.; Lawonn, J. Fatal paralytic shellfish poisoning in Kittlitz’s Murrelet (Brachyramphus brevirostris) nestlings, Alaska, USA. J. Wildl. Dis. 2014, 50, 933–937. [Google Scholar] [CrossRef]
- Band-Schmidt, C.J.; Durán-Riveroll, L.M.; Bustillos-Guzmán, J.J.; Leyva-Valencia, I.; López-Cortés, D.J.; Núñez-Vázquez, E.J.; Hernández-Sandoval, F.E.; Ramírez-Rodríguez, D.V. Paralytic toxin producing dinoflagellates in Latin America: Ecology and physiology. Front. Mar. Sci. 2019, 6, 42. [Google Scholar] [CrossRef]
- Van Hemert, C.; Dusek, R.J.; Smith, M.M.; Kaler, R.; Sheffield, G.; Divine, L.M.; Kuletz, K.J.; Knowles, S.; Lankton, J.S.; Hardison, D.R.; et al. Investigation of algal toxins in a multispecies seabird die-off in the Bering and Chukchi Seas. J. Wildl. Dis. 2021, 57, 399–407. [Google Scholar] [CrossRef]
- Jones, T.; Divine, L.M.; Renner, H.; Knowles, S.; Lefebvre, K.A.; Burgess, H.K.; Wright, C.; Parrish, J.K. Unusual mortality of Tufted puffins (Fratercula cirrhata) in the Eastern Bering Sea. PLoS ONE 2019, 14, e0216532. [Google Scholar] [CrossRef]
- Gibble, C.M.; Kudela, R.M.; Knowles, S.; Bodenstein, B.; Lefebvre, K.A. Domoic acid and saxitoxin in seabirds in the United States between 2007 and 2018. Harmful Algae 2021, 103, 101981. [Google Scholar] [CrossRef]
- Saeed, A.F.; Awan, S.A.; Ling, S.; Wang, R.; Wang, S. Domoic acid: Attributes, exposure risks, innovative detection techniques and therapeutics. Algal Res. 2017, 24, 97–110. [Google Scholar] [CrossRef]
- Kotaki, Y. Ecobiology of amnesic shellfish toxin producing diatoms. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 383–396. [Google Scholar]
- Bates, S.S.; Bird, C.J.; de Freitas, A.S.W.; Foxall, R.; Gilgan, M.; Hanic, L.A.; Johnson, G.R.; McCulloch, A.W.; Odense, P.; Pocklington, R.; et al. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from Eastern Prince Edward Island, Canada. Can. J. Fish. Aquat. Sci. 1989, 46, 1203–1215. [Google Scholar] [CrossRef]
- Núñez-Vázquez, E.J.; Gárate-Lizarraga, I.; Band-Schmidt, C.J.; Cordero-Tapia, A.; Lopez-Cortes, D.J.; Sandoval, F.E.H.; Heredia-Tapia, A.; Bustillos-Guzman, J.J. Impact of harmful algal blooms on wild and cultured animals in the Gulf of California. J. Environ. Biol. 2011, 32, 413–423. [Google Scholar]
- Bargu, S.; Silver, M.W.; Ohman, M.D.; Benitez-Nelson, C.R.; Garrison, D.L. Mystery behind Hitchcock’s birds. Nat. Geosci. 2012, 5, 2–3. [Google Scholar] [CrossRef]
- Doucette, G.; Maneiro, I.; Riveiro, I.; Svensen, C. Phycotoxin pathways in aquatic food webs: Transfer, accumulation and degradation. In Ecology of Harmful Algae. Ecological Studies (Analysis and Synthesis); Granéli, E., Turner, J.T., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2006; Volume 189, pp. 283–295. [Google Scholar]
- Shumway, S.E. Phycotoxin-related shellfish poisoning: Bivalve molluscs are not the only vectors. Rev. Fish. Sci. 1995, 3, 1–31. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Bargu, S.; Kieckhefer, T.; Silver, M.W. From sanddabs to blue whales: The pervasiveness of domoic acid. Toxicon 2002, 40, 971–977. [Google Scholar] [CrossRef]
- Jester, R.J.; Baugh, K.A.; Lefebvre, K.A. Presence of Alexandrium catenella and paralytic shellfish toxins in finfish, shellfish and rock crabs in Monterey Bay, California, USA. Mar. Biol. 2009, 156, 493–504. [Google Scholar] [CrossRef]
- Lopes, V.M.; Lopes, A.R.; Costa, P.; Rosa, R. Cephalopods as vectors of harmful algal bloom toxins in marine food webs. Mar. Drugs 2013, 11, 3381–3409. [Google Scholar] [CrossRef]
- McCabe, R.M.; Hickey, B.M.; Kudela, R.M.; Lefebvre, K.A.; Adams, N.G.; Bill, B.D.; Gulland, F.M.D.; Thomson, R.E.; Cochlan, W.P.; Trainer, V.L. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys. Res. Lett. 2016, 43, 10–366. [Google Scholar] [CrossRef]
- Costa, P.R.; Costa, S.T.; Braga, A.C.; Rodrigues, S.M.; Vale, P. Relevance and challenges in monitoring marine biotoxins in non-bivalve vectors. Food Control 2017, 76, 24–33. [Google Scholar] [CrossRef]
- Álvarez, G.; Díaz, P.A.; Godoy, M.; Araya, M.; Ganuza, I.; Pino, R.; Álvarez, F.; Rengel, J.; Hernández, C.; Uribe, E.; et al. Paralytic shellfish toxins in surf clams Mesodesma donacium during a large bloom of Alexandrium catenella dinoflagellates associated to an intense shellfish mass mortality. Toxins 2019, 11, 188. [Google Scholar] [CrossRef]
- Blanco, J. Accumulation of Dinophysis toxins in bivalve molluscs. Toxins 2018, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.I.C.; Gomes, M.T.S.R.; Botelho, M.J.; Rudnitskaya, A. Paralytic shellfish toxins (PST)-transforming enzymes: A review. Toxins 2020, 12, 344. [Google Scholar] [CrossRef] [PubMed]
- Balech, E. The genus Alexandrium or Gonyaulax of the tamarensis Group. In Proceedings of the Third International Conference on Toxic Dinoflagellates, St. Andrews, NB, Canada, 8–12 June 1985; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: New York, NY, USA, 1985; pp. 33–38. [Google Scholar]
- Montoya, N.G.; Carignan, M.O.; Carreto, J.I. Alexandrium tamarense/catenella blooms in the Southwestern Atlantic: Paralytic shellfish toxin production and its trophic transference. In Plankton Ecology of the Southwestern Atlantic. From the Subtropical to the Subantarctic Realm.; Hoffmeyer, M.S., Sabatini, M., Brandini, F.P., Calliari, D.L., Santinelli, N.H., Eds.; Springer International Publishing: Basel, Switzerland, 2018; pp. 453–476. ISBN 9783319778693. [Google Scholar]
