Morphological Analysis Reveals a Compartmentalized Duct in the Venom Apparatus of the Wasp Spider (Argiope bruennichi)
Abstract
1. Introduction
2. Results
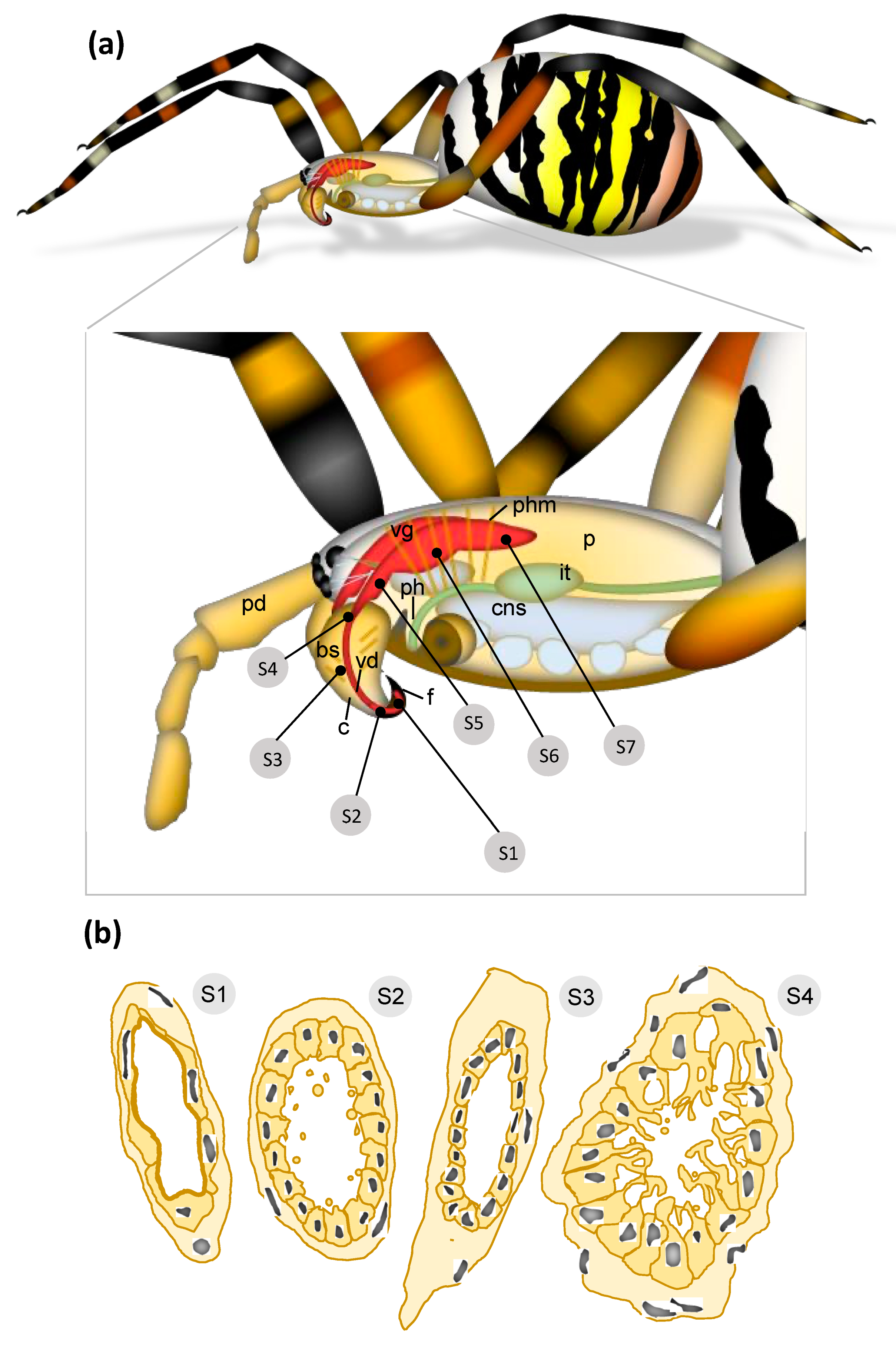
2.1. Outer Morphology
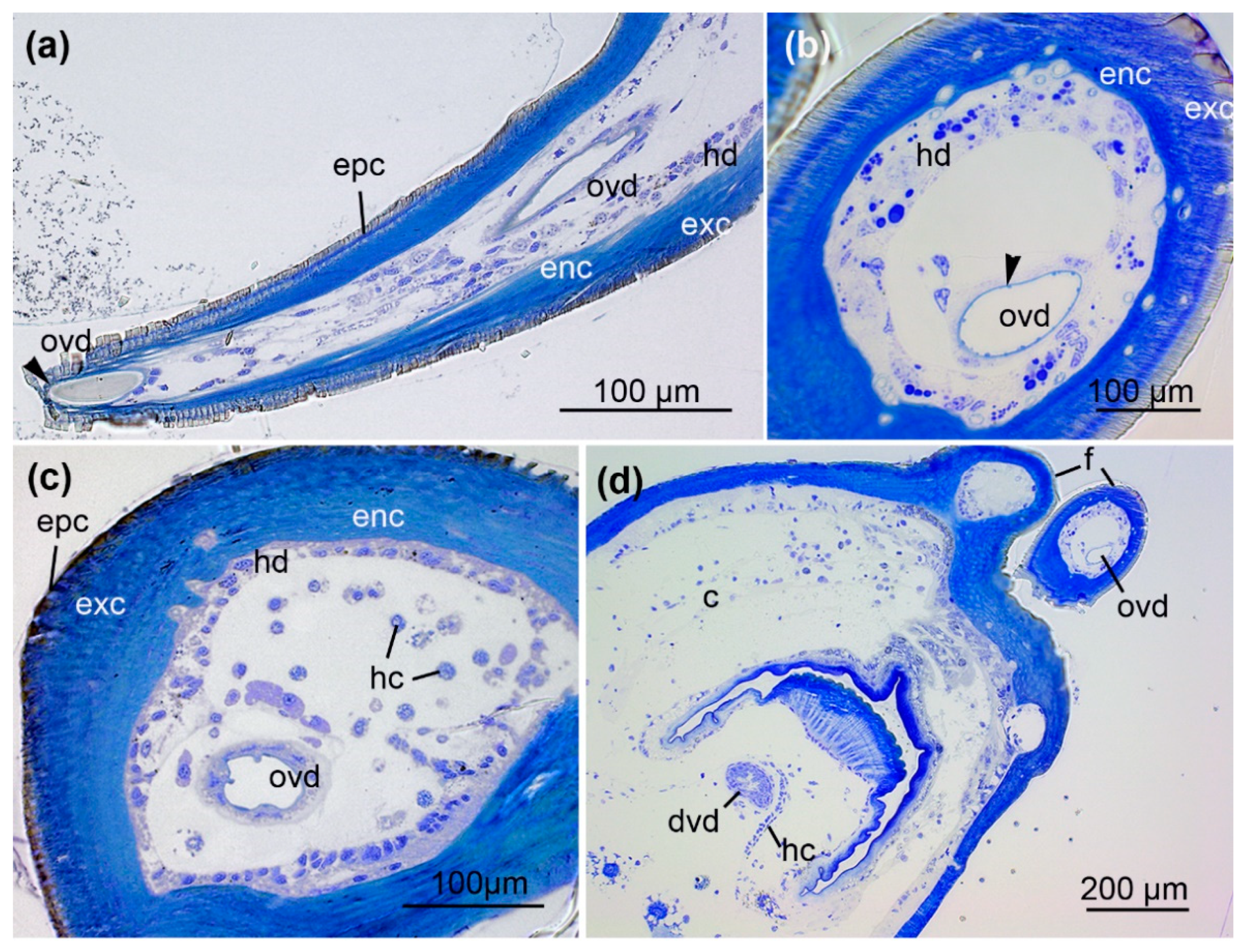
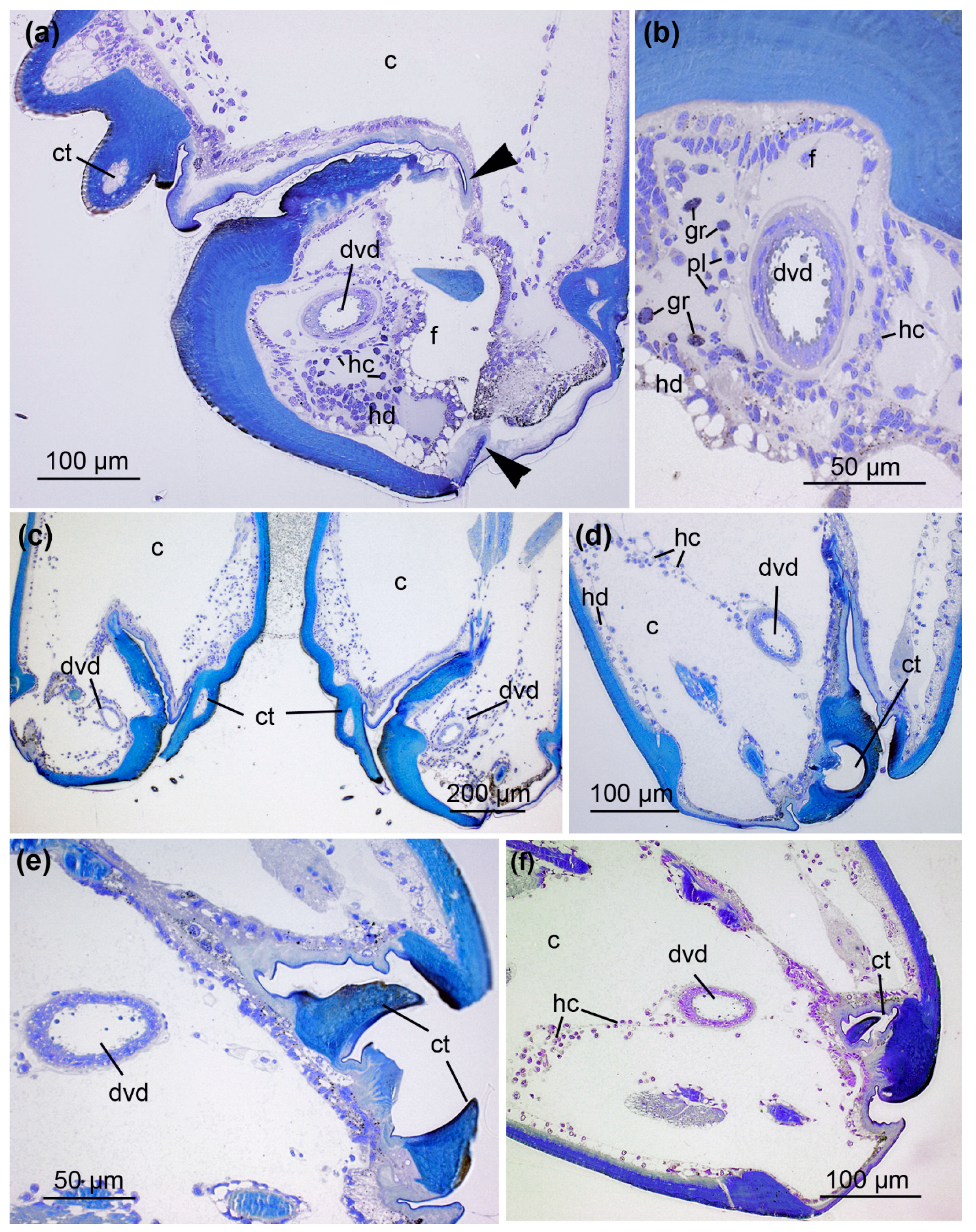
2.2. From Fang to Reservoir: Structural Analysis of the Venom Apparatus
