Abstract
Venoms of solitary wasps are utilized for prey capture (insects and spiders), paralyzing them with a stinger injection to be offered as food for their larvae. Thus, the identification and characterization of the components of solitary wasp venoms can have biotechnological application. In the present study, the venom components profile of a solitary scoliid wasp, Campsomeriella annulata annulata, was investigated through a comprehensive analysis using LC-MS and -MS/MS. Online mass fingerprinting revealed that the venom extract contains 138 components, and MS/MS analysis identified 44 complete sequences of the peptide components. The peptides are broadly divided into two classes: bradykinin-related peptides, and linear α-helical peptides. Among the components of the first class, the two main peptides, α-campsomerin (PRLRRLTGLSPLR) and β-campsomerin (PRLRRLTGLSPLRAP), had their biological activities evaluated. Both peptides had no effects on metallopeptidases [human neprilysin (NEP) and angiotensin-converting enzyme (ACE)] and acetylcholinesterase (AChE), and had no cytotoxic effects. Studies with PC12 neuronal cells showed that only α-campsomerin was able to enhance cell viability, while β-campsomerin had no effect. It is noteworthy that the only difference between the primary structures from these peptides is the presence of the AP extension at the C-terminus of β-campsomerin, compared to α-campsomerin. Among the linear α-helical peptides, annulatin (ISEALKSIIVG-NH2) was evaluated for its biological activities. Annulatin showed histamine releasing activity from mast cells and low hemolytic activity, but no antimicrobial activities against all microbes tested were observed. Thus, in addition to providing unprecedented information on the whole components, the three peptides selected for the study suggest that molecules present in solitary scoliid wasp venoms may have interesting biological activities.
Keywords:
solitary scoliid wasp; venom; comprehensive analysis; LC-MS; bradykinin-related peptide; linear α-helical peptide Key Contribution:
Comprehensive LC-MS and -MS/MS analyses of the venom extract of a solitary scoliid wasp Campsomeriella annulata annulata led to find two major peptides, α-campsomerin and β-campsomerin, and a linear α-helical peptide, annulatin.
1. Introduction
Arthropod venoms have been a subject of toxinological and pharmacological investigation, which have revealed that venoms are a rich source of pharmacologically and medically useful peptides [1]. Among them, arachnid (scorpions and spiders) venoms are the best studied, and a variety of peptides that could be developed for pharmacological tools, and medical and agricultural applications have been found [2,3,4,5]. Hymenopteran insects (bees and wasps) are also arthropods, and their venoms are another rich source of bioactive peptides (melittin, mastoparan, and waspkinins) and proteins (phospholipase C) [6,7].
Solitary wasps belong to the Hymenoptera order, but, in contrast to social wasps and bees, their venoms, and components, have been much less investigated. That may be due to their solitary lifestyle, living alone [8], and accordingly, making it difficult to collect enough venom (large numbers of wasp individuals) for chemical and pharmacological analyses. In recent years, however, toxinological and pharmacological investigation of solitary wasp venoms has advanced considerably, mainly due to remarkable progress of analytical methods using mass spectrometry [9,10,11,12,13,14].
We have surveyed solitary wasp venoms from Japan for the last decades, with interest in neuroactive substances contained in their venoms. Venoms of solitary wasps are utilized for prey capture (insects and spiders), paralyzing them with a stinger injection to be offered as food for their larvae. [8]. Therefore, solitary wasp venoms may contain neurotoxins and/or neuroactive molecules as important components. In fact, we found novel neurotoxins, pompilidotoxins (PMTXs) in spider wasp venoms [15], blocking both mammalian and insect sodium-channels [16,17]. Another neurotoxin, named Sa12b, is an FMRFamide-like peptide in sphecid wasp venoms, and inhibits acid-sensing ion channels (ASICs) [18]. In addition to these neurotoxins, we have found other types of bioactive peptides: antimicrobial peptides [19,20]; bradykinin-related peptides [21]; FMRFamide-like peptides [22]. Thus, our studies of solitary wasp venom components revealed not only the presence of neuroactive peptides, but also a variety of bioactive peptides [23].
Scoliid wasp is one of the groups of solitary wasps, belonging to the Scoliidae family, which hunt and sting beetle larva under the ground. Scoliid wasp venom components were investigated for the first time in 1987. From the scoliid wasp venoms of Colpa interrupta and Megascolia flavifrons, from Europe, two kinins, threonine6-bradykinin (Thr6-BK) and megascoliakinin, were isolated and pharmacologically characterized [24,25]. These kinins irreversibly block the synaptic transmission of the nicotinic acetylcholine receptor (nAChR) in the insect central nervous system [25,26]. Thr6-BK was identified in three scoliid wasp venoms from Japan through screening with MALDI-TOF MS [27]. Recently, we investigated the venom components of another Japanese scoliid wasp, Scolia decorate ventralis, and found a novel neuroprotective peptide, β-scoliidine, in a cellular stress model based on the H2O2-induced oxidative stress, which stimulates the excessive production of reactive oxygen species (ROS) [28].
The solitary scoliid wasp Campsomeriella annulata annulata is one of three species with Thr6-BK identified in their venom [27]. In this study, we investigated the venom components of this wasp in detail. The component profile was attained by comprehensive LC-MS and MS/MS analysis of crude venom extracts, which identified small molecules (amino acids, biogenic amines and nucleic acids), and many short peptide sequences. Two major structural types of peptides, bradykinin-related peptides, and linear α-helical peptides were identified, from which three novel peptides (α-campsomerin, β-campsomerin, and annulatin) were synthesized and biologically evaluated. Regarding bradykinin-related peptides, only α-campsomerin showed cell viability potentiating effect in neuronal PC12 cells, whereas β-campsomerin had no effect. Moreover, despite their structural resemblance to another neuroprotective peptide from the Scolia decorata ventralis wasp, different concentrations of two peptides did not show neuroprotective benefits against H2O2-induced oxidative stress in PC12 neuronal cells. Annulatin, classified as linear α-helical peptides, showed histamine releasing activity from mast cells. This is the first case that linear α-helical peptides were found in scoliid wasp venoms.
2. Results
2.1. Comprehensive Analysis of Venom Extract from Campsomeriella annulata annulata
2.1.1. On-Line Mass Fingerprinting by LC-MS
The component profile (number of components and its molecular mass determination) was obtained by LC-ESI-MS analysis of the crude venom extract. Only 10% of the amount of venom sac extracts from a single specimen was sufficient for mass fingerprinting and peptide sequencing by LC-ESI-MS analysis. The TIC is shown in Figure 1.
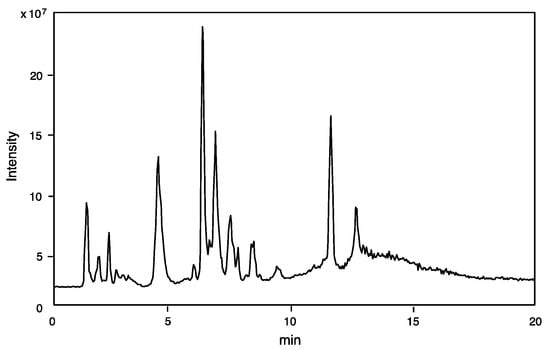
Figure 1.