- Sunesen, I.; Méndez, S.M.; Mancera-Pineda, J.E.; Dechraoui Bottein, M.Y.; Enevoldsen, H. The Latin America and Caribbean HAB status report based on OBIS and HAEDAT maps and databases. Harmful Algae 2021, 102, 101920. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, G.C.; Louw, D.C. Harmful algal blooms of the Benguela eastern boundary upwelling system. Harmful Algae 2020, 102, 101898. [Google Scholar] [CrossRef] [PubMed]
- Horstman, D.A. Reported red-water outbreaks and their effects on fauna of the West and South Coasts of South Africa, 1959–1980. Fish. Bull. S. Afr. 1981, 15, 71–88. [Google Scholar]
- Gilchrist, J.D.F. An enquiry into fluctuations in fish supply on the South African coast, Part 2. Mar. Biol. Rep. Cape T. 1914, 2, 8–35. [Google Scholar]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef]
- Wood, P.C.; Mason, J. Paralytic Shellfish Poisoning: A Short Account of an Outbreak Occurring on the North-East Coast of Britain in May 1968; ICES CM 1968/K:16; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1968; pp. 1–8. [Google Scholar]
- Bicknell, W.J.; Collins, J.C. The Paralytic Shellfish Poisoning Incident in Massachusetts; Massachusetts Department of Public Health: Boston, MA, USA, 1972; p. 21. [Google Scholar]
- Sasner, J.J.; Miyoshi, I.; Barret, B.E. The 1972 red tide in New Hampshire. In Proceedings of the First International Conference on Toxic Dinoflagellate Blooms; LoCicero, V.R., Wakefield, M., Eds.; Massachusetts Science and Technology Foundation: Wakefield, MA, USA, 1975; pp. 517–523. [Google Scholar]
- Bicknell, W.J.; Walsh, D.C. The first “red tide” in recorded Massachusetts history. Managing an acute and unexpected public health emergency. In Proceedings of the First International Conference on Toxic Dinoflagellate Blooms; LoCicero, V.R., Ed.; Massachusetts Science and Technology Foundation: Wakefield, MA, USA, 1975; pp. 447–458. [Google Scholar]
- Hockey, P.A.R.; Cooper, J. Paralytic shellfish poisoning—A controlling factor in Black Oystercatcher populations? Ostrich 1980, 51, 188–190. [Google Scholar]
- Popkiss, M.E.E.; Horstman, D.A.; Harpur, D. Paralytic shellfish poisoning. A report of 17 cases in Cape Town. S. Afr. Med. J. 1979, 55, 1017–1023. [Google Scholar]
- Fritz, L.; Quilliam, M.A.; Wright, J.L.C.; Beale, A.M.; Work, T.M. An outbreak of domoic scid poisoning attibuted to the pennate diatom Pseudonitzschia australis. J. Phycol. 1992, 28, 439–442. [Google Scholar] [CrossRef]
- Sierra-Beltrán, A.P.; Cortés-Altamirano, R.; Gallo-Reynoso, J.P.; Licea-Duran, S.; Égido-Villareal, J. Is Pseudo-nitzschia pseudodelicatissima toxin the principal cause of sardines, dolphins, sea lions and pelicans mortality in 2004 in Mexico? Harmful Algae News 2005, 29, 6–8. [Google Scholar]
- Sonne, C.; Alstrup, A.K.O.; Therkildsen, O.R. A review of the factors causing paralysis in wild birds: Implications for the paralytic syndrome observed in the Baltic Sea. Sci. Total Environ. 2012, 416, 32–39. [Google Scholar] [CrossRef]
- Silvagni, P.A. Comparative Pathology and Diagnosis of Domoic Acid Toxicity; Universitu of California: Davis, CA, USA, 2003. [Google Scholar]
- Gibble, C.; Duerr, R.; Bodenstein, B.; Lindquist, K.; Lindsey, J.; Beck, J.; Henkel, L.; Roletto, J.; Harvey, J.; Kudela, R. Investigation of a largescale Common Murre (Uria aalge) mortality event in California, USA, in 2015. J. Wildl. Dis. 2018, 54, 569–574. [Google Scholar] [CrossRef]
- Hunt, G.L.; Montevecchi, W.A.; Leopold, M.F. A review of issues related to seabird consumption of fish and shellfish stocks, discards and mariculture as well as the trophic role and ecology of seabirds and waders. ICES Coop. Res. Rep. 1999, 232, 2–5. [Google Scholar]
- Newman, S.H.; Chmura, A.; Converse, K.; Kilpatrick, A.M.; Patel, N.; Lammers, E.; Daszak, P. Aquatic bird disease and mortality as an indicator of changing ecosystem health. Mar. Ecol. Prog. Ser. 2007, 352, 299–309. [Google Scholar] [CrossRef]
- Jones, T.; Parrish, J.K.; Peterson, W.T.; Bjorkstedt, E.P.; Bond, N.A.; Ballance, L.T.; Bowes, V.; Hipfner, J.M.; Burgess, H.K.; Dolliver, J.E.; et al. Massive mortality of a planktivorous seabird in response to a marine heatwave. Geophys. Res. Lett. 2018, 45, 3193–3202. [Google Scholar] [CrossRef]
- Vandersea, M.W.; Kibler, S.R.; Tester, P.A.; Holderied, K.; Hondolero, D.E.; Powell, K.; Baird, S.; Doroff, A.; Dugan, D.; Litaker, R.W. Environmental factors influencing the distribution and abundance of Alexandrium catenella in Kachemak bay and lower cook inlet, Alaska. Harmful Algae 2018, 77, 81–92. [Google Scholar] [CrossRef]
- Casero, M. V Paretic syndrome in Larus michahellis and Larus fuscus in southern Portugal between 2010 and 2019. Master’s Thesis, Huelva University, Huelva, Spain, December 2020. [Google Scholar]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef]
- AOAC, Official Method 959.08. Paralytic shellfish poison, biological method. In Official Methods of Analysis of Association of Official Analytical Chemists; Trucksess, M.W., Ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Mons, M.; van Egmond, H.; Speijers, G. Paralytic Shellfish Poisoning: A Review; Report 388802005; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Utrecht, The Netherlands, 1998; pp. 1–47. [Google Scholar]
