First Report of Paralytic Shellfish Toxins in Marine Invertebrates and Fish in Spain
Abstract
1. Introduction
2. Results
2.1. Analysis of PSTs in Cultures from A. minutum Strains by HPLC-PCOX-FLD
2.2. Analysis of PSTs in Fish, Invertebrates and Dolphins by HPLC-PCOX-FLD
3. Discussion
3.1. PSTs in Cultures from Alexandrium minutum Strains by HPLC-PCOX-FLD
3.2. PSTs in Fish, Invertebrates and Dolphins by HPLC-PCOX-FLD
4. Materials and Methods
4.1. Marine Fauna Sampling
4.2. Isolation of A. minutum Strains
4.3. PSTs Analyses (A. minutum Strains and Marine Fauna)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martínez, A.; Garrido-Maestu, A.; Ben-Gigirey, B.; Chapela, M.J.; González, V.; Vieites, J.M.; Cabado, A.G. Marine biotoxins. In Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer: Heidelberg, Germany, 2015; pp. 869–904. [Google Scholar]
- Khora, S.S. Marine dinoflagellate associated human poisoning. In Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer: Heidelberg, Germany, 2015; pp. 789–814. [Google Scholar]
- Vilariño, N.; Louzao, M.C.; Abal, P.; Cagide, E.; Carrera, C.; Vieytes, M.R.; Botana, L.M. Human poisoning from marine toxins: Unknowns for optimal consumer protection. Toxins 2018, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.T.; Tester, P.A. Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnol. Oceanogr. 1997, 42, 1203–1214. [Google Scholar] [CrossRef]
- Shumway, S.E.; Allen, S.M.; Boersma, P.D. Marine birds and harmful algal blooms: Sporadic victims or under-reported events? Harmful Algae 2003, 2, 1–17. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Lefebvre, K.A.; Flewelling, L.J. Effects of Toxic Microalgae on Marine Organisms. In Toxins and Biologically Active Compounds from Microalgae, Volume 2; Rossini, G.P., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 379–449. ISBN 9781482231472. [Google Scholar]
- Deeds, J.R.; Landsberg, J.H.; Stacey, M.E.; Pitcher, G.C.; Longan, S.W. Non-Traditional Vectors for Paralytic Shellfish Poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations. Marine Biotoxins. Paralytic Shellfish Poisoning; Food and Nutrition Paper; FAO: Rome, Italy, 2004; Volume 80, pp. 5–52. [Google Scholar]
- Ben-Gigirey, B.; Turner, A.D.; Gago-Martínez, A. Instrumental Methods for Paralytic Shellfish Toxins. In Marine and Freshwater Toxins, Springer; Gopalakrishnakone, P., Haddad, V., Jr., Tubaro, A., Kim, E., Kem, W.R., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 43–69. ISBN 9789400766501. [Google Scholar]
- European Parliament Regulation (EC) N° 853/2004 of the European Parlamient and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. Off. J. Eur. Union 2004, L 139, 55–205.