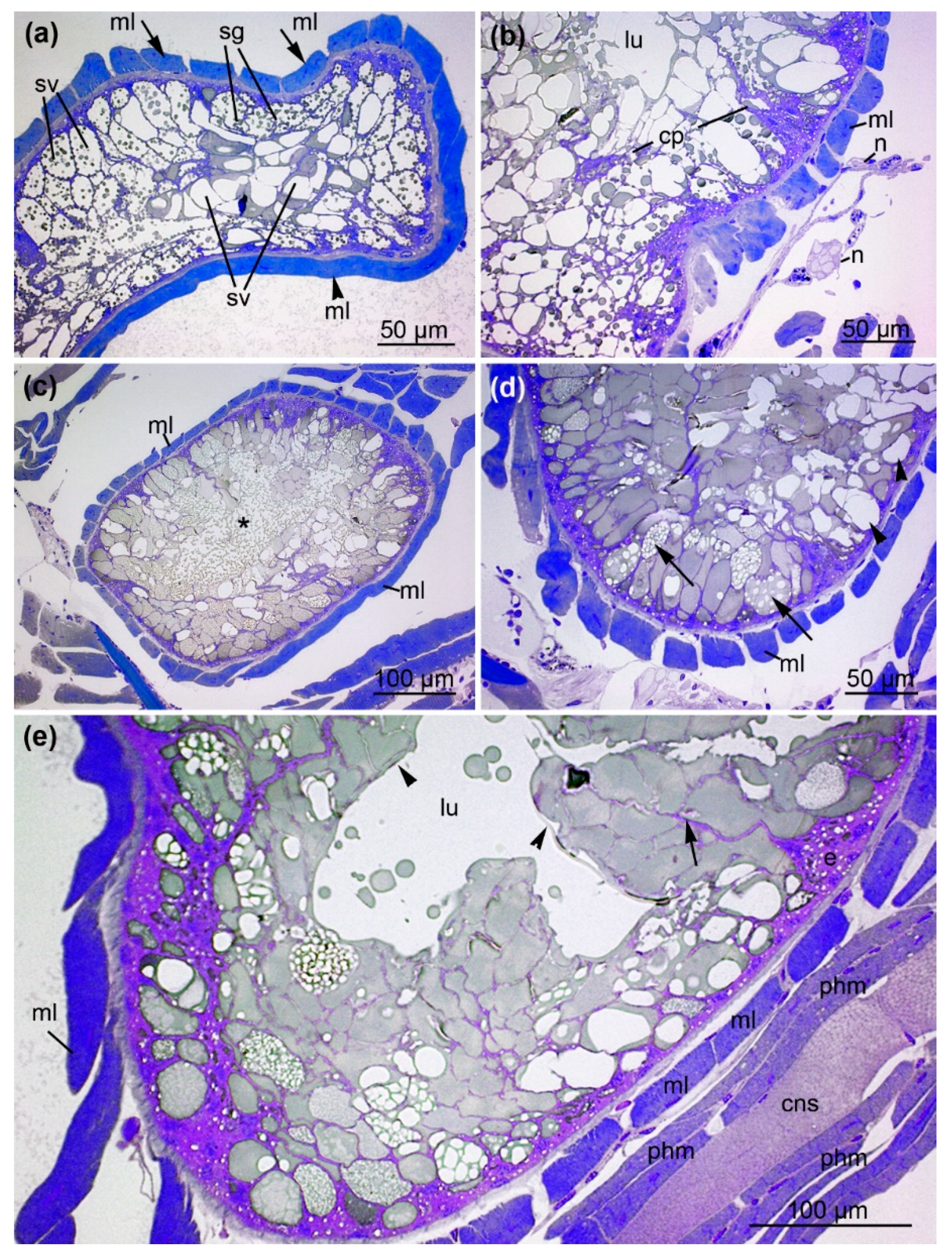
2.3. Architecture of the Venom Glands
3. Discussion
3.1. Overview of the Wasp Spider Venom System
3.2. Histology of the Venom System
3.3. Hidden Complexity of the Venom Duct
4. Conclusions
5. Materials and Methods
5.1. Preparation of Histological Sections
5.2. Preparation of Histological Sections
5.3. Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schendel, V.; Rash, L.D.; Jenner, R.A.; Undheim, E.A.B. The diversity of venom: The importance of behavior and venom system morphology in understanding its ecology and evolution. Toxins 2019, 11, 666. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.A.; Norton, R.S.; et al. The Toxicogenomic Multiverse: Convergent Recruitment of Proteins into Animal Venoms. Annu. Rev. Genom. Hum. Genet. 2009. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013. [Google Scholar] [CrossRef]
- World Spider Catalog Version 22.0. Nature Histori Sches Museum Bern. 2021. Available online: http://wsc.nmbe.ch (accessed on 31 March 2021).