- McCall, J.R.; Holland, W.C.; Keeler, D.M.; Hardison, D.R.; Litaker, R.W. Improved accuracy of saxitoxin measurement using an optimized enzyme-linked immunosorbent assay. Toxins 2019, 11, 632. [Google Scholar] [CrossRef]
- Bates, H.A.; Rapoport, H. A chemical assay for saxitoxin, the paralytic shellfish poison. J. Agric. Food Chem. 1975, 23, 237–239. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Villar-González, A. Chemical analysis. Paralytic shellfish poisoning (PSP). In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 177–196. [Google Scholar]
- Rourke, W.A.; Murphy, C.J.; Pitcher, G.; Van De Riet, J.M.; Burns, B.G.; Thomas, K.M.; Quilliam, M.A. Rapid postcolumn methodology for determination of paralytic shellfish toxins in shellfish tissue. J. AOAC Int. 2008, 91, 589–597. [Google Scholar] [CrossRef]
- Van de Riet, J.M.; Gibbs, R.S.; Chou, F.W.; Muggah, P.M.; Rourke, W.A.; Burns, G.; Thomas, K.; Quilliam, M.A. Liquid chromatographic post-column oxidation method for analysis of paralytic shellfish toxins in mussels, clams, scallops, and oysters: Single-laboratory validation. J. AOAC Int. 2009, 92, 1690–1704. [Google Scholar]
- Lawrence, J.F.; Ménard, C.; Cleroux, C. Evaluation of prechromatographic oxidation for liquid chromatographic determination of paralytic shellfish poisons in shellfish. J. AOAC Int. 1995, 78, 514–520. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Niedzwiadek, B. Quantitative determination of paralytic shellfish poisoning toxins in shellfish by using prechromatographic oxidation and liquid chromatography with fluorescence detection. J. AOAC Int. 2001, 84, 1099–1108. [Google Scholar] [CrossRef]
- AOAC Official Method 2005.06. Paralytic shellfish poisoning toxins in shellfish. Prechromatographic oxidation and liquid chromatography with fluorescence detection. First Action. In Official methods of analysis of Association of Official Analytical Chemists; Horwitz, W., Latimer, G.W., Eds.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- AOAC Official Method 2011.02. Paralytic shellfish toxins in mussels, clams, oysters, and scallops. PostColumn oxidation (PCOX) method. First action. In Official Methods of Analysis of Association of Official Analytical Chemists; AOAC International: Gaithersburg, MD, USA, 2011.
- Ben-Gigirey, B.; Rodríguez-Velasco, M.L.; Gago-Martínez, A. Extension of the validation of AOAC Official Method 2005.06 for dc-GTX2,3: Interlaboratory study. J. AOAC Int. 2012, 95, 111–121. [Google Scholar] [CrossRef]
- Rossignoli, A.E.; Ben-Gigirey, B.; Gago-Martínez, A. A Comparison of the results obtained for the direct quantification of GTX6 and C3,4 toxins and after hydrolisis. In Proceedings of the Marine and Freshwater Toxin Analysis, Fourth Joint Symposium and AOAC Task Force Meeting, Baiona, Spain, 5–9 May 2013; p. 99. [Google Scholar]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a sensitive and selective liquid chromatography-mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef]
- Gartrell, B.; Agnew, D.; Alley, M.; Carpenter, T.; Ha, H.J.; Howe, L.; Hunter, S.; McInnes, K.; Munday, R.; Roe, W.; et al. Investigation of a mortality cluster in wild adult yellow-eyed penguins (Megadyptes antipodes) at Otago Peninsula, New Zealand. Avian Pathol. 2017, 46, 278–288. [Google Scholar] [CrossRef]
- Dell’Aversano, C.; Hess, P.; Quilliam, M.A. Hydrophilic interaction liquid chromatography-mass spectrometry for the analysis of paralytic shellfish poisoning (PSP) toxins. J. Chromatogr. A 2005, 1081, 190–201. [Google Scholar] [CrossRef]
- Rodríguez, F.; Garrido, J.L.; Sobrino, C.; Johnsen, G.; Riobó, P.; Franco, J.; Aamot, I.; Ramilo, I.; Sanz, N.; Kremp, A. Divinyl chlorophyll a in the marine eukaryotic protist Alexandrium ostenfeldii (Dinophyceae). Environ. Microbiol. 2016, 18, 627–643. [Google Scholar] [CrossRef]
- Wright, J.L.C.; Boyd, R.K.; de Freitas, A.S.W.; Falk, M.; Foxall, R.A.; Jamieson, W.D.; Laycock, M.V.; McCulloch, A.W.; McInnes, A.G.; Odense, P.; et al. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from eastern Prince Edward Island. Can. J. Chem. 1989, 67, 481–490. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on marine biotoxins in shellfish—Domoic acid. EFSA J. 2009, 1181, 1–61. [Google Scholar]
- Quilliam, M.A.; Sim, P.G.; Mcculloch, A.W.; Mcinnes, A.G. High-performance liquid chromatography of domoic acid, a marine neurotoxin, with application to shellfish and plankton. Int. J. Environ. Anal. Chem. 1989, 36, 139–154. [Google Scholar] [CrossRef]
- Wright, J.L.C.; Falk, M.; McInnes, A.G.; Walter, J.A. Identification of isodomoic acid D and two new geometrical isomers of domoic acid in toxic mussels. Can. J. Chem. 1990, 68, 22–25. [Google Scholar] [CrossRef]
- Djaoued, Y.; Thibodeau, M.; Robichaud, J.; Balaji, S.; Priya, S.; Tchoukanova, N.; Bates, S.S. Photocatalytic degradation of domoic acid using nanocrystalline TiO2 thin films. J. Photochem. Photobiol. A Chem. 2008, 193, 271–283. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Centro Oceanográfico de Vigo (IEO, CSIC), Vigo, Spain. Personal communication, 2021.