TIC profile from LC-ESI-MS of venom extracts of Campsomeriella annulata annulata by reverse-phase HPLC using CAPCELL PAK C18 (1.5 × 150 mm) with linear gradient of 5–65% CH3CN/H2O/0.1% formic acid over 20 min at flow rate of 200 μL/min.
Online mass fingerprint was prepared from TIC by “virtual fractionation”, collecting MS spectra from certain range of retention time (fractions), and then the molecular mass was analyzed in each fraction. The results are summarized in Table 1. A total of 138 components were found from 20 virtual fractions with a molecular mass range of m/z 90 to 6300. Most of the low molecular mass components of m/z 90–400 in the earlier fractions (Fr. 1–2, RT 0.8–2.0) may be small molecules (free amino acids, biogenic amines, and nucleic acids). Those in higher mass range and the later fractions can be peptides, in particular, 82 components are in the range of m/z 500–2000, accounting for 59% of total components, should be small peptides. Accordingly, a majority of the venom components may be small peptides, which are the subject of structural determination as follows.

Table 1.
On-line mass fingerprinting of crude venom extract from Campsomeriella annulata annulata by LC-ESI-MS.
2.1.2. Identification of Small Molecules (Amino Acids, Biogenic Amines and Nucleic Acids)
As summarized in Table 2, Table 3 and Table 4, 13 amino acids, 5 biogenic amines, and 7 nucleic acids were identified. The identification was made by the previously reported method based on elemental composition analysis of molecular ion (M + H)+ with an error limit of 0.005 Da [19,28]. Concomitant detection of iminium ion and deamination (-NH3) peak in some case of amino acids and biogenic amines, respectively, was supportive and useful. For nucleic acids (AMP, ADP, and NAD), MS/MS spectra were obtained by data-dependent MS/MS measurement, which confirmed the structure of these compounds.

Table 2.
Amino acids in the crude venom extract from Campsomeriella annulata annulata by LC-ESI-MS.

Table 3.
Biogenic amines in the crude venom extract from Campsomeriella annulata annulata by LC-ESI-MS.

Table 4.
Nucleic acids in the crude venom extract from Campsomeriella annulata annulata by LC-ESI-MS.
2.1.3. Peptide Sequencing by MS/MS Analysis
Peptide sequences were manually analyzed their MS/MS spectra obtained by data-dependent MS/MS measurement, which revealed the full sequence of 44 small peptides. The analyzed full sequences are shown in Table 5. These small peptides can be classified according to structural similarity as shown in Table 6. They are broadly divided into two classes: bradykinin-related peptides, and linear α-helical peptides. In each class, there are peptides seemed to be truncated from N- and C-terminus of the “parent peptide” (the longest sequence). It is not sure whether they are originally contained in the venom or cleavage products in some way.

Table 5.
Peptide sequences analyzed from MS/MS spectra.

Table 6.
Classification of the peptide sequences.
The bradykinin-related peptides can be further divided into three subclasses. The first is Thr6-BK and its close relatives. As mentioned in the Introduction section, we already reported the identification of Thr6-BK in this venom, but it was only detection of (M + H)+ by MALDI-TOF MS [27]. In this study, it was verified by sequence analysis as RPPGFTPFR (m/z 1074.574). Two closely related peptides were found: Ca-969 (m/z 969.590, PATLPAPFR: where L = either L or I) and Ca-1056 (m/z 1056.622, RLPGLTPFR: where L = either L or I). Both of them have the same amino acid length and 3–4 C-terminal sequence as those of Thr6-BK, and the rest of N-terminal sequence is different from Thr6-BK. The second subclass consists of two peptides: Ca-1365 (m/z 1365.787, DALPRLLPAPFR: where L = either L or I), and Ca-1452 (m/z 1365.787, DALPRLLPGTPFR: where L = either L or I). They are quite similar to each other, having the same N-terminal 8 amino acids (DALPRLL), and the C-terminal 3 amino acids (PFR) are the same as Thr6-BK. The third subclass includes the two major peptide components: α-campsomerin (m/z 1534.865, PRLRRLTGLSPLR: where L = either L or I), and β-campsomerin (m/z 1702.955, PRLRRLTGLSPLRAP: where L = either L or I), which correspond to the two most intense peaks at RT 6.45 min, Fr. 9 and 7.02 min, Fr.10, respectively. They are different each other only at the C-terminal: β-campsomerin has the dipeptide AP at the C-terminal of α-campsomerin. Determination of L (leucine) or I (isoleucine) at the positions 3, 6, 9, and 12 of both peptides was performed by MALDI TOF/TOF MS analysis. The MALDI TOF/TOF spectrum of α-campsomerin afforded d-ion peaks at m/z 297.3 (d3), 722.6 (d6), and 993.7(d9), and w-ion peaks at m/z 229.3 (w2) and 526.4 (w5), which clearly showed that all these residues are L (leucine), not I (isoleucine). Similarly, all the L/I residues were determined as L by MALDI TOF/TOF spectral analysis of β-campsomerin, observing d-ion peaks at m/z 297.1 (d3), 722.3 (d6), 993.4 (d9), and 1290.8 (d12), and w-ion peaks at m/z 398.1 (w4) and 694.3 (w7). Finally, solid-phase synthesis of these peptides, and the HPLC and MS/MS comparisons of the synthetic specimens with the natural peptides corroborated the sequences. The C-terminal sequence, GLSPL, of these peptides is similar to that, GFSPL, of Cd-146, α-scoliidine, β-scoliidine, bradykinin-related peptides already known in solitary wasp venoms (Table 7).

Table 7.
Bradykinin-related peptides in solitary wasp venoms.
The third most intense, but rather minor peak at RT 8.57, Fr. 13 contained annulatin (m/z 1128.687, LSEALKSLLVG-NH2: where L = either L or I). The MALDI TOF/TOF spectrum of annulatin afforded d-ion peaks at m/z 786.2 (d8a), 800.2 (d8b), and 913.3 (d9b), and w-ion peaks at m/z 669.2 (w7) and 1083.6 (w11a), which indicated 1I, 7L, 8I, and 9I. Accordingly, the exact structure should be ISEALKSIIVG-NH2. It was confirmed by solid-phase synthesis of this peptide, and the HPLC and MS/MS comparisons of the synthetic specimens with the natural peptide. This peptide may belong to linear α-helical peptides since it can adopt amphiphilic α-helical secondary structure as shown in Figure 2. A large number of this type of peptides are found in natural sources, including arthropod venoms. In case of solitary wasp venoms, we already discovered several peptides of such a type (Table 8), but this is the first example found in scoliid wasp venom. Two other peptides, Ca-1281 (m/z 1281.875, FLLPLLKGLLVG-NH2: where L = either L or I) and Ca-1415 (m/z 1415.896, GLLTDLRKFLLK-NH2: where L = either L or I) may also be linear α-helical peptides, as they can be predicted to adopt α-helical secondary structure.
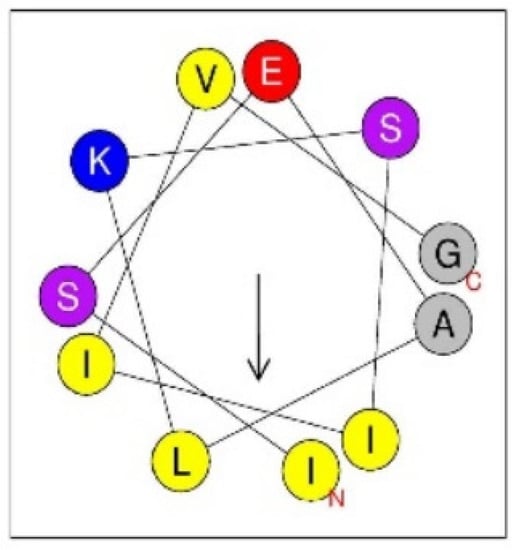
Figure 2.