- Saez, N.J.; Senff, S.; Jensen, J.E.; Yan, S.; Herzig, V.; Rash, L.D.; King, G.F. Spider-venom peptides as therapeutics. Toxins 2010, 21, 2851–2871. [Google Scholar] [CrossRef] [PubMed]
- Langenegger, N.; Nentwig, W.; Kuhn-Nentwig, L. Spider venom: Components, modes of action, and novel strategies in transcriptomic and proteomic analyses. Toxins 2019, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Saez, N.J.; Herzig, V. Versatile spider venom peptides and their medical and agricultural applications. Toxicon 2019. [Google Scholar] [CrossRef]
- Richards, K.L.; Milligan, C.J.; Richardson, R.J.; Jancovski, N.; Grunnet, M.; Jacobson, L.H.; Undheim, E.A.B.; Mobli, M.; Chow, C.Y.; Herzig, V.; et al. Selective NaV1.1 activation rescues Dravet syndrome mice from seizures and premature death. Proc. Natl. Acad. Sci. USA 2018. [Google Scholar] [CrossRef] [PubMed]
- King, G.F.; Hardy, M.C. Spider-Venom Peptides: Structure, Pharmacology, and Potential for Control of Insect Pests. Annu. Rev. Entomol. 2013. [Google Scholar] [CrossRef]
- Chassagnon, I.R.; McCarty, C.A.; Chin, Y.K.Y.; Pineda, S.S.; Keramidas, A.; Mobli, M.; Pham, V.; De Silva, T.M.; Lynch, J.W.; Widdop, R.E.; et al. Potent neuroprotection after stroke afforded by a double-knot spider-venom peptide that inhibits acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef]
- Herzig, V.; King, G.F.; Undheim, E.A.B. Can we resolve the taxonomic bias in spider venom research? Toxicon X 2019. [Google Scholar] [CrossRef]
- Lüddecke, T.; Vilcinskas, A.; Lemke, S. Phylogeny-guided selection of priority groups for venom bioprospecting: Harvesting toxin sequences in tarantulas as a case study. Toxins 2019, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, V.L.; Franco, C.R.; Viggiano, R.L.; da Silveira, R.B.; Cantão, M.P.; Mangili, O.C.; Veiga, S.S.; Gremski, W. Structural and ultrastructural description of the venom gland of Loxosceles intermedia (brown spider). Toxicon 2000, 38, 265–285. [Google Scholar] [CrossRef]
- Silva, L.M.; Botelho, A.C.; Nacif-Pimenta, R.; Martins, G.F.; Alves, L.C.; Brayner, F.A.; Fortes-Dias, C.L.; Pimenta, P.F. Structural analysis of the venom glands of the armed spider Phoneutria nigriventer (Keyserling, 1891): Microanatomy, fine structure and confocal observations. Toxicon 2008, 51, 693–706. [Google Scholar] [CrossRef]
- Grishin, E.V. Black widow spider toxins: The present and the future. Toxicon 1998, 11, 1693–1701. [Google Scholar] [CrossRef]
- Garb, J.E. Extraction of venom and venom gland microdissections from spiders for proteomic and transcriptomic analyses. J. Vis. Exp. 2014. [Google Scholar] [CrossRef]
- Järlfors, U.; Smith, D.S.; Russell, F.E. Nerve endings in the venom gland of the spider Latrodectus mactans. Toxicon 1969, 7, 263–265. [Google Scholar] [CrossRef]
- Smith, D.S.; Russel, F.E. Structure of the venom gland of the black widow spider Latrodectus mactans. A preliminary light and electron microscopic study. In Animal Toxins; Russel, F.E., Saunders, P.R., Eds.; Pergamon: Oxford, UK, 1967; pp. 1–15. [Google Scholar]
- Kovoor, J.; Muñoz-Cuevas, A. Comparative histology of the venom glands in a lycosid and several oxyopid spiders (Araneae). Ekol. Bratisl. 2000, 19, 129–140. [Google Scholar]
