Variation of Deoxynivalenol Levels in Corn and Its Products Available in Retail Markets of Punjab, Pakistan, and Estimation of Risk Assessment
Abstract
1. Introduction
2. Results
2.1. Method Validation
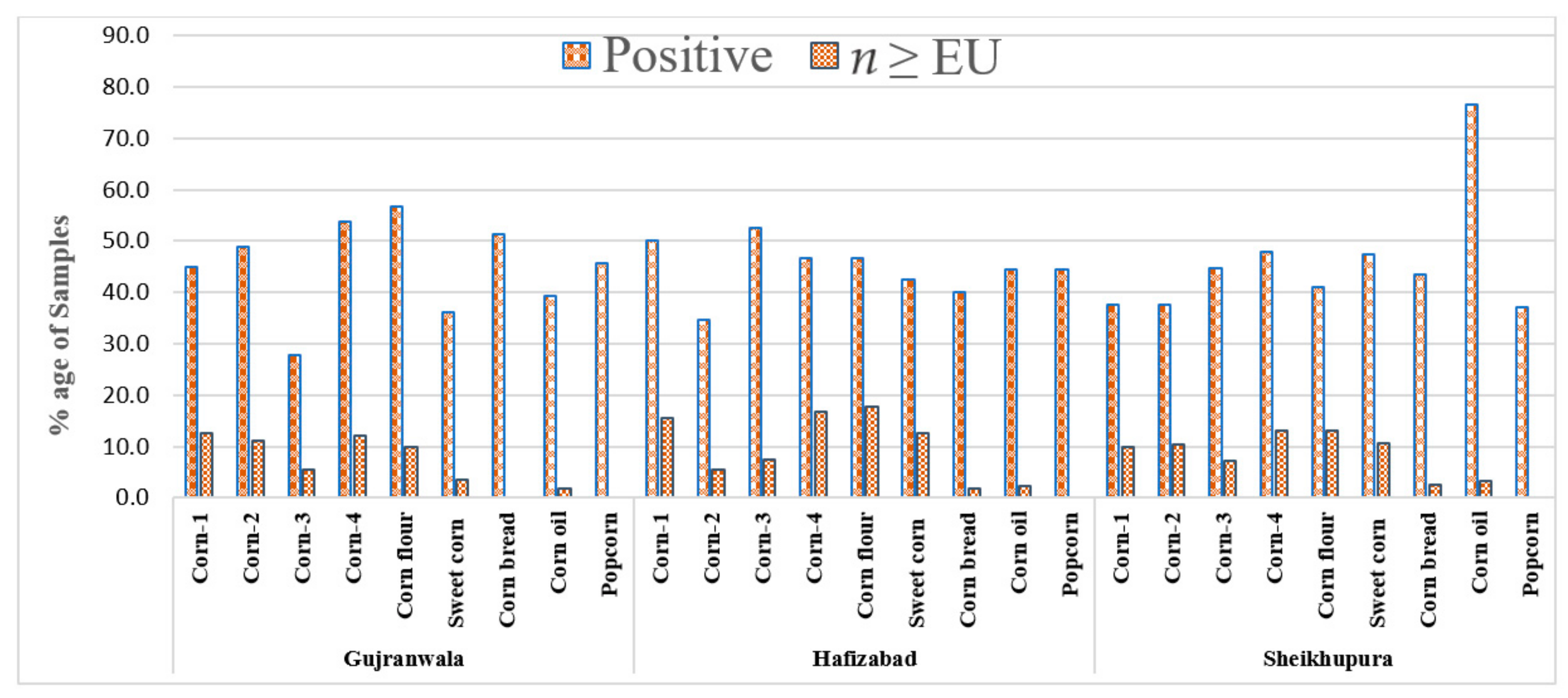
2.2. Incidence of DON in Corn and Corn Products
2.3. Estimation of Exposure Assessment of DON in Corn Flour
3. Discussion
3.1. Method Validation
3.2. Incidence of DON in Corn and Corn Products
3.3. Exposure Assessment of DON in Corn Flour
4. Conclusions
5. Materials and Methods
5.1. Sampling
5.2. Chemicals and Reagents
5.3. Extraction and HPLC Parameters
5.4. Exposure Assessment
5.5. Method Validation
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA. Pakistan: Grain and Feed Annual. GAIN Report Number: PK1707. 2017. Available online: https://www.fas.usda.gov/data/pakistan-grain-and-feed-annual-3 (accessed on 24 January 2021).