Helical wheel projection of the sequence of annulatin (ISEALKSIIVG-NH2). In this view through the helix axis, the hydrophilic Ser (S), Glu (E), and Lys (K) residues are located on one side and the hydrophobic Ala (A), Ile (I), and Leu (L) residues on the other side of the helix.

Table 8.
Linear α-helical peptides in solitary wasp venoms.
The rest of the peptides included in the Miscellaneous section are not classified into any groups since they have no similarity to bradykinin-related peptides, linear α-helical peptides, nor any known peptides. Accordingly, chemical and biological characteristics of these peptides are not known.
2.2. Biological Characterization of α-Campsomerin and β-Campsomerin
2.2.1. Interaction with NEP and ACE
NEP and ACE catalytic activity was not inhibited by either peptide. Furthermore, neither peptide was broken by the metallopeptidases, indicating that they do not interact with the metallopeptidases under investigation (Table 9).

Table 9.
Hydrolyses of α-campsomerin and β-campsomerin by NEP and ACE.
2.2.2. AChE Activity
The α-campsomerin and β-campsomerin were tested for AChE inhibitory activities in vitro compared to the tetraethyl pyrophosphate (TEPP; positive control), and untreated AChE reaction (negative control). Both peptides did not exhibit anti-AChE activities compared to the negative control. However, TEPP reduced the AChE activity with an inhibition percentage of 76.20 ± 1.42% (Figure 3).
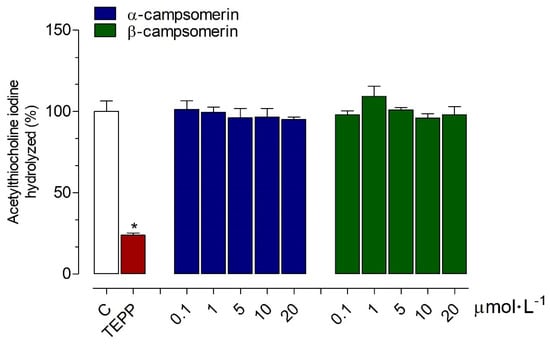
Figure 3.
Inhibitory effects of α-campsomerin and β-campsomerin on the activity of acetylcholine esterase (AChE). The extent of AChE activity inhibition was expressed as a percentage of acetylthiocholine iodine substrate hydrolyzed in relation to control (C; blank box). Data from three independent experiments in triplicate are expressed as mean ± standard deviation, and evaluated using one-way ANOVA followed by Tukey’s post-test. * p < 0.05 for differences between the control groups; Tetraethyl pyrophosphate (TEPP; red box).
2.2.3. Cytotoxic Effects
The cytotoxic effects of α-campsomerin and β-campsomerin were assessed in two different cell types: a typical fibroblastic cell (Vero cells,) and a neuronal cell line (PC12 cells). Both peptides showed no significant cytotoxicity (p > 0.05) in Vero cells after 1, 6, 24, and 48 h of treatment when compared to their respective control groups (untreated cells) at all doses tested (Figure 4).
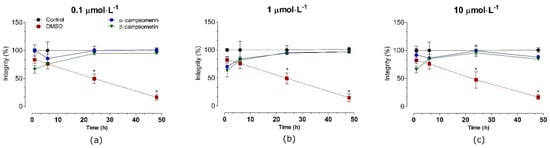
Figure 4.
Toxicity of α-campsomerin or β-campsomerin on cell integrity in Vero cells. Cells were treated with peptides 0.1 μmol·L−1 (a), 1 μmol·L−1 (b) and 10 μmol·L−1 (c) for 1, 6, 12, 24, and 48 h. Control and DMSO groups represent cells without treatment and treated with DMSO 5%, respectively. Values are expressed as mean ± SD (n = 3 in triplicate) and analyzed by one-way ANOVA followed by Tukey’s post-test. * p < 0.05 in relation to the control group.
In PC12 cells, α–campsomerin reduced cell integrity after 6 h of treatment in all concentrations tested (Figure 5). In spite of that, cells integrity increased significantly (p < 0.05) after 24 and 48 h of treatment with this peptide, especially at 1 and 10 µmol·L−1, in relation to the control and β–campsomerin groups. When compared to the untreated cell (control) or DMSO, the β-campsomerin showed cytotoxic effects at 10 µmol·L−1 after 24 h of treatment (Figure 4). DMSO (positive control) decreased cell integrity in Vero and PC12 cells after 6, 24 and 48 h in comparison to the control group (Figure 4 and Figure 5).
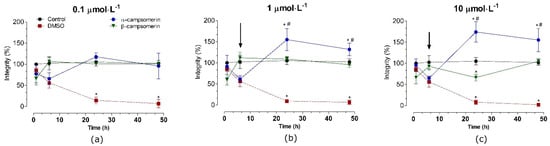
Figure 5.
Toxicity of α-campsomerin and β-campsomerin on PC12 cell integrity. Cells were treated with peptides 0.1 μmol·L−1 (a), 1 μmol·L−1 (b) and 10 μmol·L−1 (c) for 1, 6, 12, 24, and 48 h. Control and DMSO groups represent cells without treatment and treated with DMSO 5%, respectively. Data were obtained from three independent experiments in triplicate, expressed as mean ± SD, and analyzed by one-way ANOVA followed by Tukey’s post-test. * p < 0.05 compared to the control group; # p < 0.05 compared to the β-campsomerin. Arrows indicate the integrity cells reduced by α-campsomerin, but not β-campsomerin, at 6 h of treatment.
2.2.4. Neuroprotective Effects
The neuroprotective effects against H2O2-induced oxidative stress in the presence of α-campsomerin or β-campsomerin under different concentrations were studied in PC12 cells (Figure 6). Initially, we evaluated the chronic cytotoxic effects promoted by H2O2 (concentrations varying between 1–0.06 mmol·L−1) on PC12 cells after 20 h of treatment. H2O2 promoted cell death at concentrations greater than 0.3 mmol·L−1 in a dose-dependent manner. The 0.5 mmol·L−1 concentration of H2O2 reduced 60.5 ± 4.0% of cell integrity and it was chosen for studies of protective effects of α-campsomerin or β-campsomerin against oxidative stress-induced neurotoxicity. Our results demonstrated that both peptides did not show neuroprotective effects against the H2O2-induced damage in PC12 cells after chronic treatment (Figure 6).
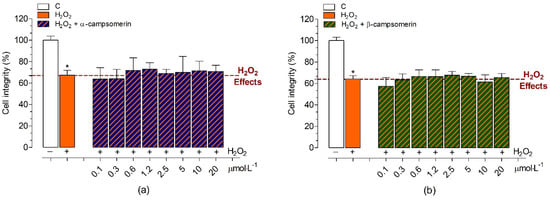
Figure 6.