- Bayram, Y.A.; Danisman, T.; Sancak, Z.; Tel, M.G. Morphological characterization of the venom apparatus in the wolf spider Lycosa singoriensis (Laxmann, 1770). J. Venom. Anim. Toxins Incl. Trop. Dis. 2009. [Google Scholar] [CrossRef]
- Çavuşoǧlu, K.; Bayram, Y.A.; Maraş, M.; Kirindi, T. A morphological study on the venom apparatus of spider Larinioides cornustus (Araneae, Araneidae). Turk. J. Zool. 2005, 29, 351–356. [Google Scholar]
- Yiǧit, N.; Bayram, A.; Danişman, T.; Sancak, Z. Functional morphology of the venom apparatus of Larinioides ixobolus (Araneae: Araneidae). Pak. J. Biol. Sci. 2006, 9, 1975–1978. [Google Scholar] [CrossRef]
- Rocha-e-Silva, T.A.A.; Collares-Buzato, C.B.; da Cruz-Höfling, M.A.; Hyslop, S. Venom apparatus of the brazilian tarantula Vitalius dubius Mello-Leitão 1923 (Theraphosidae). Cell Tissue Res. 2009, 335, 617–629. [Google Scholar] [CrossRef]
- Benli, M.; Karakas, M.; Yigit, N.; Cebesoy, S. Determining with SEM, structure of the venom apparatus in the tube web spider, Segestria florentina (Araneae: Segestriidae). J. Entomol. Zool. Stud. 2013, 1, 61–65. [Google Scholar]
- Suter, B.; Stratton, G.E. Predation by spitting spiders: Elaborate venom gland, intricate delivery system. In Ecophysiology of Spiders; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar] [CrossRef]
- Pekár, S.; Bočánek, O.; Michálek, O.; Petráková, L.; Haddad, C.R.; Šedo, O.; Zdráhal, Z. Venom gland size and venom complexity-essential trophic adaptations of venomous predators: A case study using spiders. Mol. Ecol. 2018, 27, 4257–4269. [Google Scholar] [CrossRef]
- Malt, S.; Sander, F.W.; Schaller, G. Contribution to foraging ecology of selected Araneidae in xerophil grasslands with particular consideration of Argiope brunnichii Scop. Zool. Jahrbücher Abt. Für Syst. Ökol. Und Geogr. Der Tiere 1990, 117, 237–260. [Google Scholar]
- Gomes, D.G.E. Orb-weaving spiders are fewer but larger and catch more prey in lit bridge panels from a natural artificial light experiment. PeerJ 2020. [Google Scholar] [CrossRef]
- Buskirk, R.E. Coloniality, activity patterns and feeding in a tropical orb-weaving spider. Ecology 1975, 56, 1314–1328. [Google Scholar] [CrossRef]
- Heiling, A.M. Why do nocturnal orb-web spiders (Araneidae) search for light? Behav. Ecol. Sociobiol. 1999, 46, 43–49. [Google Scholar] [CrossRef]
- Biere, J.M.; Uetz, G.W. Web Orientation in the Spider Micrathena Gracilis (Araneae: Araneidae). Ecology 1981, 62, 336–344. [Google Scholar] [CrossRef]
- Welke, K.W.; Schneider, J.M. Sexual cannibalism benefits offspring survival. Anim. Behav. 2012, 83, 201–207. [Google Scholar] [CrossRef]
- Nyffeler, M.; Benz, G. Foraging ecology and predatory importance of a guild of orb-weaving spiders in a grassland habitat. J. Appl. Entomol. 1989, 107, 166–184. [Google Scholar] [CrossRef]
- Eisner, T.; Dean, J. Ploy and counterploy in predator—prey interactions: Orb weaving spiders versus bombardier beetles. Proc. Natl. Acad. Sci. USA 1976, 73, 1365–1367. [Google Scholar] [CrossRef] [PubMed]
- Václav, R.; Prokop, P. Does the appearance of orbweaving spiders attract prey? Ann. Zool. Fenn. 2006, 43, 65–71. [Google Scholar]