- Aniołowska, M.; Steininger, M. Determination of trichothecenes and zearalenone in different corn (Zea mays) cultivars for human consumption in Poland. J. Food Comp. Anal. 2014, 33, 14–19. [Google Scholar] [CrossRef]
- Escobar, J.; Loran, S.; Gimenez, I.; Ferraz, E.; Herrera, M.; Herrera, A.; Arino, A. Occurrence and exposure assessment of Fusarium mycotoxins in maize germ, refined corn oil and margarine. Food Chem. Toxicol. 2013, 62, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.J.; Dobson, A.D. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef]
- Majeed, S.; Asi, M.R.; Iqbal, M.; Iqbal, S.Z.; Jinap, S. Analysis of nutritional traits and comparison of aflatoxins contamination in selected maize varieties from Pakistan. J. Food Prot. 2017, 80, 1993–1998. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Jinap, S. Detection of aflatoxins and zearalenone contamination in wheat-derived products. Food Control 2014, 35, 223–226. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- Martins, H.M.; Almeida, I.; Marques, M.F.; Guerra, M.M. Fumonisins and deoxynivalenol in corn-based food products in Portugal. Food Chem. Toxicol. 2008, 46, 2585–2587. [Google Scholar] [CrossRef]
- Marasas, W.F.O. Toxigenic Fusaria. In Mycotoxins and Animal Foods; Smith, J.E., Ed.; CRS Press Inc.: Boston, MA, USA, 1991; pp. 216–252. [Google Scholar]
- Tanaka, T.; Yamamoto, S.; Hasegawa, A.; Aoki, N.; Besling, J.R.; Sugiura, Y.; Ueno, Y. A survey of the natural occurrence of Fusarium mycotoxins, deoxynivalenol, nivalenol and zearalenone, in cereal harvested in the Netherlands. Mycopathologia 1990, 110, 19–22. [Google Scholar] [CrossRef]
- Trucksess, M.W.; Thomas, F.; Young, K.; Stack, M.E.; Fulguers, W.J.; Page, S.W. Survey of deoxynivalenol in US 1993 wheat and barley crop by enzyme-linked-immunosorbent-assay. J. AOAC Int. 1995, 78, 631–636. [Google Scholar] [CrossRef]
- Placenta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Animal Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Rehman, B.; Jinap, S.; Akram, N.; Ahmad, M.N.; Sanny, M.; Sukor, R.; Samsudin, N.I. Assessment of fumonisin B1 concentrations in wheat and barley products in the Punjab region of Pakistan. J. Food Prot. 2020, 83, 1284–1288. [Google Scholar] [CrossRef]
- Pestka, J.J.; Bondy, J.S. Alterations of immune function following dietary mycotoxin exposure. Can. J. Physiol. Pharmacol. 1990, 68, 1009–1016. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health Part B 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Raza, H.M.F.; Asi, M.R.; Maqbool, U. Assessment of deoxynivalenol (don) mycotoxin in corn and wheat grains consumed in central Punjab, Pakistan. Pak. J. Bot. 2020, 52, 2205–2210. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Usman, S.; Razis, A.F.A.; Ali, N.B.; Saif, T.; Asi, M.R. Assessment of deoxynivalenol in wheat, corn and products and estimation of dietary intake. Int. J. Environ. Res. Public Health 2020, 17, 5602. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuff. Eur. J. Union 2006, 364, 5–24. [Google Scholar]
- Iqbal, S.Z.; Asi, M.R.; Ariño, A.; Akram, N.; Zuber, M. Aflatoxin contamination in different fractions of rice from Pakistan and estimation of dietary intakes. Mycotoxin Res. 2012, 28, 175–180. [Google Scholar] [CrossRef]
- Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives (JECFA). Evaluation of Certain Food Additives and Contaminants (Report of the 72nd Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA)); WHO Technical Report Series; WHO: Geneva, Switzerland, 2010; p. 958. [Google Scholar]
- European Commission. Commission Regulation (EC) No. 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Eur. Comm. 2006, 70, 12–35. [Google Scholar]
- Ok, H.E.; Lee, S.Y.; Chun, H.S. Occurrence and simultaneous determination of nivalenol and deoxynivalenol in rice and bran by HPLC-UV detection and immunoaffinity cleanup. Food Control 2018, 87, 53–59. [Google Scholar] [CrossRef]
- Yang, D.; Geng, Z.M.; Ma, H.X.; Yao, J.B.; Zhang, X.; Zhang, P.P.; Zhang, P. Establishment of a HPLC-UV method for simultaneous determination of DON, 15AcDON, and 3AcDON in wheat. Acta Agron. Sin. 2012, 38, 186–189. [Google Scholar] [CrossRef]
- Thammawong, M.; Okabe, M.; Kawasaki, T.; Nakagawa, H.; Nagashima, H.; Okadome, H.; Nakajima, T.; Kushiro, M. Distribution of deoxynivalenol and nivalenol in milling fractions from Fusarium-infected Japanese wheat cultivars. J. Food Prot. 2010, 73, 1817–1823. [Google Scholar] [CrossRef]
- Golge, O.; Kabak, B. Occurrence of deoxynivalenol and zearalenone in cereals and cereal products from Turkey. Food Control 2020, 110, 106982. [Google Scholar] [CrossRef]
- Cerveró, M.C.; Castillo, M.Ã.; Montes, R.; Hernández, E. Determination of trichothecenes, zearalenone and zearalenols in commercially available corn-based foods in Spain. Rev. Iberoam. Micol. 2007, 24, 52–55. [Google Scholar] [CrossRef]
- Berthiller, F.; Dall’asta, C.; Corradini, R.; Marchelli, R.; Sulyok, M.; Krska, R.; Adam, G.; Schuhmacher, R. Occurrence of deoxynivalenol and its 3-β-D-glucoside in wheat and maize. Food Addict. Contam. Part A 2009, 26, 507–511. [Google Scholar] [CrossRef]
- Nobel, P.B.; Dutton, M.F.; Koch, S.H.; Chuturgoon, A.A.; Stoev, S.D.; Mosonik, J.S. Simultaneous occurrence of mycotoxins in human food commodities from Cameroon. Mycotoxin Res. 2010, 26, 47–57. [Google Scholar]
- Paladin, J.; Sokolović, M.; Perši, N.; Zadravec, M.; Jaki, V.; Vulić, A. Contamination of maize with deoxynivalenol and zearalenone in Croatia. Food Control 2012, 28, 94–98. [Google Scholar] [CrossRef]
- Marques, M.F.; Martins, H.M.; Costa, J.M.; Bernardo, F. Co-occurrence of deoxynivalenol and zearalenone in crops marketed in Portugal. Food Addict. Contam. Part B 2008, 1, 130–133. [Google Scholar] [CrossRef]
- Vidal, A.; Marín, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Determination of aflatoxins, deoxynivalenol, ochratoxin A and zearalenone in wheat and oat-based bran supplements sold in the Spanish market. Food Chem. Toxicol. 2013, 53, 133–138. [Google Scholar] [CrossRef]
- Jajić, I.; Jurić, V.; Glamočić, D.; Abramović, B. Occurrence of deoxynivalenol in maize and wheat in Serbia. Int. J. Mol. Sci. 2008, 9, 2114–2126. [Google Scholar] [CrossRef]
