Abstract
Soybean is an important, high protein source of food and feed. However, like other agricultural grains, soybean may pose a risk to human and animal health due to contamination of the grains with toxigenic Fusaria and associated mycotoxins. In this study, we investigated the diversity of Fusaria on a panel of 104 field isolates obtained from soybean grains during the growing seasons in 2017–2020. The results of species-specific PCR analyses showed that Fusarium avenaceum was the most common (n = 40) species associated with soybean grains in Poland, followed by F. equiseti (n = 22) and F. sporotrichioides (11 isolates). A set of isolates, which was not determined based on PCR analyses, was whole genome sequenced. Multiple sequence analyses using tef-1α, top1, rpb1, rpb2, tub2, pgk, cam and lsu genes showed that most of them belonged to Equiseti clade. Three cryptic species from this clade: F. clavum, F. flagelliforme and FIESC 31 (lacking Latin binomial) were found on soybean for the first time. This is the first report demonstrating the prevalence of Fusaria on soybean grains in Poland.
Key Contribution:
Fusarium avenaceum is the most common Fusarium species associated with soybean grains in Poland, followed by F. equiseti and F. sporotrichioides. Four cryptic species from Equiseti clade: F. equiseti, F. clavum, F. flagelliforme and FIESC 31 can contaminate soybean grains.
1. Introduction
The continuous growth of the global population demands an improvement of protein production with an environmentally friendly and energy-efficient practice. The integration of protein-rich legumes into cropping systems appears to be among the most promising strategies to bridge the gap between global food and feed demand and supply. Soybean is one of the most important crops worldwide with the highest protein content (40–42%) of all crops and is the second, after groundnut, to oil content (18–22%) of legumes [1,2]. It is currently the most widely cultivated legume crop occupying around 6% of the total land surface [3]. However, soybean production is threatened by a variety of pathogens [4,5]. Among the most economically important are fungi belonging to the Fusarium solani species complex responsible for soybean sudden death syndrome [6] and the Fusarium oxysporum species complex causing soybean root rot and seedling blight [7].
In addition, a range of other Fusaria such as F. verticillioides (Sacc.) Nirenberg [8], F. sporotrichioides Scherb [9], F. equiseti (Corda) Sacc. [10], F. semitectum Berk. and Ravenel [11], F. fujikuroi Nirenberg [8], F. graminearum Schwabe [12], F. proliferatum (Matsush.) Nirenberg [8] and fungi from F. incarnatum-equiseti species complex may be involved in the contamination of soybean grains posing threat to human and animal health due to mycotoxin production [13,14,15,16,17,18,19] (Table 1).

Table 1.
Fusarium species and associated mycotoxins previously reported on soybean grains.
However, it is worth noting that, in contrast to other grains such as wheat [20], barley [21] or corn [22], knowledge of Fusarium fungi and associated mycotoxins on soybean grains is scarce (Table 1).
To fill this gap, we studied the diversity of this group of toxigenic fungi on a panel of 104 field isolates recovered from soybean grains during the 2017–2020 growing seasons. Contrary to previous studies, our results highlight the predominance of enniatin genotypes of F. avenaceum in Polish soybean grains. We also showed that nearly one-fifth of isolates tested by species-specific assays did not give any positive results preventing their identification. Therefore, whole-genome sequencing was performed to clarify their taxonomic status. Multiple sequence comparisons using tef-1α, top1, rpb1, rpb2, tub2, pgk, cam and lsu genes showed that most of them belonged to Equiseti clade. Newly assembled genomes provide great scope for comparative genomics and characterization of mycotoxin gene clusters. This issue will be addressed in a future study.
2. Results
2.1. Identification of Fusaria by Species-Specific PCR Assays
The plating of diseased soybean grains on PDA plates allowed to isolate a total of 104 Fusarium-like colonies, which were then subjected to molecular analyses (Table S1). PCR analyses using species-specific primers allowed determining 80 isolates to the species level. Forty isolates were identified as F. avenaceum (Fr.) Sacc. [10], 22 isolates as F. equiseti, 11 isolates as F. sporotrichioides, six isolates as F. graminearum and one isolate as F. culmorum (Wm.G. Sm.) Sacc. [23]. Each isolate of F. avenaceum gave a positive result with the assay determining esyn1 genotype.