Neuroprotective property of α-campsomerin (a) and β-campsomerin (b) on cell integrity of the PC12 cell line against H2O2-induced oxidative stress. PC12 cells were plated at 2 × 104 cells per well in a 96-well plate, and were pre-treated with peptides for 4 h at 37 °C. After that, the medium was replaced containing peptide and H2O2 0.5 mmol·L−1) and incubated for more 20 h. Data are expressed as the mean ± standard deviation from three independent experiments in triplicate and analyzed using one-way ANOVA followed by Dunnett’s post-test. * p < 0.05 for differences between the control and experimental groups.
2.3. Antimicrobial, Hemolytic, and Histamine-Releasing Activities of Annulatin and Related Peptides
Annulatin displayed no antimicrobial activities against all the microbes tested in this study, low hemolytic activity, and comparable histamine-releasing activity with mastoparan, famous wasp peptides, in a dose-dependent manner (Table 10). Since many linear α-helical antimicrobial peptides are basic peptides, it is assumed that the lower pI of annulatin displayed no antimicrobial activity. The hydrophobicity of annulatin (0.69) is lower than that of the highest hydrophobic region in melittin (0.88), honeybee hemolytic peptide. Previous studies suggested a correlation between peptide hydrophobicity and hemolytic activity [33].

Table 10.
Antimicrobial, hemolytic, and histamine-releasing activities of annulatin and related peptides.
3. Discussion
We have studied venom components of solitary hunting wasps with a special interest in the neuroactive substances due to their functional role, which is to paralyze the prey (spiders and insects). Initially, and until recently, studies were conducted by the conventional way: HPLC purification, followed by chemical characterization of the isolated compounds [15,19,21,22,29,30,31,32]. In this way, however, only a few major components were successfully characterized, despite the large numbers present in the venom. Furthermore, for this, a significant amount of venom extracts is required. This means a collection of a number of individuals at least 20–30 insects, which is usually very difficult because of the lifestyle of solitary wasps. The remarkable progress of mass spectrometry in sensitivity and resolution made it possible to accomplish this type of analysis with a minute amount of venom. Indeed, we reported comprehensive analysis of venom components of solitary scoliid wasp Scolia ventralis using only 10% of the amount of a single venom contents [28].
This highly efficient analytical means was used again for this study. Comprehensive analysis of the Campsomeriella annulata annulata venom extract by using LC-ESI-MS attained the component profile, consisting of 138 molecules. Peptide sequences were manually analyzed their MS/MS spectra obtained by data-dependent MS/MS measurement, which revealed the full sequence of 44 small peptides. They are broadly classified into two major structural types: bradykinin-related peptides, and linear α-helical peptides. The bradykinin-related peptides can be further divided into three subclasses. The first one has a high similarity to bradykinin, and the others are only partially similar to the three C-terminal amino acids of bradykinin. Three species of scoliid wasp venoms have so far been studied, and all of these have bradykinin-related peptides [24,25,27,28]. Accordingly, bradykinin-related peptides are common components of scoliid wasp venoms. Another class of peptides, linear α-helical peptides, have mostly been found in eumenine wasp venoms, and this is the first time that such peptides were found in scoliid wasp venoms. With this comprehensive analysis, small molecules (amino acids, biogenic amines, and nucleic acids) could also be identified as previously reported [19,28]. Some of them were reported to be present and functional in solitary wasp venoms. Histamine and tyramine play a role in pain-producing activity [35]. Dopamine in the venom of the emerald jewel wasp Ampulex compressa is implicated in a unique behavior of its prey, the American cockroach [11]. In this particular venom, however, their functional role is not yet known. Any other small molecules identified in this venom may give physiological effects when injected into the beetle larvae prey, which remains to be studied.
The two main peptide components identified from the Campsomeriella annulata annulata solitary wasp venom, α-campsomerin (PRLRRLTGLSPLR) and β-campsomerin (PRLRRLTGLSPLRAP), were studied in two relevant therapeutic targets related to hypertension, and cardiovascular and renal diseases. ACE (EC 3.4.15.1) and NEP (EC 3.4.24.11) are membrane-anchored vasopeptidases important in blood pressure control. While ACE produces angiotensin II from the hydrolysis of angiotensin I and inactivates bradykinin, NEP degrades natriuretic peptides (ANP, BNP and CNP), in addition to bradykinin and substance P [36]. Interestingly, despite the similarity of both peptides with the C-terminus of bradykinin (RPPGFSPFR), our results demonstrated that they did not behave as substrates or inhibitors of the catalytic activities of NEP and ACE, indicating that they do not interact with the studied metallopeptidases.
Continuing the studies of biological effects of α-campsomerin and β-campsomerin, we investigated the possible interaction of these molecules with AChE (E.C.3.1.1.7). AChE is a well-known serine hydrolase that catalyzes the hydrolysis of the neurotransmitter acetylcholine into choline and acetic acid [37]; inhibiting it would increase acetylcholine levels in the brain, enhancing cholinergic synapses in Alzheimer’s disease patients [38,39]. Natural peptides have garnered a lot of attention as AChE inhibitors [38], but in our study, both peptides did not exhibit anti-AChE activities, as seen by TEPP, an organophosphate pesticide that induces excessive stimulation of the central nervous system leading to respiratory failure and death by irreversibly inhibiting AChE [40].
The venom of solitary wasps is a rich source of neuroactive chemicals [7], including bradykinin-related peptides, which disrupt the synaptic transmission of the nAChR in the central nervous system of prey [24,25,26]. Our group has studied the neuroprotective effects of natural peptides from solitary wasp venoms against the H2O2-induced oxidative stress in neuronal cell lines after different treatments for acute and chronic conditions. [28]. This cell model, which promotes excessive ROS generation [41,42], with consequent neurotoxic effects [43,44], represents typical characteristics of different neurodegenerative diseases. In a previous study, the neuroprotective effects of the two main peptides α-scoliidine (DYVTVKGFSPLR) and β-scoliidine (DYVTVKGFSPLRKA) identified from the Scolia decorata ventralis venom, with significant similarity between the C-terminal of campsomerins, showed that small structural differences of natural peptides can result in significant differences in biological activities [28]. β-scoliidine, but not α-scoliidine, showed neuroprotective effects against H2O2-induced neurotoxicity in prolonged therapy, preserving neuronal cell integrity and mitochondrial metabolism [28]. It is worth noting that the effects of β-scoliidine were only reliant on the presence of two extra amino acid residues (KA) at the C-terminal on its main molecular sequence [28], and this would explain its protective effects against oxidative stress-induced neurotoxicity in these cells. In the present study, we also investigated the neuroprotective effects against H2O2-induced stress in the presence of α-campsomerin or β-campsomerin, at different concentrations, in PC12 neuronal cells. Interestingly, despite the relative similarity between the C-terminal of campsomerins and scoliidines, these peptides did not show neuroprotective effects against damage induced by oxidative stress after chronic treatment.