- Roje-Busatto, R.; Ujević, I. PSP toxins profile in ascidian Microcosmus vulgaris (Heller, 1877) after human poisoning in Croatia (Adriatic Sea). Toxicon 2014, 79, 28–36. [Google Scholar] [CrossRef]
- Silva, M.; Rey, V.; Barreiro, A.; Kaufmann, M.; Neto, A.I.; Hassouani, M.; Sabour, B.; Botana, A.; Botana, L.M.; Vasconcelos, V. Paralytic shellfish toxins occurrence in non-traditional invertebrate vectors from north Atlantic waters (Azores, Madeira, and Morocco). Toxins 2018, 10, 362. [Google Scholar] [CrossRef]
- Dean, K.J.; Hatfield, R.G.; Lee, V.; Alexander, R.P.; Lewis, A.M.; Maskrey, B.H.; Alves, M.T.; Hatton, B.; Coates, L.N.; Capuzzo, E.; et al. Multiple new paralytic shellfish toxin vectors in offshore North Sea benthos, a deep secret exposed. Mar. Drugs 2020, 18, 400. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Marine Biotoxins in Shellfish—Saxitoxin Group. Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on Marine Biotoxins in Shellfish. EFSA J. 2009, 1019, 1–76. [Google Scholar]
- Ben-Gigirey, B.; Rodríguez-Velasco, M.L.; Otero, A.; Vieites, J.M.; Cabado, A.G. A comparative study for PSP toxins quantification by using MBA and HPLC official methods in shellfish. Toxicon 2012, 60, 864–873. [Google Scholar] [CrossRef]
- Kodama, M. Paralytic Shellfish Poisoning. Ecobiology, classification and origin. In Seafood and Freshwater Toxins. Pharmacology, Physiology and Detection; Botana, L.M., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2000; pp. 125–149. [Google Scholar]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.; Daugbjerg, N.; Franco, J.M. Morphology, toxin composition and LSU rDNA phylogeny of Alexandrium minutum (Dinophyceae) from Denmark, with some morphological observations on other European strains. Harmful Algae 2003, 2, 317–335. [Google Scholar] [CrossRef]
- Lewis, A.M.; Coates, L.N.; Turner, A.D.; Percy, L.; Lewis, J. A review of the global distribution of Alexandrium minutum (Dinophyceae) and comments on ecology and associated paralytic shellfish toxin profiles, with a focus on Northern Europe. J. Phycol. 2018, 54, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.; Malhi, N.; Seger, A.; Harwood, T.; Jolley, J.; Fitzgibbon, Q.; Hallegraeff, G. Paralytic shellfish toxin uptake, tissue distribution, and depuration in the Southern Rock Lobster Jasus edwardsii Hutton. Harmful Algae 2020, 95, 101818. [Google Scholar] [CrossRef] [PubMed]
- Castonguay, M.; Levasseur, M.; Beaulieu, J.L.; Grégoire, F.; Michaud, S.; Bonneau, E.; Bates, S.S. Accumulation of PSP toxins in Atlantic mackerel: Seasonal and ontogenetic variations. J. Fish Biol. 1997, 50, 1203–1213. [Google Scholar] [CrossRef]
- Reyero, M.; Cacho, E.; Martínez, A.; Vázquez, J.; Marina, A.; Fraga, S.; Franco, J.M. Evidence of saxitoxin derivatives as causative agents in the 1997 mass mortality of monk seals in the Cape Blanc peninsula. Nat. Toxins 1999, 7, 311–315. [Google Scholar] [CrossRef]