2.2. Identification of Fusaria through Sequence Comparisons
Nineteen isolates that did not give positive signals with qPCR as well as five isolates from the 2020 growing season (which were not subjected to PCR) were whole genome sequenced. For the purpose of sequence comparison, an additional 16 isolates that were identified using PCR were also sequenced. To determine their taxonomic affiliation, we performed BLASTn searches against the NCBI database using eight genes: tef-1α (translation elongation factor 1 alpha), top1 (topoisomerase 1), tub2 (tubulin beta chain), pgk (phosphoglycerate kinase), rpb1 (DNA-directed RNA polymerase II largest subunit), and rpb2 (DNA-directed RNA polymerase II second largest subunit), cam (calmodulin) and lsu (large-subunit rRNA gene) genes. Selected genes have been previously shown to resolve phylogenetic relationships of diverse Fusaria [24,25,26]. The results of BLAST searches are shown in Table S2. Twenty-one isolates were determined to belong to Equiseti clade, 5 isolates were identified as F. avenaceum, five isolates as F. oxysporum, one as F. sporotrichioides and one as F. cerealis (Cooke) Sacc [10].
Assuming > 99% identity match and ≥75% query coverage, tef-1α was the most effective in identifying phylogenetic species from Equiseti clade (Table S2). It is worth noting, however, that the GenBank database provides an informal classification system based on a haplotype nomenclature. In addition, most GenBank entries are assigned a single latin binomial F. equiseti, which refers to the morphological species concept (morphospecies). In most cases, BLAST searches using other genes did not allow resolving taxonomic issues in this clade mostly due to the lack of reference sequences in the GenBank database. Tef-1α-based analysis showed that, among 21 isolates from Equiseti clade, 12 were determined as F. equiseti, six as F. flagelliforme (J.W. Xia, L. Lombard, Sand.-Den., X.G. Zhang and Crous) [27], two as FIESC 31 (lacking latin binomial) (J.W. Xia, L. Lombard, Sand.-Den., X.G. Zhang and Crous) [27] and one as F. clavum (J.W. Xia, L. Lombard, Sand.-Den., X.G. Zhang and Crous) [27].
To determine trichothecene genotypes of F. cerealis, F. culmorum and F. graminearum, we performed sequence comparisons against the ToxGen database [28] using complete sequence of Tri12 gene. Results of analyses showed that both F. cerealis (S18/34) and F. culmorum (S18/1) yielded 100% sequence identity to NIV genotypes: AY102569 and KU572425, respectively. An isolate S18/4 of F. graminearum yielded 100% sequence identity to 3ADON genotype (KU572434), while the remaining three isolates S18/49, S18/55 and S18/66 had the highest identity to the 15ADON genotype (HG970333).
2.3. Phylogenetic Analysis
Phylogenetic analyses were performed using nucleotide sequences of tef-1α, top1, rpb1, rpb2, tub2, pgk, cam and lsu genes. Estimates of genetic diversity (indels, SNPs, nucleotide diversity values and the percent of polymorphic sites) are provided in Table 2.

Table 2.
Variation in tef-1α, top1, rpb1, rpb2, tub2, pgk, cam and lsu among isolates from Equiseti clade.
The phylogenetic relationships among isolates were inferred using Bayesian inference (BI). Strains were resolved into two main sister clades by nucleotide variations within the sequence of tef-1α. The first clade included isolates of F. clavum, F. flagelliforme and FIESC 31 in three species specific clades, while the second sister clade included all F. equiseti isolates (Figure S1). Similar topologies were also found with phylogenetic trees for rpb1 (Figure S2), rpb2 (Figure S3) and cam (Figure S4).
A tree based on tub2 sequences showed a slightly different topology and showed a closer relationship of F. clavum to F. equiseti compared to the remaining two species (Figure S5). A similar finding was also evident for top1 by clustering F. clavum (S19/5) into the second sister clade together with all F. equiseti isolates (Figure S6). Phylogenetic analysis of pgk sequences showed contrasting results and grouped F. clavum into a well-supported clade together with isolates of FIESC 31 (Figure S7). The lsu tree failed to resolve strains of F. clavum and FIESC 31, presumably due to the low number of SNPs (Figure S8, Table 2).