- Perl, T.M.; Bédard, L.; Kosatsky, T.; Hockin, J.C.; Todd, E.C.D.; Remis, R.S. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N. Engl. J. Med. 1990, 322, 1775–1780. [Google Scholar] [CrossRef]
- Strain, S.M.; Tasker, R.A.R. Hippocampal damage produced by systemic injections of domoic acid in mice. Neuroscience 1991, 44, 343–352. [Google Scholar] [CrossRef]
- Tasker, R.A.R.; Connell, B.J.; Strain, S.M. Pharmacology of systemically administered domoic acid in mice. Can. J. Physiol. Pharmacol. 1991, 69, 378–382. [Google Scholar] [CrossRef]
- Garthwaite, L.; Ross, K.M.; Miles, C.O.; Hansen, R.P.; Foster, D.; Wilkins, A.L.; Towers, N.R. Polyclonal antibodies to domoic acid, and their use in immunoassays for domoic acid in sea water and shellfish. Nat. Toxins 1998, 6, 93–104. [Google Scholar] [CrossRef]
- Van Dolah, F.M.; Leighfield, T.A.; Haynes, B.L.; Hampson, D.R.; Ramsdell, J.S. A microplate receptor assay for the amnesic shellfish poisoning toxin, domoic acid, utilizing a cloned glutamate receptor. Anal. Biochem. 1997, 245, 102–105. [Google Scholar] [CrossRef]
- Kleivdal, H.; Kristiansen, S.I.; Nilsen, M.V.; Briggs, L. Single-laboratory validation of the biosense direct competitive Enzyme-Linked Immunosorbent Assay (ELISA) for determination of domoic acid toxins in shellfish. J. AOAC Int. 2007, 90, 1000–1010. [Google Scholar] [CrossRef]
- Kleivdal, H.; Kristiansen, S.-I.; Nilsen, M.V.; Goksyr, A.; Briggs, L.; Holland, P.; McNabb, P.; Aasheim, A.; Aune, T.; Bates, S.; et al. Determination of domoic acid toxins in shellfish by Biosense ASP ELISA -A direct competitive Enzyme-Linked Immunosorbent Assay: Collaborative study. J. AOAC Int. 2007, 90, 1011–1027. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Robertson, A.; Frame, E.R.; Colegrove, K.M.; Nance, S.; Baugh, K.A.; Wiedenhoft, H.; Gulland, F.M.D. Clinical signs and histopathology associated with domoic acid poisoning in northern fur seals (Callorhinus ursinus) and comparison of toxin detection methods. Harmful Algae 2010, 9, 374–383. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Hendrix, A.; Halaska, B.; Duignan, P.; Shum, S.; Isoherranen, N.; Marcinek, D.J.; Gulland, F.M.D. Domoic acid in California sea lion fetal fluids indicates continuous exposure to a neuroteratogen poses risks to mammals. Harmful Algae 2018, 79, 53–57. [Google Scholar] [CrossRef]
- Shum, S.; Kirkwood, J.S.; Jing, J.; Petroff, R.; Crouthamel, B.; Grant, K.S.; Burbacher, T.M.; Nelson, W.L.; Isoherranen, N. Validated HPLC-MS/MS method to quantify low levels of domoic acid in plasma and urine after subacute exposure. ACS Omega 2018, 3, 12079–12088. [Google Scholar] [CrossRef]
- AOAC Official Method 991.26. Domoic acid in mussels, liquid chromatographic method. In Official Methods of Analysis of Association of Official Analytical Chemists; Horwitz, W., Latimer, G.W., Eds.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Quilliam, M.A.; Xie, M.; Hardstaff, W.R. Rapid extraction and cleanup for liquid chromatographic determination of domoic acid in unsalted seafood. J. AOAC Int. 1995, 78, 543–554. [Google Scholar] [CrossRef]
- López-Rivera, A.; Suárez-Isla, B.A.; Eilers, P.P.; Beaudry, C.G.; Hall, S.; Fernández Amandi, M.; Furey, A.; James, K.J. Improved high-performance liquid chromatographic method for the determination of domoic acid and analogues in shellfish: Effect of pH. Anal. Bioanal. Chem. 2005, 381, 1540–1545. [Google Scholar] [CrossRef]
- Pocklington, R.; Milley, J.E.; Batesf, S.S.; Bird, C.J.; De Freitas, A.S.W.; Quilliamt, M.A. Trace determination of domoic acid in seawater and phytoplankton by high-performance liquid chromatography of the fluorenylmethoxycarbonyl (FMOC) derivative. Int. J. Environ. Anal. Chem. 1990, 38, 351–368. [Google Scholar] [CrossRef]
- Maroulis, M.; Monemvasios, I.; Vardaka, E.; Rigas, P. Determination of domoic acid in mussels by HPLC with post-column derivatization using 4-chloro-7-nitrobenzo-2-oxa-1,3-diazole (NBD-Cl) and fluorescence detection. J. Chromatogr. B 2008, 876, 245–251. [Google Scholar] [CrossRef]
- Hess, P.; Morris, S.; Stobo, L.A.; Brown, N.A.; McEvoy, J.D.G.; Kennedy, G.; Young, P.B.; Slattery, D.; McGovern, E.; McMahon, T.; et al. LC-UV and LC-MS methods for the determination of domoic acid. TrAC Trends Anal. Chem. 2005, 24, 358–367. [Google Scholar] [CrossRef]