- Tima, H.; Brückner, A.; Mohácsi-Farkas, C.; Kiskó, G. Fusarium mycotoxins in cereals harvested from Hungarian fields. Food Addict. Contam. Part B 2016, 9, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Giménez, I.; Herrera, M.; Escobar, J.; Ferraz, E.; Lorán, S.; Herrera, A.; Ariño, A. Distribution of deoxynivalenol and zearalenone in milled germ during wheat milling and analysis of toxin levels in wheat germ and wheat germ oil. Food Control 2013, 34, 268–273. [Google Scholar] [CrossRef]
- Hirooka, E.Y.; Yamaguchi, M.M.; Aoyama, S.; Sugiura, Y.; Ueno, Y. The natural occurrence of fumonisins in Brazilian corn kernels. Food Addict. Contam. 1996, 13, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Stack, M.E.; Musser, S.M. Determination of deoxynivalenol in 1991 in US winter and spring wheat by high-performance thin-layer chromatography. J. Assoc. Off. Anal. Chem. Int. 1994, 77, 628–630. [Google Scholar]
- Ngoko, Z.; Marasas, W.F.O.; Rheeder, J.P.; Shephard, G.S.; Wingfield, M.J.; Cardwell, K.F. Fungal infection and mycotoxin contamination of maize in the humid forest and the western highlands of Cameroon. Phytoparacitica 2001, 29, 352–360. [Google Scholar] [CrossRef]
- Devegowda, G.; Raju, M.V.; Nazar, A.; Swamy, H.V. Mycotoxin picture worldwide: Novel solutions for their counteraction. In Biotechnology in the Feed Industry. Proceedings of Alltech’s 14th Annual Symposium; Nottingham University Press: Nottingham, UK, 1998; pp. 241–255. [Google Scholar]
- Soriano, J.M.; Dragacci, S. Occurrence of fumonisins in foods. Food Res. Int. 2004, 37, 985–1000. [Google Scholar] [CrossRef]
- Whitaker, T.B.; Truckess, W.M.; Johansson, A.S.; Giesbrecht, F.G.; Hagler, W.M., Jr.; Bowman, D.T. Variability associated with testing shelled corn for fumonisin. J. Assoc. Off. Anal. Chem. Int. 1998, 81, 1162–1168. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Rabbani, T.; Asi, M.R.; Jinap, S. Assessment of aflatoxins, ochratoxin A and zearalenone in breakfast cereals. Food Chem. 2014, 157, 257–262. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Principles and Methods for the Risk Assessment of Chemicals in Food; World Health Organization: Geneva, Switzerland, 2009; Volume 240. [Google Scholar]
- Raad, F.; Nasreddine, L.; Hilan, C.; Bartosik, M.; Parent-Massin, D. Dietary exposure to aflatoxins, ochratoxin A and deoxynivalenol from a total diet study in an adult urban Lebanese population. Food Chem. Toxicol. 2014, 73, 35–43. [Google Scholar] [CrossRef]
- Cano-Sancho, G.; Ramos, V.; Marín, S.; Sanchis, V. Micotoxinas. Estudio de dieta Total en Cataluña 2008–2009; Generalitat de Cataluña: Barcelona, Spain, 2012; pp. 135–140. [Google Scholar]
| DON Level | Corn (Mix) | Corn Flour | Corn Bread | Sweet Corn | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery | RSD | Precision (%) | Recovery | RSD | Precision (%) | Recovery | RSD | Precision (%) | Recovery | RSD | Precision (%) | |||||
| µg/kg | (%) | (%) | Rep a | Reprod b | (%) | (%) | Rep a | Reprod b | (%) | (%) | Rep a | Reprod b | (%) | (%) | Rep a | Reprod b |
| 100 | 84.5 | 16 | 11 | 21 | 81.5 | 11 | 12 | 19 | 89.5 | 14 | 9 | 20 | 85.2 | 16 | 10 | 21 |
| 150 | 89.5 | 11 | 18 | 19 | 86.5 | 14 | 09 | 15 | 83.5 | 18 | 15 | 26 | 89.5 | 17 | 14 | 17 |
| 300 | 88.0 | 22 | 15 | 22 | 87.4 | 17 | 16 | 22 | 90.5 | 23 | 10 | 18 | 89.5 | 15 | 9 | 21 |
| 400 | 91.0 | 13 | 18 | 24 | 90.9 | 12 | 14 | 20 | 89.5 | 22 | 12 | 22 | 88.4 | 19 | 17 | 26 |