The differences in phylogenetic relationships among these cryptic species could be explained by incomplete lineage sorting or more recent inter-species gene exchange. The impact of incomplete lineage sorting and recombination on the evolution of Equiseti clade could also be observed on top1, which failed to group all strains of F. flagelliforme into a species-specific clade (Figure S6). Phylogenetic analysis of pgk sequences showed a different topology than the remaining trees and placed F. flagelliforme isolates into divergent clades occupying the basal position in the phylogenetic tree (Figure S7). A combined phylogenetic tree provided similar topology to tef-1α, rpb1, rpb2 and cam trees, and grouped all isolates into four well-supported species-specific clades (Figure 1).
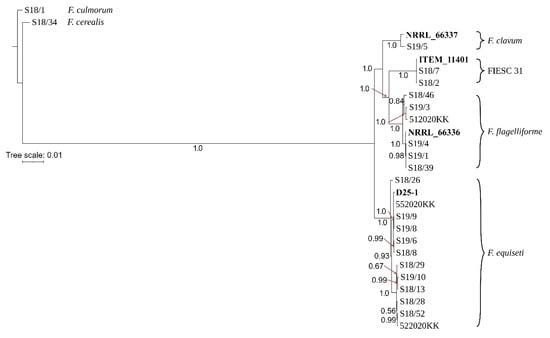
Figure 1.
The phylogenetic tree resulting from a Bayesian analysis on the combined alignment of eight loci (tef-1α, top1, rpb1, rpb2, tub2, pgk, cam and lsu) for Fusarium spp. Bayesian posterior probability scores are shown at the nodes. The scale bar represents the expected number of changes per site. The reference strains are indicated in bold. The tree was rooted to F. culmorum S 18/1.
3. Discussion
The knowledge of fungal patterns contaminating crops is fundamental for understanding the population ecology, dynamics and evolutionary relationships of fungi [29]. Soybean grains may be contaminated by a range of Fusaria [13,14,15,16,17,18,19] (Table 1). However, contrary to previous studies, our results highlight the predominance of F. avenaceum, which, to date, was rarely reported on soybean [30]. It is worth noting that the high prevalence of F. avenaceum in tested isolates is in line with our previous study on other protein-rich crops, such as common vetch, faba bean and blue lupine [31]. In small-grain cereals, F. avenaceum appears to be more commonly responsible for the crown rot and head blight that negatively results in yield and quality of grain [32]. F. avenaceum was recently detected during FHB epidemics in Poland, although with far less frequency than F. graminearum [33].
In this study, 37 isolates recovered from soybean were determined to belong to the Equiseti clade. This clade, together with the Incarnatum clade, forms the FIESC complex involving 33 phylogenetically distinct species, which can be resolved based on Multi-Locus Sequence Typing (MLST) [34,35,36]. Members from both Equiseti and Incarnatum clades are mainly associated with crops and soil [37]. Several reports have documented a prevalence of fungi from the FIESC complex on soybean [14,18,19,37]. However, for some (especially older) reports, it is impossible to gather information on the cryptic diversity within the FIESC due to the fact that a number of species have been previously treated as synonyms of F. equiseti. A more recent MLST-based characterization of the FIESC complex showed that F. ipomoeae (M.M. Wang, Qian Chen and L. Cai) [36], F. sulawesiense (Maryani, Sand.-Den., L. Lombard, Kema and Crous) [38] and F. luffae (M.M. Wang, Qian Chen and L. Cai) [36] are mainly associated with soybean in China [19]. Surveys from Ethiopia and Ghana showed that most of the isolates recovered from soybean roots represented novel, undescribed species [37]. The complex nature of FIESC from soybean was also highlighted in this study. We showed that four species from the Equiseti clade are responsible for the contamination of soybean grains; however, with variable species richness patterns. No members from Incarnatum clade were detected. Among the 21 isolates subjected to whole-genome sequencing, more than half were determined as F. equiseti. This cryptic species appears to be broadly distributed in agroecosystems. To date, the vast majority of characterized F. equiseti strains were recovered from either plant material or soil/sediment substrates [27]. Six isolates recovered in this study were identified as F. flagelliforme. This cryptic species appears to be restricted to Europe, and according to our knowledge, there are no reports showing the incidence of this species on hosts other than cereals [27]. Two remaining species, F. clavum and FIESC 31, were also found to be associated with soybean for the first time. The broad distribution of F. clavum was recently indicated by screening a number of isolates recovered from environmental, plant and human samples in Africa, Asia, Europe and North America [27,34]. Knowledge on the geographic distribution of FIESC 31 is scarce. To date, only two strains of this cryptic species have been described [39].