- Núñez-Vázquez, E.J.; Gárate-Lizarraga, I.; Band-Schmidt, C.J.; Cordero-Tapia, A.; Lopez-Cortes, D.J.; Sandoval, F.E.H.; Heredia-Tapia, A.; Bustillos-Guzman, J.J. Impact of Harmful Algal Blooms on wild and cultured animals in the Gulf of California. J. Environ. Biol. 2011, 32, 413–423. [Google Scholar]
- Van Hemert, C.; Schoen, S.K.; Litaker, R.W.; Smith, M.M.; Arimitsu, M.L.; Piatt, J.F.; Holland, W.C.; Ransom Hardison, D.; Pearce, J.M. Algal toxins in Alaskan seabirds: Evaluating the role of saxitoxin and domoic acid in a large-scale die-off of Common Murres. Harmful Algae 2020, 92, 101730. [Google Scholar] [CrossRef]
- Hashimoto, K.; Noguchi, T. Recent studies on paralytic shellfish poison in Japan. Pure Appl. Chem. 1989, 61, 7–18. [Google Scholar] [CrossRef]
- Ito, K.; Asakawa, M.; Sida, Y.; Miyazawa, K. Occurrence of paralytic shellfish poison (PSP) in the starfish Asterina pectinifera collected from the Kure Bay, Hiroshima Prefecture, Japan. Toxicon 2003, 41, 291–295. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; Dean, K.; Milligan, S.; Hamilton, M.; Thomas, J.; Poole, C.; Haycock, J.; Spelman-Marriott, J.; Watson, A.; et al. Fatal canine intoxications linked to the presence of saxitoxins in stranded marine organisms following winter storm activity. Toxins 2018, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Braid, H.E.; Deeds, J.; DeGrasse, S.L.; Wilson, J.J.; Osborne, J.; Hanner, R.H. Preying on commercial fisheries and accumulating paralytic shellfish toxins: A dietary analysis of invasive Dosidicus gigas (Cephalopoda Ommastrephidae) stranded in Pacific Canada. Mar. Biol. 2012, 159, 25–31. [Google Scholar] [CrossRef]
- Lopes, V.M.; Lopes, A.R.; Costa, P.; Rosa, R. Cephalopods as vectors of harmful algal bloom toxins in marine food webs. Mar. Drugs 2013, 11, 3381–3409. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.R.; Costa, S.T.; Braga, A.C.; Rodrigues, S.M.; Vale, P. Relevance and challenges in monitoring marine biotoxins in non-bivalve vectors. Food Control 2017, 76, 24–33. [Google Scholar] [CrossRef]
- Fritz, L.; Triemer, R.E. A Rapid Simple Technique Utilizing Calcofluor White M2R for the Visualization of Dinoflagellate Thecal Plates. J. Phycol. 1985, 21, 662–664. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Rodríguez-Velasco, M.L.; Gago-Martínez, A. Extension of the Validation of AOAC Official Method 2005.06 for dc-GTX2,3: Interlaboratory Study. J. AOAC Int. 2012, 95, 111–121. [Google Scholar] [CrossRef]
- Van De Riet, J.; Gibbs, R.S.; Muggah, P.M.; Rourke, W.A.; MacNeil, J.D.; Quilliam, M.A. Liquid chromatography post-column oxidation (PCOX) method for the determination of paralytic shellfish toxins in mussels, clams, oysters, and scallops: Collaborative study. J. AOAC Int. 2011, 94, 1154–1176. [Google Scholar] [CrossRef]