| 800 | 88.8 | 23 | 10 | 22 | 86.1 | 16 | 18 | 26 | 88.4 | 21 | 15 | 20 | 85.2 | 21 | 15 | 24 |
| 3000 | 85.0 | 21 | 18 | 28 | 84.0 | 20 | 20 | 27 | 85.5 | 20 | 18 | 27 | 81.3 | 28 | 18 | 28 |
| Sample Type | Samples N | Positive N (%) | Mean (µg/kg) ± S.D. | Range (µg/kg) |
|---|---|---|---|---|
| Corn 1 | 40 | 18 (45.0) | 1256.2 ± 124.5 | 25–5687.5 |
| Corn 2 | 45 | 22 (48.9) | 1126.2 ± 90.8 | 25–5568.6 |
| Corn 3 | 54 | 15 (27.8) | 1049.7 ± 110.5 | 25–5678.5 |
| Corn 4 | 41 | 22 (53.7) | 909.8 ± 120.5 | 25–4550.5 |
| Corn flour | 60 | 34 (57.6) | 854.7 ± 40.7 | 25–8990.5 |
| Sweet corn | 83 | 30 (36.1) | 556.5 ± 80.6 | 25–4550.5 |
| Corn bread | 35 | 18 (51.4) | 86.6 ± 14.5 | 25–450.5 |
| Corn oil | 56 | 22 (39.3) | 88.8 ± 15.5 | 25–980.5 |
| Popcorn | 35 | 16 (45.7 | 41.4 ± 10.5 | 25–95.5 |
| Total | 449 | 197 (43.9) | 25–8990.5 |
| Sample Type | Samples N | Positive N (%) | Mean (µg/kg) ± S.D. | Range (µg/kg) |
|---|---|---|---|---|
| Corn 1 | 48 | 15 (37.5 | 1187.3 ± 230 | 25–6660.5 |
| Corn 2 | 48 | 18 (37.5) | 1081.6 ± 145.5 | 25–6590.6 |
| Corn 3 | 56 | 25 (44.6) | 808.2 ± 60.5 | 25–5640.5 |
| Corn 4 | 46 | 22 (47.8) | 1489.4 ± 180.5 | 25–5540.5 |
| Corn flour | 61 | 25 (41.0) | 1030.8 ± 170.5 | 25–7860.5 |
| Sweet corn | 38 | 18 (47.4) | 471.1 ± 30.5 | 25–2345.5 |
| Corn bread | 39 | 17 (43.6) | 97.3 ± 15.5 | 25–500.5 |
| Corn oil | 30 | 23 (76.7) | 88.3 ± 9.5 | 25–540.5 |
| Popcorn | 35 | 13 (37.1) | 42.1 ± 4.5 | 25–90.5 |
| Total | 393 | 176 (44.8) | 25–7860 |
| Sample Type | Samples N | Positive N (%) | Mean (µg/kg) ± S.D. | Range (µg/kg) |
|---|---|---|---|---|
| Corn 1 | 32 | 16 (50.0) | 1187.3 ± 210.5 | 25–6660.5 |
| Corn 2 | 55 | 19 (34.5) | 1081.6 ± 140.3 | 25–6590.6 |
| Corn 3 | 40 | 21 (52.5) | 808.2 ± 70.5 | 25–5640.5 |
| Corn 4 | 30 | 14 (46.7) | 1489.4 ± 190.8 | 25–5540.5 |
| Corn flour | 45 | 21 (46.7) | 1030.8 ± 105.4 | 25–7860.5 |
| Sweet corn | 40 | 17 (42.5) | 471.1 ± 20.5 | 25–2345.5 |
| Corn bread | 55 | 22 (40.0) | 97.3 ± 15.5 | 25–500.5 |
| Corn oil | 45 | 20 (44.4) | 88.3 ± 9.5 | 25–540.5 |
| Popcorn | 36 | 16 (44.4) | 42.2 ± 4.5 | 25–80.5 |
| Total | 378 | 166 (43.9) | 25–7860.5 |
| Sum of Squares | df | Mean Square | F | Sig | ||
|---|---|---|---|---|---|---|
| DON Level | Between groups | 96,192,972.7 | 8 | 12,024,121.6 | 8.132 | 0.000 |
| Within groups | 783,676,623.6 | 530 | 1,478,635.1 | |||
| Total | 879,869,596.4 | 538 | ||||
| Location | Between groups | 4.737 | 8 | |||
| Within groups | 367.4 | 530 | 0.592 | 0.854 | 0.555 | |
| Total | 372.2 | 538 | 0.693 |
| Corn Products | Mean Difference | Std. Error | Significance | ||
|---|---|---|---|---|---|
| Don Level | Corn 1 | Sweet corn | 629.92 | 230.05 | 0.006 |
| Corn bread | 1066.63 | 236.89 | 0.000 | ||
| Corn oil | 1073.58 | 230.05 | 0.000 | ||
| Popcorn | 1130.06 | 251.06 | 0.000 | ||
| Corn 2 | Sweet corn | 542.88 | 218.65 | 0.013 | |
| Corn bread | 979.59 | 225.83 | 0.000 | ||
| Corn oil | 986.53 | 218.65 | 0.000 | ||
| Popcorn | 1043.03 | 240.66 | 0.000 | ||
| Corn 3 | Corn bread | 842.21 | 224.01 | 0.000 | |
| Corn oil | 849.16 | 216.76 | 0.000 | ||
| Popcorn | 9.05.65 | 238.95 | 0.000 | ||
| Corn 4 | Sweet corn | 483.11 | 219.64 | 0.028 | |