Cytotoxicity assays evaluate the effects of a compound on different mammalian cell lines, providing vital information on the biological characteristics and basic tolerance of a new molecule—an important aspect of the modern pharmaceutical development process [45]. Here, the cytotoxic effects of α-campsomerin and β-campsomerin were studied using two different cell types: a typical fibroblastic cell (Vero cells), and a neuronal PC12 cell line. Both peptides were not cytotoxic in Vero cells in concentrations up to 10 µmol.L−1 after 48 h of incubation, in contrast to the DMSO, which reduced cell integrity, in agreement with the literature [46]. In PC12 cells, β-campsomerin was also not toxic under the conditions tested, but α-campsomerin increased the number of cells after 24 to 48 h of treatment. Different biological effects of natural components on cell damage or toxicity in tumor (PC12, HepG2, Caco-2 and 4T1) and non-tumor (Vero, CHO, 3T3, MCDK and BHK2) cell lines have been reported in the literature [47,48]. Pseudocerastes venoms—desert snakes of the Viperidae family—reduced the viability of the tumor cells, while having a limited effect on healthy cells, showing the specificity of their effects on tumor cells [47]. Interestingly, we also found that the α-campsomerin increased the viability of neuronal PC12 cells by more than 50% when compared to the β-campsomerin, while it had essentially no effect on the typical fibroblasts (Vero cell line). These results suggest that α-campsomerin appears to stimulate cell viability up-regulation (mitogenic agent) in tumoral cells. It is important to note that the only difference between the primary structures from the studied peptides is the presence of the AP extension at the C-terminus of β-campsomerin compared to α-campsomerin, indicating, again, that small structural differences of natural peptides lead to different biological activities. Hence, for these reasons, more research is needed to understand the potentiating effects of cell viability in the presence of α-campsomerin.
Annulatin is unique as a linear α-helical peptide. Structurally, it has a short length (11 amino acids length), and only one cationic residue (6K) with one anionic residue (3E). Usually, this type of peptide has 2–4 cationic residues (K and R) without any anionic residues, and a length of 10–30 amino acids. In biological activities, linear α-helical peptides commonly show antimicrobial, hemolytic, and histamine-releasing activities [20,29,30,31,32]. Annulatin also showed histamine-releasing activity and weak hemolytic activity, but no antimicrobial activity. Linear α-helical peptides are present in many arthropod venoms, such as scorpion and spider venoms [49,50]. They may be involved in functional roles in preventing the prey from microbial infection during long-time storage, and potentiating venom toxicity by disturbing excitable membranes [49,50]. It is the case for those in solitary wasp venoms, as we have reported previously [20,29,30,31,32] (Table 8). However, in this annulatin case, there is no antimicrobial activity, and accordingly, it may have some other role in venom function, which is a subject of further study. In this sense, future in silico analysis using platforms to predict the possible antihypertensive and antimicrobial activities of all peptides present in C. annulata annulata venom are being carried out to define the next molecules to be synthesized.
4. Conclusions
Component profile of the venom from the solitary scoliid wasp Campsomeriella annulata annulata was revealed by comprehensive LC-MS and MS/MS analyses. The two major peptide components, α-campsomerin (PRLRRLTGLSPLR) and β-campsomerin (PRLRRLTGLSPLRAP), are bradykinin-related peptides. α-Campsomerin increased the viability of neuronal PC12 cells, whereas β-campsomerin had no effect. A minor peptide component annulatin (ISEALKSIIVG-NH2) is a linear α-helical peptide with a unique biological activity profile, showing histamine-releasing activity from mast cells. This is the first case in which linear α-helical peptides were found in scoliid wasp venoms.
5. Materials and Methods
5.1. Materials
All chemicals used in the present study were of analytical reagent grade (purity higher than 95%) and purchased from Calbiochem-Novabiochem Corporation (San Diego, CA, USA), Gibco BRL (New York, NY, USA), Fluka Chemical Corp. (Buchs, Switzerland) or Sigma-Aldrich Corporation (St. Louis, MO, USA). The ACE I from rabbit lung and AChE from Electrophorus electricus (electric eel) Type VI-S were purchased from Sigma-Aldrich. Neprilysin and the Fluorescence Resonance Energy Transfer (FRET) substrates, Abz-FRK (Dnp) P-OH (for ACE I assays) and Abz-RGFK (Dnp)-OH (for NEP assays) were provided by Prof. Adriana Carmona, from the Department of Biophysics of UNIFESP-EPM, São Paulo, SP, Brazil. For the reverse phase chromatography, acetonitrile and TFA were acquired from J.T. Baker.
5.2. Wasp Collection
For this study, five female wasp individuals of Campsomeriella annulata annulata were collected manually by an insect-catching net in Kyoto, Japan, in August 2010. The venom sacs were dissected under a low temperature anesthetization and extracted with 50% MeCN (acetonitrile)/water. The extracts were lyophilized and stored at −35 °C until use.
5.3. Cell Lines
Two types of cell line were used in the present study: PC12 cells (ATCC® CRL-1721™ from the American Type Culture Collection—ATCC, Manassas, VA, USA) was obtained from a transplantable rat pheochromocytoma, and Vero cells (Cercopithecus aethiops—CCIAL 057, provided by the Adolfo Lutz Institute, São Paulo, Brazil) from kidney cells of the African green monkey. PC12 cells were cultured in DMEM medium (Sigma-Aldrich), while the Vero cells were maintained in Ham-F-10 medium (Sigma-Aldrich, St. Louis, MO, USA). Both cell cultures were supplemented with 10% fetal bovine serum (FBS; Gibco, Waltham, MA, USA), 1% (v/v) of penicillin (10,000 U·mL−1), streptomycin (10 mg·mL−1), and amphotericin B solution (25 μg·mL−1) (Sigma-Aldrich, St. Louis, MO, USA). The cultures were kept at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air (Water Jacketed CO2 Incubator, Thermo Scientific). Cells were passaged using trypsin-EDTA solution [0.05% (m/v) trypsin and 0.02% (m/v) EDTA] at 80 percent confluence after the culture medium was replenished every 2–3 days.
5.4. LC-ESI-MS
The crude venom was analyzed with a LC (Accela 600 Pump, Thermo Scientific) connected with ESI-FTMS (LTQ Orbitrap XL, Thermo Scientific). The lyophilized venom sac extracts were dissolved into 500 μL of water, and, from this solution, 10 μL (corresponding to 10% amount of crude venom sac extracts from a single specimen) was subjected to reversed-phase HPLC using CAPCELL PAK C18 UG 120, 1.5 × 150 mm (SHISEIDO Co., Ltd., Tokyo, Japan) with linear gradient from 5% to 65% CH3CN/H2O/0.1% (v/v) formic acid at a flow rate of 200 µL·min−1 over 20 min at 25 °C. ESI-FTMS was operated by XcaliburTM software (Thermo Scientific) as: capillary voltage, +4.6 kV; capillary temp., 350 °C; sheath and aux gas flow, 50 and 30, respectively (arbitrary units); resolution, 5 ppm. MS/MS spectra were obtained by data-dependent MS/MS mode (the two most intense peaks by HCD), and the obtained spectra were manually analyzed to give peptide sequences, which were confirmed by MS-Product in ProteinProspector program (http://prospector.ucsf.edu/prospector/cgi-bin/msform.cgi?form=msproduct, accessed on 8 November 2021).
5.5. MALDI-TOF MS
MALDI-TOF MS spectra were acquired on an Autoflex TOF/TOF mass spectrometer (Bruker Daltonics, Yokohama, Japan) equipped with 337 nm pulsed nitrogen laser under reflector mode. The resolution and accuracy of MS were 18,000 full width at half maximum (m/z 3000) and 10 ppm, respectively. The accelerating voltage was 20 kV. Matrix, α-cyano-4-hydroxycinnamic acid (Sigma-Aldrich, St. Louis, MO, USA), was prepared at a concentration of 10 mg·mL−1 in 1:1 CH3CN/ 0.1% (v/v) TFA. External calibration was performed with [Ile7]-angiotensin III (m/z 897.51, monoisotopic; Sigma, St. Louis, MO, USA ) and human ACTH fragment 18–39 (m/z 2465.19, monoisotopic; Sigma, St. Louis, MO, USA). The sample solution (0.5 µL) dropped onto the MALDI sample plate was added to the matrix solution (0.5 µL) and allowed to dry at room temperature. For TOF/TOF measurement, argon was used as a collision gas and ions were accelerated at 19 kV. The series of b and y ions were afforded, which enabled identification of whole amino acid sequence by manual analysis.