- Wang, Z.; Maucher-Fuquay, J.; Fire, S.E.; Mikulski, C.M.; Haynes, B.; Doucette, G.J.; Ramsdell, J.S. Optimization of solid-phase extraction and liquid chromatography-tandem mass spectrometry for the determination of domoic acid in seawater, phytoplankton, and mammalian fluids and tissues. Anal. Chim. Acta 2012, 715, 71–79. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Charbonneau, C.F.; Ménard, C. Liquid chromatographic determination of domoic acid in mussels, using AOAC paralytic shellfish poison extraction procedure: Collaborative study. J. Assoc. Off. Anal. Chem. 1991, 74, 68–72. [Google Scholar] [CrossRef]
- Tor, E.R.; Puschner, B.; Whitehead, W.E. Rapid determination of domoic acid in serum and urine by liquid chromatography-electrospray tandem mass spectrometry. J. Agric. Food Chem. 2003, 51, 1791–1796. [Google Scholar] [CrossRef]
- Truelove, J.; Iverson, F. Serum domoic acid clearance and clinical observations in the Cynomolgus Monkey and Sprague-Dawley Rat following a single IV dose. Bull. Environ. Contam. Toxicol. 1994, 52, 479–486. [Google Scholar] [CrossRef]
- Maucher, J.M.; Ramsdell, J.S. Ultrasensitive detection of domoic acid in mouse blood by competitive ELISA using blood collection cards. Toxicon 2005, 45, 607–613. [Google Scholar] [CrossRef]
- Anonymous. Avian Mortality Event Response Plan; Region 7; U.S. Fish and Wildlife Service: Washington, DA, USA, 2015; pp. 1–50.
- McKechnie, A.E.; Wolf, B.O. Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 2010, 6, 253–256. [Google Scholar] [CrossRef]
- Fey, S.B.; Siepielski, A.M.; Nusslé, S.; Cervantes-Yoshida, K.; Hwan, J.L.; Huber, E.R.; Fey, M.J.; Catenazzi, A.; Carlson, S.M. Recent shifts in the occurrence, cause, and magnitude of animal mass mortality events. Proc. Natl. Acad. Sci. USA 2015, 112, 1083–1088. [Google Scholar] [CrossRef]
- Velarde, E.; Anderson, D.W.; Ezcurra, E. Seabird clues to ecosystem health. Science 2019, 365, 116–117. [Google Scholar] [CrossRef]
- Alaska Harmful Algal Bloom Network. Available online: https://legacy.aoos.org/alaska-hab-network/ (accessed on 17 June 2021).
- U.S. Geological Survey, National Wildlife Health Center. Available online: https://www.usgs.gov/centers/nwhc/science/report-mortality-events-and-submit-specimens?qt-science_center_objects=0#qt-science_center_objects (accessed on 14 May 2021).
- Materna, E.; Buerger, T.; Buck, J.; Lowe, R.; Newman, S.H. Investigation of persistent seabird mortalities along the Oregon coast. In Environmental Contaminants Program. On-Refuge Investigations Sub-Activity; U.S. Fish and Wildlife Service: Sacramento, CA, USA, 2011; Volume 48, pp. 1–69. [Google Scholar]
- Seabird Health Program. California Department of Fish and Wildlife. Available online: https://wildlife.ca.gov/OSPR/Science/MWVCRC/Sea-Bird-Health-Study (accessed on 14 May 2021).
- Gibble, C.M.; Henkel, L.A.; Nevins, H.M.; Miller, M.A.; Ziccardi, M.H. Summary of California Brown Pelican Mortality: An Evaluation of Live and Dead Strandings in California during 2014; U.S. Fish and Wildlife Service, Pacific Southwest Region: Sacramento, CA, USA, 2016; pp. 1–39.
- Roletto, J.; Mortenson, J.; Harrald, I.; Hall, J.; Grella, L. Beached bird surveys and chronic oil pollution in Central California. Mar. Ornithol. 2003, 31, 21–28. [Google Scholar]
- Parrish, J.K.; Bond, N.; Nevins, H.; Mantua, N.; Loeffel, R.; Peterson, W.T.; Harvey, J.T. Beached birds and physical forcing in the California Current System. Mar. Ecol. Prog. Ser. 2007, 352, 275–288. [Google Scholar] [CrossRef]
- Newton, K.M.; Croll, D.A.; Nevins, H.M.; Benson, S.R.; Harvey, J.T.; Tershy, B.R. At-sea mortality of seabirds based on beachcast and offshore surveys. Mar. Ecol. Prog. Ser. 2009, 392, 295–305. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention, U.S.A. Available online: https://www.cdc.gov/habs/ohhabs.html (accessed on 14 May 2021).
- Coastal Observation and Seabird Survey Team (COASST). Available online: https://coasst.org/ (accessed on 17 June 2021).
- Mosites, E.; Lujan, E.; Brook, M.; Brubaker, M.; Roehl, D.; Tcheripanoff, M.; Hennessy, T. Environmental observation, social media, and One Health action: A description of the Local Environmental Observer (LEO) Network. One Health 2018, 6, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Flores, M. Grupo de trabajo de Aves Marinas. In Programas de Seguimiento de Avifauna y Grupos de Trabajo; SEO/BirdLife (Sociedad Española de Ornitología): Madrid, Spain, 2019; pp. 56–57. [Google Scholar]
- Sea Alarm Foundation. Available online: https://www.sea-alarm.org/ (accessed on 17 May 2021).