| Corn bread | 919.82 | 226.79 | 0.000 | ||
| Corn oil | 926.77 | 219.64 | 0.000 | ||
| Popcorn | 983.26 | 241.56 | 0.000 | ||
| Corn flour | Corn bread | 772.55 | 210.76 | 0.000 | |
| Corn oil | 779.50 | 203.05 | 0.000 | ||
| Corn popcorn | 8.35.99 | 226.58 | 0.000 | ||
| Sweet corn | Corn type 1 | −629.91 | 230.05 | 0.006 | |
| Corn type 2 | −542.88 | 218.65 | 0.013 | ||
| Corn type 4 | −483.11 | 219.64 | 0.028 | ||
| Corn bread | 436.70 | 220.65 | 0.048 | ||
| Corn oil | 443.65 | 213.29 | 0.038 | ||
| Popcorn | 500.15 | 235.81 | 0.034 | ||
| Corn bread | Corn type 1 | −1066.62 | 236.89 | 0.000 | |
| Corn type 2 | −979.59 | 225.83 | 0.000 | ||
| Corn type 3 | −842.21 | 224.01 | 0.000 | ||
| Corn type 4 | −919.82 | 226.79 | 0.000 | ||
| Corn flour | −772.55 | 210.76 | 0.000 | ||
| Sweet corn | −436.70 | 220.65 | 0.048 | ||
| Corn oil | Corn type 1 | −1073.57 | 230.05 | 0.000 | |
| Corn type 2 | −986.53 | 218.65 | 0.000 | ||
| Corn type 3 | −849.15 | 216.76 | 0.000 | ||
| Corn type 4 | −926.73 | 219.64 | 0.000 | ||
| Corn flour | −779.50 | 203.05 | 0.000 | ||
| Sweet corn | −443.65 | 213.29 | 0.038 | ||
| Popcorn | Corn type 1 | −1130.06 | 251.06 | 0.000 | |
| Corn type 2 | −1043.03 | 240.66 | 0.000 | ||
| Corn type 3 | −905.65 | 238.95 | 0.000 | ||
| Corn type 4 | −983.26 | 241.56 | 0.000 | ||
| Corn flour | −835.99 | 226.58 | 0.000 | ||
| Sweet corn | −500.15 | 235.81 | 0.034 |
| Consumption (kg) | Gujranwala | Hafizabad | Sheikhupura | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DON Levels | Exposure | DON Levels | Exposure | DON Levels | Exposure | ||||||||
| Mean µg/kg | Highest Level µg/kg | Mean µg/kg bw/day | Highest µg/kg bw/day | Mean µg/kg | Highest Level µg/kg | Mean µg/kg bw/day | Highest µg/kg bw/day | Mean µg/kg | Highest Level µg/kg | Mean µg/kg bw/day | Highest µg/kg bw/day | ||
| Corn flour | 0.07 | 854.7 | 8990.5 | 0.92 | 9.68 | 1030.8 | 7860.5 | 1.11 | 8.47 | 1030.8 | 7860.5 | 1.11 | 8.47 |
| HQ 1 | 0.92 | 1.11 | 1.11 | ||||||||||
| HQ 2 | 9.68 | 8.47 | 8.47 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, S.Z.; Abdull Razis, A.F.; Usman, S.; Ali, N.B.; Asi, M.R. Variation of Deoxynivalenol Levels in Corn and Its Products Available in Retail Markets of Punjab, Pakistan, and Estimation of Risk Assessment. Toxins 2021, 13, 296. https://doi.org/10.3390/toxins13050296
Iqbal SZ, Abdull Razis AF, Usman S, Ali NB, Asi MR. Variation of Deoxynivalenol Levels in Corn and Its Products Available in Retail Markets of Punjab, Pakistan, and Estimation of Risk Assessment. Toxins. 2021; 13(5):296. https://doi.org/10.3390/toxins13050296
Chicago/Turabian StyleIqbal, Shahzad Zafar, Ahmad Faizal Abdull Razis, Sunusi Usman, Nada Basheir Ali, and Muhammad Rafique Asi. 2021. "Variation of Deoxynivalenol Levels in Corn and Its Products Available in Retail Markets of Punjab, Pakistan, and Estimation of Risk Assessment" Toxins 13, no. 5: 296. https://doi.org/10.3390/toxins13050296
APA StyleIqbal, S. Z., Abdull Razis, A. F., Usman, S., Ali, N. B., & Asi, M. R. (2021). Variation of Deoxynivalenol Levels in Corn and Its Products Available in Retail Markets of Punjab, Pakistan, and Estimation of Risk Assessment. Toxins, 13(5), 296. https://doi.org/10.3390/toxins13050296