- Rourke, W.A.; Murphy, C.J.; Pitcher, G.; Van De Riet, J.M.; Burns, B.G.; Thomas, K.M.; Quilliam, M.A. Rapid postcolumn methodology for determination of paralytic shellfish toxins in shellfish tissue. J. AOAC Int. 2008, 91, 589–597. [Google Scholar] [CrossRef]
- Rodríguez, F.; Garrido, J.L.; Sobrino, C.; Johnsen, G.; Riobó, P.; Franco, J.; Aamot, I.; Ramilo, I.; Sanz, N.; Kremp, A. Divinyl chlorophyll a in the marine eukaryotic protist Alexandrium ostenfeldii (Dinophyceae). Environ. Microbiol. 2016, 18, 627–643. [Google Scholar] [CrossRef]
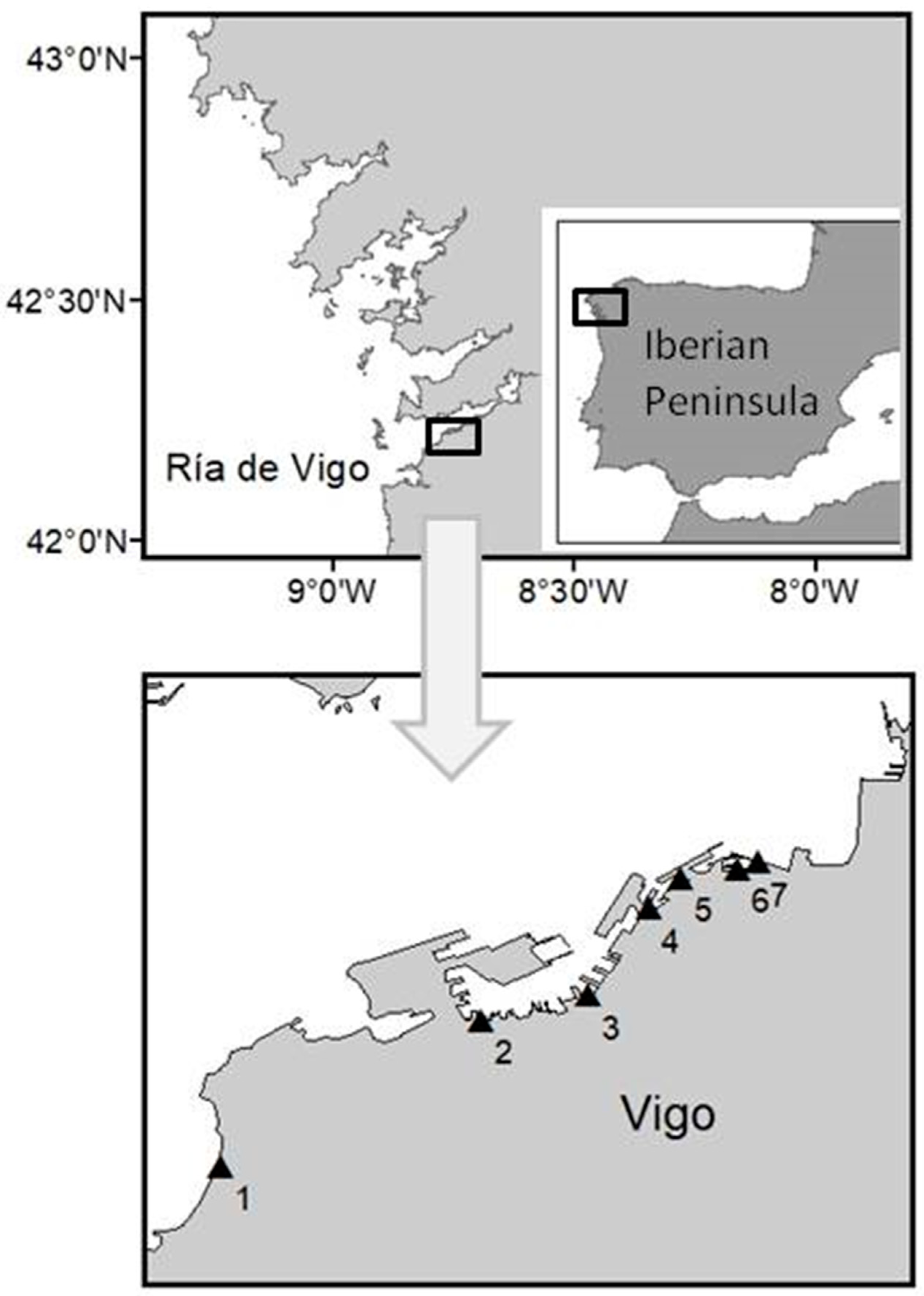


| Strain Code | Sampling Date | Sampling Point and Coordinates | Toxic Profile |
|---|---|---|---|
| SA2B | 28/06/2018 | 1: Samil Beach 42°12′38.0″ N, 8°46′34.3″ W | GTX3, GTX4 |
| SA2C | 28/06/2018 | GTX2, GTX3, GTX4 | |
| SA3B | 28/06/2018 | - | |
| SA3C | 28/06/2018 | GTX4 | |
| SA5B | 28/06/2018 | - | |
| SFA | 15/07/2018 | GTX3, GTX4 | |
| S1 | 28/06/2018 | GTX3, GTX4 | |
| PC4B | 16/07/2018 | 2: Vigo marina (dockyards) 42°13′32.2″ N, 8°44′57.2″ W | GTX1, GTX2, GTX3, GTX4 |
| PC4D | 16/07/2018 | GTX3, GTX4 | |
| P1FA | 18/07/2018 | 3: Vigo marina (Beiramar) 42°13′41.5″ N, 8°44′17.2″ W | GTX4 |
| P1FA2 | 18/07/2018 | GTX4 | |
| P13A | 18/07/2018 | GTX4 | |
| P15C | 18/07/2018 | - | |
| P14A | 18/07/2018 | - | |
| P12A | 18/07/2018 | GTX3, GTX4 | |
| P2FB1 | 18/07/2018 | - | |
| P21B | 18/07/2018 | - | |
| P2FA | 18/07/2018 | GTX3, GTX4 | |
| P21A | 18/07/2018 | GTX1, GTX2, GTX3, GTX4 | |
| P3FB2 | 18/07/2018 | GTX2, GTX3, GTX4 | |
| P31C | 18/07/2018 | GTX2, GTX3, GTX4 | |
| P32D | 18/07/2018 | GTX1, GTX2, GTX3, GTX4 | |
| P3FA | 18/07/2018 | GTX3, GTX4 | |
| P31D | 18/07/2018 | - | |
| P36A | 18/07/2018 | GTX4 | |
| P36B | 18/07/2018 | - | |
| P4FA | 18/07/2018 | GTX3, GTX4 | |
| PFB3 | 18/07/2018 | - | |
| PB7F | 09/07/2018 | 4: Vigo marina (inshore pier) 42°14′13.9″ N, 8°43′55.0″ W | GTX4 |
| PB6F | 09/07/2018 | GTX4 | |
| PB9 | 09/07/2018 | - | |
| ALB6 | 17/07/2018 | 5: Vigo marina (A Laxe) 42°14′25.1″ N, 8°43′43.2″ W | GTX2, GTX3, GTX4 |
| ALB7 | 17/07/2018 | GTX2, GTX3, GTX4 | |
| ALC7 | 17/07/2018 | GTX3, GTX4 | |
| P1 | 09/07/2018 | 6: Vigo marina (Náutico) 42°14′28.5″ N, 8°43′21.7″ W | GTX3, GTX4 |
| P4 | 09/07/2018 | GTX2, GTX3, GTX4 | |
| NA2 | 12/07/2018 | GTX3, GTX4 | |
| NA3 | 12/07/2018 | GTX3, GTX4 | |
| NA4 | 12/07/2018 | GTX3, GTX4 | |
| NA5 | 12/07/2018 | GTX1, GTX2, GTX3, GTX4 | |
| NB3 | 12/07/2018 | - | |
| NB4 | 12/07/2018 | GTX4 | |
| NB5 | 12/07/2018 | GTX4 | |
| NC2 | 12/07/2018 | GTX3, GTX4 | |
| FP2 | 09/07/2018 | 7: Vigo marina (As Avenidas) 42°14′31.1″ N, 8°43′14.4″ W | GTX4 |