- Sea Alarm Foundation. Country Wildlife Response Profiles. Available online: https://www.sea-alarm.org/100-country-wildlife-response-profiles-and-counting/ (accessed on 17 May 2021).
- International Tanker Owners Pollution Federation Limited (ITOPF). Country and Territory Profiles. Available online: https://www.itopf.org/knowledge-resources/countries-territories-regions/ (accessed on 17 May 2021).

| Species | Location, Year | Tissue | Conc. Ranges (μg STX·eq·kg−1) | Observations | Refs. | |
|---|---|---|---|---|---|---|
| Scientific Name | Common Name | |||||
| Alca torda | Razorbill | St. Lawrence Estuary, Quebec, 2008 | Digestive tract | <LOD–960 | - | [35] |
| Liver | <LOD–150 | - | ||||
| Ardea herodias | Great blue heron | St. Lawrence Estuary, Quebec, 2008 | Several tissues | <LOD | - | [35] |
| Ardenna tenuirostris | Short-tailed shearwater | Gambell and Shishmaref, North Berin Sea, Alaska, 2017 | Several tissues | <LOD | - | [62] |
| St. Paul Island, Pribilof Islands, Alaska, 2017 | Stomach and cloaca contents | <LOQ | Pooled samples from several species | |||
| Liver | <LOD | - | ||||
| Brachyramphus brevirostris | Kittlitz’s murrelet | Kodiak Island, Alaska, 2011–2012 | Upper gastrointestinal content | <LOD–216 | Dead chicks. Values probably underestimated | [60] |
| Liver | 56.3–106.4 | |||||
| Kidney | 27.9 | |||||
| Cepphus grylle | Black guillemot | St. Lawrence Estuary, Quebec, 2008 | Digestive tract | 64–700 | - | [35] |
| Liver | <LOD–410 | - | ||||
| Fratercula cirrhata | Tufted puffin | St. Paul Island, Alaska, 2016 | Stomach and cloaca contents | 3.1–9.5 | Concentrations for each tissue not specified | [63] |
| Fratercula corniculata | Horned puffin | Shishmaref, North Berin Sea, Alaska, 2017 | Stomach and cloaca contents | <LOQ | Pooled samples from several species | [62] |
| Several tissues | <LOD | - | ||||
| Fulmarus glacialis | Northern fulmar | St. Lawrence Estuary, Quebec, 2008 | Several tissues | <LOD | - | [35] |
| Gambell and Shishmaref, North Berin Sea, Alaska, 2017 | Cloaca and stomach contents | 46.0 | Pooled sample | [62] | ||
| Stomach content | <LOQ–149 | - | ||||
| Stomach | 12–53 | - | ||||
| Intestinal contents | 21–111 | - | ||||
| Intestine | 15–129 | - | ||||
| Liver | <LOQ–59 | - | ||||
| Muscle | <LOQ–15 | - | ||||
| St. Paul Island and St. George Island, Pribilof Islands, Alaska, 2017 | Cloaca and stomach contents | 46–305 | Pooled sample | |||
| Stomach contents | <LOD–633 | - | ||||
| Intestine | <LOD–145 | - | ||||
| Liver | <LOD–44 | - | ||||
| Several tissues | <LOQ | - | ||||
| San Luis Obispo County, California, 2018 | Liver | 6.9 | - | [64] | ||
| Kidney | 8.8–9.6 | - | ||||
| Bile | 21 | - | ||||
| Gavia immer | Common loon | St. Lawrence Estuary, Quebec, 2008 | Digestive tract | 45, 19 | Results from 1 sample. Conc. for ELISA and HPLC, respectively | [35] |
| Liver | <LOD | |||||
| Gavia stellate | Red-throated loon | St. Lawrence Estuary, Quebec, 2008 | Digestive tract | 61 | - | [35] |
| Liver | <LOD | - | ||||
| Hydrobates furcatus | Fork-tailed Storm-petrel | Unalaska and Aleutian Islands, Alaska, 2017 | Several tissues | <LOQ | - | [62] |
| Larus argentatus | Herring gull | St. Lawrence Estuary, Quebec, 1996 | Intestine | 110 | - | [58] |
| Brain | 48 | - | ||||
| St. Lawrence Estuary, Quebec, 2008 | Digestive tract | 47–690 | - | [35] | ||
| Liver | 100 | - | ||||
| Larus delawarensis | Ring-billed gull | St. Lawrence Estuary, Quebec, 2008 | Digestive tract | 420 | - | [35] |
| Liver | <LOD | - | ||||
| Providence County, Rhode island, 2016 | Cloaca contents | <LOD | - | [64] | ||
| Larus fuscus | Black-backed gull | Ria Formosa, Olhão, Portugal, 2020 | Several tissues | <LOD | - | [38] |
| Larus marinus | Great black-backed gull | St. Lawrence Estuary, Quebec, 2008 | Several tissues | <LOD | - | [35] |
| Larus michahellis | Yellow-legged gull | Ria Formosa, Olhão, Portugal, 2020 | Several tissues | <LOD | - | [38] |
| Larus philadelphia | Bonaparte’s gull | St. Lawrence Estuary, Quebec, 2008 | Digestive tract | <LOD–31 | Results from 1 sample. Conc. for ELISA and HPLC, respectively | [35] |
| Larus sp. | Gull (not identified) | St. Lawrence Estuary, Quebec, 2008 | Liver | 337 | - | [35] |
| Digestive tract | 54.7 | - | ||||
| Melanita deglandi | White-winged scoter | Grays Harbor County, Washington, 2009 | Liver | <LOD–6.4 | - | [64] |
| Bile | <LOD–6.2 | - | ||||
| Several tissues | <LOD | - | ||||
| Melanita perspicillata | Surf scoter | Grays Harbor County, Washington, 2009 | Intestinal contents | <LOD–4.7 | [64] | |
| Morus bassanus | Northern gannet | St. Lawrence Estuary, Quebec, 2008 | Digestive tract | 110–850 | - | [35] |
| Liver | 850 | - | ||||
| Kidney | <LOD–63 | - | ||||
| Muscle | <LOD–87 | - | ||||
| Phalacrocorax auritus | Double-crested cormorant | St. Lawrence Estuary, Quebec, 2008 | Digestive tract | <LOD–370 | - | [35] |
| Liver | <LOD–58 | - | ||||