5.6. Peptide Synthesis
The peptides were synthesized using Fmoc chemistry by GenScript (Nanjing, China). The crude products were purified by RP-HPLC with a preparative C18 column, and the purity and molecular weight of the final peptides were verified by HPLC and MS.
5.7. ACE and NEP Activities
Experiments were performed using different concentrations of α-campsomerin and β-campsomerin with ACE and NEP, and their FRET substrates, FRK(Dnp)P-OH and Abz-RGFK (Dnp)-OH, respectively. The assays used 7.5 ng of both peptidases and the substrates were added in a 100 mmol·L−1 Tris-HCl buffer containing 50 mmol·L−1 NaCl, 10 μmol·L−1 ZnCl2, pH 7.0 (for ACE assays) and Tris HCl 50 mmol·L−1, pH 7.5 buffer (for NEP assays). All experiments were carried out at 37 °C and at a final volume of 100 µL Three FRETs substrate concentrations were used (2 μmol·L−1, 4 μmol·L−1 and 8 μmol·L−1), and were incubated with three concentrations of both peptides (20 μmol·L−1, 30 μmol·L−1 and 50 μmol·L−1). Controls without the peptides were also performed in all assays. The reactions were monitored for 15 min on fluorimeter (Victor 3—Perkin–Elmer) and the results were analyzed on GraFit 3.0 from Erithacus Software. All assays were performed in triplicate.
5.8. Stability Tests of Peptides
Both peptides (30 µmol·L−1) were incubated for four hours at 37 °C with ACE and NEP (7.5 n). As a negative control, samples containing only synthetic peptides were employed. After incubation, samples were analyzed on a Shim-pack VP-ODS C-18 column (4.6 150 mm) utilizing reverse-phase chromatography on HPLC (Prominence, Shimadzu). Solvent A was 0.1% TFA in water (solvent A), and solvent B was acetonitrile plus solvent A (9:1). Over a 20-min period, separations were carried out at a flow rate of 1 mL/min with a 10–60 percent gradient of solvent B. Elution was always followed by an assessment of UV absorption (214 nm).
5.9. Measurement of AChE Activity
The AChE activity was evaluated spectrophotometrically using Ellman’s technique, as previously reported by Harry et al. [51], with slight modifications. Both peptides (20 μL) at varied concentrations (20, 10, 5, 1, and 0.1 µmol·L−1), 10 μL of AChE (0.5 U·mL−1) (Sigma, St. Louis, MO, USA) and 160 μL of 5,5-dithiobis-2-nitrobenzoic acid (DTNB, Ellman’s reagent) 0.33 mM (Sigma, St. Louis, MO, USA) in phosphate buffer (0.1 mo·L−1, pH 8.0) were placed in triplicate in a microplate, and incubated at room temperature for 10 min after adding 10 μL of acetylthiocholine iodide (20 mmol·L−1) (Sigma, St. Louis, MO, USA). The hydrolysis of acetylthiocholine iodide leads to production of acetic acid and thiocholine, which reacts with DTNB producing the anion 5-thio-2-nitrobenzoic acid, which was monitored at 412 nm during 20 min in microplate reader (BioTek Instruments, Inc., Winooski, VT, USA. Tetraethyl pyrophosphate (TEPP; Sigma, St. Louis, MO, USA) was used as a positive control. IAChE = [(Ac − As)/Ac] × 100, where Ac = absorbance for the control and As = absorbance for the sample was used to compute the percentage of inhibition (I) of AChE.
5.10. Toxicity Studies on the Integrity Cell
The cytotoxic effects of α-campsomerin and β-campsomerin were determined by the staining of attached cells with crystal violet dye, according to the literature [52]. In a 96-well plate (Nest Biotechnology, Rahway, USA), PC12 and Vero cells were put at 2.0 × 104 and 1.0 × 104 cells/well, respectively. Cells were treated with different concentrations (0.1 to 10 µmol·L−1) of peptides in 0.10 mL. The plate was incubated for 1, 6, 24, and 48 h at 37 °C. There were control and DMSO groups for each concentration and time course tested, representing untreated cells (just one equal volume of culture medium) and cells treated with DMSO (5%; v/v) diluted in the medium culture, respectively. The media was then aspirated, and the cells were stained with a 0.5 percent crystal violet staining solution (0.5%; m/v), washed, and air-dried. Afterwards, methanol (200 μL) was added, and the absorbance measured at 570 nm using a SpectraMax reader (Molecular Devices, CA, USA). Data were obtained from three independent experiments in triplicate, and were expressed by percentage of cell viability, calculated using the control group as 100% of integrity.
5.11. Neuroprotective Assay
Initially, PC12 cells were seeded at 2 × 104 cells/well in a 96-well plate (Nest Biotechnology, Rahway, NJ, USA) for 24 h. To evaluate the neuroprotective effects of peptides, cells were pre-treated at 37 °C with α-campsomerin and β-campsomerin (0.1 to 20 µmol·L−1), diluted in DMEM medium supplemented with antibiotics without fetal bovine serum for 4 h. Afterwards, the solutions were replaced by medium containing the peptides and H2O2 (0.5 mmol·L−1) and incubated for 20 h more. Cells untreated with H2O2 represent control group in all experiments. Next, all groups were analyzed by the cell integrity.
5.12. Antimicrobial, Hemolytic, and Histamine Releasing Activities
Antimicrobial, hemolytic, and histamine-releasing activities were measured as described previously [53]. The microbial species used in this study were: Escherichia coli (NBRC 14237), Staphylococcus aureus (NBRC 12732), and Saccharomyces cerevisiae (NBRC 10217), which were purchased from the Biological Resource Center (BRC, Kisarazu, Japan), National Institute of Technology and Evaluation. The pI of peptides was calculated with IPC (http://isoelectric.org/index.html, accessed on 8 November 2021). The physicochemical properties were examined using HeliQuest (http://heliquest.ipmc.cnrs.fr/, accessed on 8 November 2021).
5.13. Statistical Analyses
All data are shown in triplicate as the mean ± SD of three different experiments (n = 3). For between-groups comparisons, one-way analysis of variance (ANOVA) was used, followed by a Tukey’s post-hoc test for multiple comparisons. Statistical significance was defined as a value of p < 0.05. GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used to conduct the analysis.
Author Contributions
Designed this work, and were responsible for drafting the manuscript, C.A.-S., F.C.V.P. and K.K.; performed MALDI TOF/TOF MS experiment and analysis, K.-i.N.; performed LC-ESI-MS and MS/MS experiments and analysis, K.K., performed all experiments with cellular culture, C.A.-S. and H.Q.P.; performed enzymatic assays, F.C.V.P. and R.T.K.; performed histamine-releasing, hemolytic and antimicrobial assays H.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the State of São Paulo Research Foundation (FAPESP) (2015/13124-5; 2019/20832-7) and the Coordination for the Improvement of Higher Education Personnel (CAPES) (Finance Code 001).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors gratefully acknowledge the administrative and technical support from Immunochemistry Laboratory (Butantan Institute), Experimental Morphophysiology Laboratory (Federal University of ABC; UFABC).