Fusaria are well known as producers of a vast array of mycotoxins such as enniatins, trichothecenes, fumonisins and zearalenone, which are frequently found in grains and processed foods [40]. They are synthesized through a range of secondary metabolite gene clusters. The distribution of these clusters in fungal genomes is often not correlated with the phylogenetic relationships of species [39,41]. For some fungal lineages, their irregular distribution may also be observed at the strain level [41]. The results presented in this study may indicate potential contamination of soybean with enniatins and moniliformin, which are often found in cereal foods as the result of contamination of the grains with F. avenaceum [42]. Enniatins are mainly produced by strains harboring the esyn1 gene, which was detected in all examined isolates of F. avenaceum [32,42]. FIESC members are able to produce diverse mycotoxins, however, the mycotoxin contamination of crops with this fungal complex is unclear [39]. Previous studies by Barros et al. (2014) [43] and Hartman et al. (2019) [37] showed that FIESC isolates obtained from soybean produced a range of mycotoxin compounds from both type A and type B trichothecenes. However, the FIESC complex appears to exhibit remarkable variation in the distribution of SM clusters. In contrast to the trichothecene cluster, which appears to be commonly distributed, clusters responsible for the production of the enniatin, fusarin and zearalenone display mosaic distribution [39]. A more comprehensive understanding of the diversity and origin of SM clusters requires analysis of a larger set of genomes. However, for many cryptic species from the FIESC complex, genome characterization has been largely limited by the absence of genomes in the GenBank database. Our study may provide a valuable genomic resource for such a study. Further studies will address this issue by incorporating a larger set of strains from the Equiseti clade recovered from various cereals. Whole genome comparisons will provide an unprecedented opportunity to study their patterns of diversity and evolution.
4. Materials and Methods
4.1. Field Isolates
Field isolates were obtained from 17 soybean grain samples (0.5 kg) harvested in 2017–2020 in different regions of Poland (Figure 2). Fifty grains from each sample showing visible symptoms of fungal infection, such as discoloration, black mottling and cracked or shriveled skin, were selected and placed on Petri dishes with distilled water. After 24 h of soaking at room temperature, the grains were surface sterilized with 70% ethanol (EtOH) for 2 min and placed on potato dextrose agar (PDA) (A&A Biotechnology, Gdynia, Poland) in Petri dishes. After 4–6 days of incubation at room temperature in darkness, Fusarium resembling colonies were transferred to fresh PDA plates for further molecular analyses. A total of 104 Fusarium isolates were assigned with individual strain codes and stored at −25 °C in the fungal collection of the Department of Botany and Nature Protection, University of Warmia and Mazury in Olsztyn, Poland.
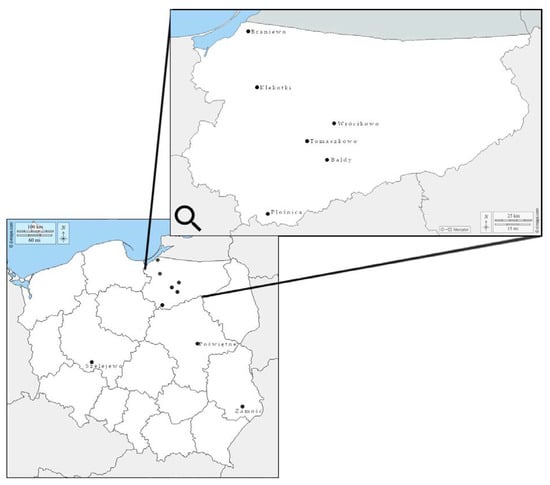
Figure 2.
Location of fields in Poland, from which soybean grain were sampled for analyses.
4.2. DNA Extraction
To obtain genomic DNA, a patch of mycelium (approximately 0.1–0.2 mg) was harvested into homogenization tubes with 1 mm silica spheres (Lysing matrix C, MP Biomedicals, Santa Ana, CA, USA). Homogenization was performed using a FastPrep-24 instrument (MP Biomedicals, Santa Ana, CA, USA). DNA from fungal isolates was extracted with the use of the Genomic Mini AX Food kit according to the manufacturer’s protocol (A&A Biotechnology, Gdynia, Poland).