| Kent County, Rhode Island, 2016 | Stomach contents | <LOD | - | [64] | ||
| Phalacrocorax penicillatus | Brandt’s cormorant | Marin County, California, 2015–2016 | Stomach contents | <LOD–2.0 | - | [64] |
| Rissa tridactyla | Black-legged kittiwake | St. Lawrence Estuary, Quebec, 2008 | Digestive tract | <LOD–1340 | - | [35] |
| Digestive tract+liver | <LOD–520 | - | ||||
| Liver | <LOD–88 | - | ||||
| Gulf of Alaska, 2015–2017 | Cloaca | <LOQ | - | [37] | ||
| Upper gastrointestinal contents | 46 | - | ||||
| Liver | 27 | Healthy animals. Minimum toxin level not provided | ||||
| Muscle | 37 | |||||
| Several tissues | <LOD | - | ||||
| Somateria mollissima | Common eider | St. Lawrence Estuary, Quebec, 2008 | Digestive tract | <LOD–740 | - | [35] |
| Liver | <LOD | - | ||||
| Sterna hirundo | Common tern | Monomoy National Wildlife Refuge, Massachusets, 1978 | Liver | <LOD | Fish vomited by birds accounted 970 μg STX equivalents·kg−1 | [46] |
| Uria aalge | Common murre | St. Lawrence Estuary, Quebec, 2008 | Several tissues | <LOD | - | [35] |
| Clallam County, Washington, 2009 | Stomach contents | <LOD | - | [64] | ||
| Gulf of Alaska, 2015–2016 | Proventriculus and cloaca | 1.4–3.9 | Toxin levels in each sample not specified | [10] | ||
| Gulf of Alaska, 2015–2017 | Cloaca | 48 | - | [37] | ||
| Upper gastrointestinal contents | 10 | 13 μg STX eq·kg−1 in healthy animals | ||||
| Liver | 108 | Minimum toxin level not provided | ||||
| Several tissues | <LOQ | - | ||||
| Shishmaref and Unalakleet, North Berin Sea, Alaska, 2017 | Cloaca and stomach content | <LOQ | Pooled samples from several species | [62] | ||
| Several tissues | <LOD | - | ||||
| Monterey County, California, 2018 | Liver | <LOD | - | |||
| Kidney | <LOD–4.9 | - | ||||
| Species | Location | Tissue | Concentration Ranges (μg DA·kg−1) | Observations | Refs. | |
|---|---|---|---|---|---|---|
| Scientific Name | Common Name | |||||
| Aechmophorus clarkii | Clark’s grebe | Monterey County, California, 2007 | Cloaca contents | <LOD | - | [64] |
| Santa Barbara County, California, 2017 | Cloaca contents | 111.2–681.2 | - | |||
| Calonectris borealis | Cory’s shearwater | Gran Canaria, Canary Island, Spain | Blood | 1.1–10.1 * | Healthy animals | [36] |
| Calonectris diomedea | Scopoli’s shearwater | Menorca, Balearic Island, Spain | Blood | 1–10.6 * | Healthy animals | [36] |
| Fulmarus glacialis | Northern fulmar | San Luis Obispo County, California, 2018 | Liver | 1.5 | - | [64] |
| Kidney | 3.5–5.7 | - | ||||
| Bile | 3.0 | - | ||||
| Gavia pacifica | Pacific loon | Monterey County, California, 2007 | Cloaca contents | <LOD–46100 | - | [64] |
| Ventura County, California, 2017 | Kidney | <LOD–33446 | - | |||
| Gavia stellata | Red-throated loon | Monterey County, California, 2007 | Cecal content | 75,300 | - | [64] |
| Bile | <LOD | - | ||||
| Ventura County, California, 2017 | Liver | 0.65–6850 | - | |||
| Bile | 82.5–49.7 | - | ||||
| Larus delawarensis | Ring-billed gull | Providence County, Rhode island, 2016 | Cloaca contents | 4.5–5.3 | - | [64] |
| Larus fuscus | Black-backed gull | Ria Formosa, Olhão, Portugal, 2020 | Several tissues | <LOD | - | [38] |
| Larus michahellis | Yellow-legged gull | Ria Formosa, Olhão, Portugal, 2020 | Several tissues | <LOD | - | [38] |
| Melanita deglandi | White-winged scoter | Grays Harbor County, Washington, 2009 | Liver | <LOD–23.2 | - | [64] |
| Kidney | <LOD–16.5 | - | ||||
| Melanita perspicillata | Surf scoter | Grays Harbor County, Washington, 2009 | Intestinal contents | <LOD–11.1 | - | [64] |
| Pelecanus occidentalis | Brown pelican | Santa Cruz County, California, 1991 | Stomach contents | <LOD–27,900 | - | [47] |
| Cabo San Lucas, Baja California, 1996 | Stomach contents | <LOD–142,850 | . | [48] | ||
| Digestive tract | 37,170 | . | ||||
| Liver | <LOQ | - | ||||
| Monterey County, California, 2007 | Intestinal contents | 14,600 | - | [64] | ||
| Several tissues | <LOD | - | ||||
| Monterey County, California, 2015–2016 | Cloaca contents | 0.00–2847 | - | |||
| Phalacrocorax auratus | Double-crested cormorant | San Luis Obispo County, California, 2015–2016 | Kidney | 0.00–82.9 | - | [64] |
| Kent County, Rhode Island, 2016 | Stomach contents | 9.0 | - | |||
| Phalacrocorax penicillatus | Brandt’s cormorant | Santa Cruz County, California, 1991 | Stomach contents | <LOD–48,000 | - | [47] |
| Monterey County, California, 2007 | Cloaca contents | <LOD | - | [64] | ||
| Stomach contents | 4000–29,000 | - | ||||
| Marin County, California, 2015–2016 | Stomach contents | 2.36–1632 | - | |||
| Los Angeles County, California, 2017 | Stomach contents | 6270–71150 | - | |||
| Ptychoramphus aleuticus | Cassin’s auklet | Humboldt County, California, 2017 | Kidney | <LOD–86.4 | - | [64] |
| Rissa tridactyla | Black-legged kittiwake | Gulf of Alaska, 2015–2017 | Several tissues | <LOD | - | [37] |