| Samples and Their Codes | Sampling Point and Date | Tissues Employed for Analysis | Total Toxicity µg eq STX·diHCl/kg | Toxic Profile | |
|---|---|---|---|---|---|
| Grey mullet-1 25 cm, 380 g Liza ramada | Vigo (a) 10/07/18 | Muscle, digestive tract, gonads | 132 | GTX3 | |
| Grey mullet-2 25 cm, 420 g Liza ramada | Vigo (a) 10/07/18 | Muscle, digestive tract, gonads | 21.7 | GTX3 | |
| Grey mullet-3 40 cm, 750 g Liza ramada | Vigo (b) 11/07/18 | Digestive tract | 151 | GTX3 | |
| Grey mullet-6 37 cm, 550 g Liza ramada | Vigo (a) 20/07/18 | Digestive tract | 67.8 | GTX3 | |
| Mackerel-2 28 cm, 200 g Scomber scombrus | Vigo (b) 10/07/18 | Muscle | 27.6 | GTX2, GTX3 | |
| Mackerel-2 28 cm, 200g Scomber scombrus | Vigo (b) 10/07/18 | Digestive tract | 292 | GTX1, GTX2, GTX3, GTX4 | |
| Mussels-1 65 g homogenate Mytilus galloprovincialis | Vigo (a) 20/07/18 | Whole body | 1877 | GTX1, GTX2, GTX3, GTX4, STX | |
| Ascidians-1 15 g homogenate Ciona intestinalis | Vigo (a) 20/07/18 | Whole body | 363 | GTX1, GTX2, GTX3, GTX4 | |
| Scallops-1 37 g homogenate Chlamys varia | Vigo (a) 20/07/18 | Whole body | 3437 | GTX1, GTX2, GTX3, GTX4, STX, NEO | |
| Scallops-2 33 g homogenate Chlamys varia | Vigo (a) 20/07/18 | Whole body | 2670 | GTX1, GTX2, GTX3, GTX4, STX, NEO | |
| Oysters-1 24 g homogenate Ostrea edulis | Vigo (a) 20/07/18 | Whole body | 485 | GTX1, GTX2, GTX3, GTX4 | |
| Oysters-2 5 g homogenate Ostrea edulis | Vigo (a) 20/07/18 | Whole body | 805 | GTX1, GTX2, GTX3, GTX4 | |
| Squids-5 17 g homogenate Loligo vulgaris | Vigo (a) 18/07/18 | Whole body | 16.3 | GTX3 | |
| Starfish-1 Marthasterias glacialis 18 cm | Vigo (a) 20/07/18 | Ambulacral groove | 38.4 | GTX2, GTX3 | |
| Starfish-2 Marthasterias glacialis 16 cm | Vigo (a) 20/07/18 | Ambulacral groove | 57.8 | GTX2, GTX3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Gigirey, B.; Rossignoli, A.E.; Riobó, P.; Rodríguez, F. First Report of Paralytic Shellfish Toxins in Marine Invertebrates and Fish in Spain. Toxins 2020, 12, 723. https://doi.org/10.3390/toxins12110723
Ben-Gigirey B, Rossignoli AE, Riobó P, Rodríguez F. First Report of Paralytic Shellfish Toxins in Marine Invertebrates and Fish in Spain. Toxins. 2020; 12(11):723. https://doi.org/10.3390/toxins12110723
Chicago/Turabian StyleBen-Gigirey, Begoña, Araceli E. Rossignoli, Pilar Riobó, and Francisco Rodríguez. 2020. "First Report of Paralytic Shellfish Toxins in Marine Invertebrates and Fish in Spain" Toxins 12, no. 11: 723. https://doi.org/10.3390/toxins12110723
APA StyleBen-Gigirey, B., Rossignoli, A. E., Riobó, P., & Rodríguez, F. (2020). First Report of Paralytic Shellfish Toxins in Marine Invertebrates and Fish in Spain. Toxins, 12(11), 723. https://doi.org/10.3390/toxins12110723