- Fromhage, L.; Uhl, G.; Schneider, J.M. Fitness consequences of sexual cannibalism in female Argiope bruennichi. Behav. Ecol. Sociobiol. 2003, 55, 60–64. [Google Scholar] [CrossRef]
- Chinta, S.P.; Goller, S.; Lux, J.; Funke, S.; Uhl, G.; Schulz, S. The sex pheromone of the wasp spider Argiope bruennichi. Angew. Chem. Int. Ed. 2010, 49, 2033–2036. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Bliss, P.; Elgar, M.A.; Moritz, R.F.A. Argiope bruennichi shows a drinking-like behaviour in web hub decorations (Araneae, Araneidae). J. Ethol. 2009, 27, 25–29. [Google Scholar] [CrossRef]
- Krehenwinkel, H.; Rödder, D.; Tautz, D. Eco-genomic analysis of the poleward range expansion of the wasp spider Argiope bruennichi shows rapid adaptation and genomic admixture. Glob. Chang. Biol. 2015, 21, 4320–4332. [Google Scholar] [CrossRef]
- Krehenwinkel, H.; Tautz, D. Northern range expansion of European populations of the wasp spider Argiope bruennichi is associated with global warming-correlated genetic admixture and population-specific temperature adaptations. Mol. Ecol. 2013, 22, 2232–2248. [Google Scholar] [CrossRef]
- Zimmer, S.M.; Krehenwinkel, H.; Schneider, J.M. Rapid range expansion is not restricted by inbreeding in a sexually cannibalistic spider. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Wawer, W.; Rutkowski, R.; Krehenwinkel, H.; Lutyk, D.; Pusz-Bocheńska, K.; Bogdanowicz, W. Population structure of the expansive wasp spider (Argiope bruennichi) at the edge of its range. J. Arachnol. 2017, 45, 361–369. [Google Scholar] [CrossRef]
- Lüddecke, T.; von Reumont, B.M.V.; Förster, F.; Billion, A.; Timm, T.; Lochnit, G.; Vilcinskas, A.; Lemke, S. An economic eilemma between molecular weapon systems may explain an arachno-atypical venom in wasp spiders (Argiope bruennichi). Biomolecules 2020, 10, 978. [Google Scholar] [CrossRef]
- Duan, Z.; Cao, R.; Jiang, L.; Liang, S. A combined de novo protein sequencing and cDNA library approach to the venomic analysis of Chinese spider Araneus ventricosus. J. Proteom. 2013, 78, 416–427. [Google Scholar] [CrossRef]
- Foelix, R.F. Biology of Spiders; Harvard University Press: London, UK, 1983. [Google Scholar]
- Zonstein, S.L. The spider chelicerae: Some problems of origin evolution. Eur. Arachnol. 2003 2004, 1, 349–366. [Google Scholar]
- Kuhn-Nentwig, L.; Stöcklin, R.; Nentwig, W. Venom composition and strategies in spiders is everything possible? Adv. Insect Physiol. 2011, 40, 1–86. [Google Scholar]
- Moon, M.J.; Yu, M.H. Fine structure of the chelicera in the spider Nephila clavata. Entomol. Res. 2007, 37, 167–172. [Google Scholar] [CrossRef]
- Millot, J. Les glandes venimeuses des araneids. Ann. Sci. Nat. Zoo. 1931, 14, 113–147. [Google Scholar]
- Vellard, J. Le venin des araigne; Monogr. De L’institut Pasteur: Paris, France, 1936. [Google Scholar]
- Soliman, B.A.; Shoukry, N.M.; Mohallal, M.E.; Fetaih, H.A.W.; Khaled, H.S. Fine structure of the stinger, histology and histochemistry of the venom gland in the scorpion Androctonus amoreuxi (Buthidae). J. Basic Appl. Zool. 2013, 66, 41–46. [Google Scholar] [CrossRef][Green Version]