| Feces and regurgitants | <LOQ | Healthy animals | ||||
| Uria aalge | Common murre | Clallam County, Washington, 2009 | Stomach contents | <LOD–12.1 | - | [64] |
| Santa Cruz County, California, 2015 | Cloaca contents | <LOD–63.2 | - | [32] | ||
| Liver | <LOD–4.0 | - | ||||
| Stomach contents | 5-36–10.8 | - | ||||
| Kidney | <LOD | - | ||||
| San Luis Obispo County, California, 2015 | Cloaca contents | 5.0–654.1 | - | |||
| Kidney | 10.7 | - | ||||
| Liver | <LOD–915.8 | - | ||||
| Monterrey County, California, 2015 | Cloaca contents | <LOD–64.1 | - | |||
| Kidney | <LOD–31.5 | - | ||||
| Liver | <LOD–9.5 | - | ||||
| Marin County, California, 2015 | Cloaca contents | <LOD–6.5 | - | |||
| San Mateo County, California, 2015–2016 | Liver | <LOD–915.8 | - | |||
| Gulf of Alaska, 2015–2016 | Proventriculus and cloaca | <LOD | - | [10] | ||
| Gulf of Alaska, 2015–2017 | Several tissues | <LOQ | - | [37] | ||
| Feces | <LOD | - | ||||
| Humboldt County, California, 2017 | Liver | <LOD–97.9 | - | |||
| Monterey County, California, 2018 | Liver | 0.00–4.9 | - | |||
| Kidney | 20.6–21.0 | - | ||||
| Vectors | Affected Birds, Place and Dates | Phytoplankton Species | Observations | References |
|---|---|---|---|---|
| Anchovies | Brown pelicans, Brandt’s cormorants; California, USA; September 1991 | Pseudo-nitzschia australis | DA detected in seabirds and fish | [47,94] |
| Mackerel and sardines | Brown pelicans; Baja California, México; January 1996 and January 2004 | Pseudo-nitzschia spp. | DA detected in seabirds and fish in 1996. Coincidence with sardine mortality and DA detected in dead dolphins in 2004 | [48,68,95] |
| Mainly anchovies, (squids and mussels also possible) | Brandt’s cormorants, brown pelicans, pacific loons, red-throated loons; Monterey County, California, USA; March–May 2007 | Pseudo-nitzschia australis | DA detected in seabirds | [64] |
| Mainly anchovies, (squids and mussels also possible) | Common murres, surf scoters, white-winged scoters; several Washington counties, USA; September–October 2009 | Pseudo-nitzschia spp | DA detected in seabirds | [64] |
| Mainly anchovies, (squids and mussels also possible) | Brandt’s cormorants, brown pelicans, double-crested cormorants, common murres; several California counties, USA; July 2015–March 2016 | Pseudo-nitzschia spp | DA detected in seabirds. In murres it could be a secondary death cause | [32,64,75] |
| Mainly anchovies, (squids and mussels also possible) | Double-crested cormorants, ring-billed gulls; Kent and Providence Counties, Rhode Island, USA; October 2016 | Pseudo-nitzschia sp | DA detected in seabirds | [64] |
| Mainly anchovies, (squids and mussels also possible) | Brandt’s cormorants, Clark’s grebes, pacific loons, Red-throated loons, Cassin’s auklets, common murres; several California counties, USA; April–May and July–August 2017 | Pseudo-nitzschia sp | DA detected in seabirds | [64] |
| Mainly anchovies, (squids and mussels also possible) | Common murres, northern fulmars; Monterey and San Luis Obispo Counties, California, USA; February 2018 | Pseudo-nitzschia sp | DA detected in seabirds. | [64] |
| Headings | Containing Information |
|---|---|
| Spill Notification Point | National contact to communicate an event |
| Response Arrangements | One or more authorities responsible for coordination in case of an event. Different levels in the command chain depending on the event seriousness |
| Response Policy | National contingency plan establishing priorities and approved or forbidden measures |
| Equipment | Government and private equipment such as boats, skimmers, dispersants, etc., and who provides it |
| Previous Spill Experience | Oil natural disasters country history |
| Hazardous and Noxious Substances | Response arrangements for other marine disasters, not oil-related |
| Conventions | International environmental conventions joined by the country |
| Regional and Bilateral Agreements | Signed by the country |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Gigirey, B.; Soliño, L.; Bravo, I.; Rodríguez, F.; Casero, M.V.M. Paralytic and Amnesic Shellfish Toxins Impacts on Seabirds, Analyses and Management. Toxins 2021, 13, 454. https://doi.org/10.3390/toxins13070454
Ben-Gigirey B, Soliño L, Bravo I, Rodríguez F, Casero MVM. Paralytic and Amnesic Shellfish Toxins Impacts on Seabirds, Analyses and Management. Toxins. 2021; 13(7):454. https://doi.org/10.3390/toxins13070454
Chicago/Turabian StyleBen-Gigirey, Begoña, Lucía Soliño, Isabel Bravo, Francisco Rodríguez, and María V. M. Casero. 2021. "Paralytic and Amnesic Shellfish Toxins Impacts on Seabirds, Analyses and Management" Toxins 13, no. 7: 454. https://doi.org/10.3390/toxins13070454
APA StyleBen-Gigirey, B., Soliño, L., Bravo, I., Rodríguez, F., & Casero, M. V. M. (2021). Paralytic and Amnesic Shellfish Toxins Impacts on Seabirds, Analyses and Management. Toxins, 13(7), 454. https://doi.org/10.3390/toxins13070454