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Rádis-Baptista, G.; Konno, K. Arthropod Venom Components and Their Potential Usage; MDPI: Basel, Switzerland, 2020; ISBN 978-3-03928-540-2 (PBK)/978-03928-541-9 (PDF). Available online: https://www.mdpi.com/2072-6651/12/2/82/htm (accessed on 8 November 2021).
- Ortiz, E.; Gurrola, G.B.; Schwartz, E.F.; Possani, L.D. Scorpion venom components as potential candidates for drug development. Toxicon 2015, 93, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Saez, N.J.; Herzig, V. Versatile spider venom peptides and their medical and agricultural applications. Toxicon 2019, 158, 109–126. [Google Scholar] [CrossRef]
- King, G. Tying pest insects in knots: The deployment of spider-venom-derived knottins as bioinsecticides. Pest Manag. Sci. 2019, 75, 2437–2445. [Google Scholar] [CrossRef]
- Cid-Uribe, J.I.; Veytia-Busheli, J.I.; Romero-Gutierres, T.; Ortiz, E.; Possani, L.D. Scorpion venomics: A 2019 overview. Exp. Rev. Proteom. 2020, 17, 67–83. [Google Scholar] [CrossRef]
- Silva, J.; Monge-Fuentes, V.; Gomes, F.; Lopes, K.; dos Anjos, L.; Campos, G.; Arena, C.; Biolochi, A.; Gonçalves, J.; Galante, P.; et al. Pharmacological alternatives for the treatment of neurodegenerative disorders: Wasp and bee venoms and their components as new neuroactive tools. Toxins 2015, 7, 3179–3209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Baek, J.H.; Yoon, K.A. Differential properties of venom peptides and proteins in solitary vs. social hunting wasps. Toxins 2016, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, K.M. Solitary Wasps: Behavior and Natural History; Cornell University Press: Ithaca, NY, USA, 2001; ISBN 0-8014-3721-0. [Google Scholar]
- White, S.R.; Kadavakollu, S. Bradykinin in Hemipepsis ustulata: A novel method for safely milking wasps. Toxicon 2016, 117, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Nolasco, M.; Biondi, I.; Pimenta, D.C.; Branco, A. Extraction and preliminary chemical characterization of the venom of the spider wasp Pepsis decorata (Hymenoptera: Pompilidae). Toxicon 2018, 150, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.L.; Arvidson, R.; Banks, C.; Urenda, J.L.; Duong, E.; Mohammed, H.; Adams, M.E. Ampulexins: A new family of peptides in venom of the emerald jewel wasp, Ampulex compressa. Biochemistry 2018, 57, 1907–1916. [Google Scholar] [CrossRef]
- Kotea, S.; Faktorb, J.; Dapica, I.; Mayordomoa, M.Y.; Kocikowskia, M.; Kagansky, A.; Goodletta, D.; Vojtesek, B.; Huppa, T.; Wilcockson, D.; et al. Analysis of venom sac constituents from the solitary, aculeate wasp Cerceris rybyensis. Toxicon 2019, 169, 1–4. [Google Scholar] [CrossRef]
- Huicab-Uribe, M.A.; Verdel-Aranda, K.; Martínez-Hernández, A.; Zamudio, F.Z.; Jiménez-Vargas, J.M.; Lara-Reyna, J. Molecular composition of the paralyzing venom of three solitary wasps (Hymenoptera: Pompilidae) collected in southeast Mexico. Toxicon 2019, 168, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Walker, A.A.; Nguyen, S.H.; Jin, A.-H.; Deuis, J.R.; Vetter, I.; King, G.F.; Schmidt, J.O.; Robinson, S.D. Venom chemistry underlying the painful stings of velvet ants (Hymenoptera: Mutillidae). Cell. Mol. Life Sci. 2021, 78, 5163–5177. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Hisada, M.; Itagaki, Y.; Naoki, H.; Kawai, N.; Miwa, A.; Yasuhara, T.; Takayama, H. Isolation and structure of pompilidotoxins (PMTXs), novel neurotoxins in solitary wasp venoms. Biochem. Biophys. Res. Commun. 1998, 250, 612–616. [Google Scholar] [CrossRef]
- Kawai, N.; Konno, K. Molecular determinants of two neurotoxins that regulate sodium current inactivation in rat hippocampus. Neurosci. Lett. 2004, 361, 44–46. [Google Scholar] [CrossRef]
- Schiavon, E.; Stevens, M.; Zaharenko, A.J.; Konno, K.; Tytgat, J.; Wanke, E. Voltage-gated sodium channels isoform-specific effects of pompilidotoxins. FEBS J. 2010, 277, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Konno, K.; Salceda, E.; Vega, R.; Zaharenko, A.J.; Soto, E. Sa12b peptide from solitary wasp inhibits ASIC currents in rat dorsal root ganglion neurons. Toxins 2019, 10, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konno, K.; Kazuma, K.; Rangel, M.; Stolarz-de-Oliveira, J.; Fontana, R.; Kawano, M.; Fuchino, H.; Hide, I.; Yasuhara, T.; Nakata, Y. New mastoparan peptides in the venom of the solitary eumenine wasp Eumenes micado. Toxins 2019, 11, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera, M.P.D.S.; Rangel, M.; Ruggiero Neto, J.; Konno, K. Chemical and biological characteristics of antimicrobial α-helical peptides found in solitary wasp venoms and their interaction with model membranes. Toxins 2019, 11, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picolo, G.; Hisada, M.; Sciani, J.M.; Conceição, I.M.; Machado, M.; Oliveira, V.; de Melo, R.L.; Cury, Y.; Konno, K.; Hayashi, M.A.F. Bradykinin-related peptides from the venom of the solitary wasp Cyphononyx fulvognathus. Biochem. Pharmacol. 2010, 79, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Nihei, K.; Peigneur, S.; Tytgat, J.; Lange, A.B.; Konno, K. Isolation and characterization of FMRFamide-like peptides in the venoms of solitary sphecid wasps. Peptides 2021, 142, 170575. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Kazuma, K.; Nihei, K. Peptide toxins in solitary wasp venoms. Toxins 2016, 8, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuhara, T.; Mantel, P.; Nakajima, T.; Piek, T. Two kinins isolated from an extract of the venom reservoirs of the solitary wasp Megascolia flavifrons. Toxicon 1987, 25, 527–535. [Google Scholar] [CrossRef]
- Piek, T.; Hue, B.; Mantel, P.; Nakajima, T.; Pelhate, M.; Yasuhara, T. Threonine6-bradykinin in the venom of the wasp Colpa interrupta (F.) presynaptically blocks nicotinic synaptic transmission in the insect CNS. Comp. Biochem. Physiol. 1990, 96, 157–162. [Google Scholar] [CrossRef]
- Piek, T.; Hue, B.; Mony, L.; Nakajima, T.; Pelhate, M.; Yasuhara, T. Block of synaptic transmission in insect CNS by toxins from the venom of the WASP Megascolia flavifrons (FAB.). Comp. Biochem. Physiol. 1987, 87, 287–295. [Google Scholar] [CrossRef]
- Konno, K.; Palma, M.S.; Hitara, I.Y.; Juliano, M.A.; Juliano, L.; Yasuhara, T. Identification of bradykinins in solitary wasp venoms. Toxicon 2002, 40, 309–312. [Google Scholar] [CrossRef]