- Yigit, N.; Guven, T. Functional structure of Agelena labyrinthica’s (Araneae: Agelenidae) venom gland and electrophoresis of venom. Toxicon 2006, 47, 58–67. [Google Scholar] [CrossRef]
- Barth, F.G. Die Feinstruktur des Spinneninteguments. Z. Zellforsch 1969, 97, 137–159. [Google Scholar] [CrossRef]
- Dalingwater, J.E. Chelicerate Cuticle Structure. In Ecophysiology of Spiders; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Erko, M.; Hartmann, M.A.; Zlotnikow, I.; Valverde Serrano, C.; Fratzl, P.; Politi, Y. Structural and mechanical properties of the arthropod cuticle: Comparison between the fang of the spider Cupiennius salei and the carapace of American lobster Homarus americanus. J. Struct. Biol. 2013, 183, 172–179. [Google Scholar] [CrossRef]
- Fukuzawa, A.H.; Vellutini, B.C.; Lorenzini, D.M.; Silva Jr, P.I.; Mortara, R.A.; da Silva, J.M.C.; Daffre, S. The role of hemocytes in the immunity of the spider Acanthoscurria gomesiana. Dev. Comp. Immunol. 2008, 32, 716–725. [Google Scholar] [CrossRef]
- Baumann, T.; Kämpfer, U.; Schürch, S.; Schaller, J.; Largiader, C.; Nentwig, W.; Kuhn-Nentwig, L. Ctenidins: Antimicrobial glycine-rich peptides from the hemocytes of the spider Cupiennius salei. Cell. Mol. Life Sci. 2010, 67, 2787–2798. [Google Scholar] [CrossRef]
- Kuhn-Nentwig, L.; Kopp, L.; Nentwig, W.; Haenni, B.; Streitberger, K.; Schürch, S.; Schaller, J. Functional differentiation of spider hemocytes by light and transmission electron microscopy, and MALDI-MS-imaging. Dev. Comp. Immunol. 2014, 43, 59–67. [Google Scholar] [CrossRef]
- Hu, H.; Bandyopadhyay, P.K.; Olivera, B.M.; Yandell, M. Characterization of the Conus bullatus genome and its venom-duct transcriptome. BMC Genom. 2011, 12. [Google Scholar] [CrossRef]
- Valente, R.H.; Sakai, F.; Portes-Junior, J.A.; Viana, L.G.; Carneiro, S.M.; Perales, J.; Yamanouye, N. The primary duct of Bothrops jararaca glandular apparatus secretes toxins. Toxins 2018, 10, 121. [Google Scholar] [CrossRef]
- Tayo, L.L.; Lu, B.; Cruz, L.J.; Yates, J.R. Proteomic analysis provides insights on venom processing in Conus textile. J. Proteome Res. 2010, 9, 2292–2301. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidtberg, H.; von Reumont, B.M.; Lemke, S.; Vilcinskas, A.; Lüddecke, T. Morphological Analysis Reveals a Compartmentalized Duct in the Venom Apparatus of the Wasp Spider (Argiope bruennichi). Toxins 2021, 13, 270. https://doi.org/10.3390/toxins13040270
Schmidtberg H, von Reumont BM, Lemke S, Vilcinskas A, Lüddecke T. Morphological Analysis Reveals a Compartmentalized Duct in the Venom Apparatus of the Wasp Spider (Argiope bruennichi). Toxins. 2021; 13(4):270. https://doi.org/10.3390/toxins13040270
Chicago/Turabian StyleSchmidtberg, Henrike, Björn M. von Reumont, Sarah Lemke, Andreas Vilcinskas, and Tim Lüddecke. 2021. "Morphological Analysis Reveals a Compartmentalized Duct in the Venom Apparatus of the Wasp Spider (Argiope bruennichi)" Toxins 13, no. 4: 270. https://doi.org/10.3390/toxins13040270
APA StyleSchmidtberg, H., von Reumont, B. M., Lemke, S., Vilcinskas, A., & Lüddecke, T. (2021). Morphological Analysis Reveals a Compartmentalized Duct in the Venom Apparatus of the Wasp Spider (Argiope bruennichi). Toxins, 13(4), 270. https://doi.org/10.3390/toxins13040270