- Alberto-Silva, C.; Portaro, F.; Kodama, R.; Pantaleão, H.; Rangel, M.; Nihei, K.; Konno, K. Novel neuroprotective peptides in the venom of the solitary scoliid wasp Scolia decorata ventralis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Hisada, M.; Fontana, R.; Lorenzi, C.C.B.; Naoki, H.; Itagaki, Y.; Miwa, A.; Kawai, N.; Nakata, Y.; Yasuhara, T.; et al. Anoplin, a novel antimicrobial peptide from the venom of the solitary wasp Anoplius samariensis. Biochim. Biophys. Acta 2001, 1550, 70–80. [Google Scholar] [CrossRef]
- Konno, K.; Rangel, M.; Oliveira, J.S.; Cabrera, M.P.S.; Fontana, R.; Hirata, I.Y.; Nakata, Y.; Mori, K.; Kawano, M.; Fuchino, H.; et al. Decoralin, a novel linear cationic α-helical peptide from the venom of the solitary eumenine wasp Oreumenes decoratus. Peptides 2007, 28, 2320–2327. [Google Scholar] [CrossRef]
- Konno, K.; Hisada, M.; Naoki, H.; Itagaki, Y.; Fontana, R.; Rangel, M.; Oliveira, J.S.; Cabrera, M.P.; Ruggiero Neto, J.; Hide, I.; et al. Eumenitin, a novel antimicrobial peptide from the venom of the solitary eumenine wasp Eumenes rubronotatus. Peptides 2006, 27, 2624–2631. [Google Scholar] [CrossRef] [PubMed]
- Rangel, M.; Cabrera, M.P.S.; Kazuma, K.; Ando, K.; Wang, X.; Kato, M.; Nihei, K.; Hirata, I.Y.; Cross, T.; Garcia, A.N.; et al. Chemical and biological characterization of four new antimicrobial and α-helical peptides from the venoms of two solitary eumenine wasps. Toxicon 2011, 57, 1081–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigeri, Y.; Horie, M.; Yoshida, T.; Hagihara, Y.; Imura, T.; Inagaki, H.; Haramoto, Y.; Ito, Y.; Asashima, M. Physicochemical and biological characterizations of Pxt peptides from amphibian (Xenopus tropicalis) skin. J. Biochem. 2016, 159, 619–629. [Google Scholar] [CrossRef]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob. Agents Chemother. 2007, 51, 1398–1406. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, T.; Yasuhara, T.; Yoshida, N.; Takemoto, Y.; Shinonaga, S.; Kano, R.; Yoshida, H. The pattern analysis of biologically active amines in some Hymenopteran venoms by high performance liquid chromatography. Med. Entomol. Zool. 1983, 34, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Arendse, L.; Danser, A.; Poglitsch, M.; Touyz, R.; Burnett, J.; Llorens-Cortes, C.; Ehlers, M.; Sturrock, E. Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacol. Rev. 2019, 71, 539–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase inhibitors in the treatment of neurodegenerative diseases and the role of acetylcholinesterase in their pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef]
- Prasasty, V.; Radifar, M.; Istyastono, E. Natural peptides in drug discovery targeting acetylcholinesterase. Molecules 2018, 23, 2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, J.; de Castro, A.; Soares, F.; da Cunha, E.; Ramalho, T. Future therapeutic perspectives into the Alzheimer’s disease targeting the oxidative stress hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, D.; Brecht, K.; Koplovitz, I.; Sweeney, R. Acetylcholinesterase inhibition: Does it explain the toxicity of organophosphorus compounds? Arch. Toxicol. 2006, 80, 756–760. [Google Scholar] [CrossRef]
- Aksenova, M.; Aksenov, M.; Mactutus, C.; Booze, R. Cell culture models of oxidative stress and injury in the central nervous system. Curr. Neurovasc. Res. 2005, 2, 73–89. [Google Scholar] [CrossRef] [Green Version]
- Querobino, S.M.; Carrettiero, D.C.; Costa, M.S.; Alberto-Silva, C. Neuroprotective property of low molecular weight fraction from B. Jararaca snake venom in H2O2-induced cytotoxicity in cultured hippocampal cells. Toxicon 2017, 129, 134–143. [Google Scholar] [CrossRef]
- Emerit, J.; Edeas, M.; Bricaire, F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004, 58, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Querobino, S.M.; Costa, M.S.; Alberto-Silva, C. Protective effects of distinct proline-rich oligopeptides from B. Jararaca snake venom against oxidative stress-induced neurotoxicity. Toxicon 2019, 167, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Bácskay, I.; Nemes, D.; Fenyvesi, F.; Váradi, J.; Vasvári, G.; Fehér, P.; Vecsernyés, M.; Ujhelyi, Z. Role of cytotoxicity experiments in pharmaceutical development. In Cytotoxicity; Çelik, T.A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Liu, M.; Luo, Q.; Zhuo, H.; Cao, H.; Wang, J.; Han, Y. Toxic effects of dimethyl sulfoxide on red blood cells, platelets, and vascular endothelial cells in vitro. FEBS Open Bio 2017, 7, 485–494. [Google Scholar] [CrossRef]
- den Brouw, B.O.; Ghezellou, P.; Casewell, N.; Ali, S.; Fathinia, B.; Fry, B.; Bos, N.; Ikonomopoulou, M. Pharmacological characterisation of Pseudocerastes and Eristicophis viper venoms reveal anticancer (Melanoma) properties and a potentially novel mode of fibrinogenolysis. Int. J. Mol. Sci. 2021, 22, 6896. [Google Scholar] [CrossRef] [PubMed]
- Rodeiro, I.; Hernández, I.; Herrera, J.; Riera, M.; Donato, M.; Tolosa, L.; González, K.; Ansoar, Y.; Gómez-Lechón, M.; Berghe, W.V.; et al. Assessment of the cytotoxic potential of an aqueous-ethanolic extract from Thalassia testudinum angiosperm marine grown in the Caribbean sea. J. Pharm. Pharmacol. 2018, 70, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.M.; Reis, P.V.; Pimenta, A.M.C. Antimicrobial peptides in spider venoms. In Spider Venoms; Gopalakrishnakone, P., Corzo, G.A., de Lima, M.E., Diego-García, E., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 361–377. ISBN 978-94-007-6389-0. [Google Scholar]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Insects, arachnids and centipedes venom: A powerful weapon against bacteria. A literature review. Toxicon 2017, 130, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Harry, J.; Tilson, H.A.; Padilla, S.; Lassiter, T.L.; Hunter, D. Biochemical measurement of cholinesterase activity. In Neurodegeneration Methods and Protocols; Humana Press: Tortowa, NJ, USA, 2003; pp. 237–246. [Google Scholar]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016, 4, 343–346. [Google Scholar] [CrossRef]
- Inagaki, H.; Akagi, M.; Imai, H.T.; Taylor, R.W.; Kubo, T. Molecular cloning and biological characterization of novel antimicrobial peptides, pilosulin 3 and pilosulin 4, from a species of the Australian ant genus Myrmecia. Arch. Biochem. Biophys. 2004, 428, 170–178. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).