4.3. Identification of Fusarium Species
To ensure recovery of DNA free of amplification inhibitors, FungiQuant assay [44] was first used. Samples with Ct-values (cycle threshold) below 25 were further analyzed with species-specific assays. Each sample was analyzed in three replicates, assuming positive signals of amplification as Ct-values below 30. Besides the identification of species, mycotoxin genotypes were also determined by using various TaqMan assays. We used marker targeting the esyn1 gene, to determine enniatin genotype for F. avenaceum (Table 3).

Table 3.
List of real-time PCR assays used to determine species and mycotoxin genotypes.
4.4. DNA Sequencing and Assembly
In total, 40 field isolates of Fusarium spp. were sequenced by the whole-genome sequencing and included: (I) a group of 19 isolates that could not be determined based on PCR assays, (II) a set of 16 isolates (two isolates per species) that were previously identified to the species level by PCR and (III) 5 isolates isolated in 2020 growing season (which were not included in PCR analyses). Sequencing was conducted by Macrogen (Seoul, South Korea). Libraries were prepared using KAPA HyperPlus Kit (Roche Sequencing Solutions, Pleasanton, CA, USA). An Illumina HiSeq X Ten was used to sequence the genomes using a paired-end read length of 2 × 150 bp with an insert size of 350 bp. The sequencing quality was assessed via FastQC (ver. 0.11.9) [50]. Low-quality reads were trimmed using Trimmomatic (v.0.36) [51] and the genome was assembled via SPAdes (v.3.13.2) [52]. The project was submitted to the NCBI BioProject under accession no: PRJNA730356.
4.5. BLAST Analysis
The complete sequences of 6 genes: tef-1α (translation elongation factor 1 alpha), top1 (topoisomerase I), rpb1, rpb2 (RNA polymerase II genes), tub2 (beta-tubulin), pgk (phosphoglycinecerate kinase), cam (calmodulin) and lsu (large-subunit rRNA gene) genes, were retrieved from genome sequences with Geneious Prime (v. 2019.0.4 created by Biomatters, Auckland, New Zealand, available from http://www.geneious.com (accessed on 1 November 2021). Identification of the isolates to the species level was done through sequence comparisons using the BLAST searches with default parameters [53]. Species were determined using thresholds of 99–100% nucleotide identity and ≥75% coverage of the query sequence length.
4.6. Phylogenetic Analysis
Phylogenetic analyses were performed using tef-1α, top1, rpb1, rpb2, tub2, pgk, cam and lsu genes of 21 field isolates from Equiseti clade. In addition, sequence data from strains: D25-1 (F. equiseti, GenBank accession no QOHM00000000.1), NRRL 66,337 (F. clavum, GenBank accession no QGEC00000000.1), NRRL 66,336 (F. flagelliforme, GenBank accession no QHHI00000000) and ITEM 11,401 (FIESC 31, GenBank accession no QHKN00000000.1) was used for comparisons. MAFFT software (v7.453) [54] was used to create sequence alignments.
The best partition schemes and corresponding substitution models for alignment were estimated by means of PartitionFinder2 [55]. Afterwards, based on the alignment and obtained models, Bayesian analysis was conducted using MrBayes 3.2.7 [56]. The Markov chain Monte Carlo (MCMC) algorithm was run for 5,000,000 generations (sampling every 500) with four incrementally heated chains (starting from random trees). The Tracer 1.7.1 [57] software was used to determine the number of generations needed to reach stationarity, which occurred at approximately 500,000 generations. Therefore, the first 1000 trees were discarded as burn-in, and the remaining trees were used to create Bayesian consensus trees. Two strains: F. cerealis (S18/34) and F. culmorum (S18/1) isolated from soybean grains were used as outgroups.
To reveal nucleotide variation, analyzed genes were extracted and aligned separately using MAFFT software (v.7.453) [54]. Gene polymorphism analyses were conducted for each gene based on the alignment of 24 strains from Equiseti clade. Variation within each gene was identified as a SNP or indel and counted with the use of an in-house Python script. Nucleotide diversity values (π) for each gene were calculated with TASSEL software (v.5.2.40) [58]. As nucleotide diversity is based only on nucleotide substitutions, the number of indels and percentage of polymorphic sites are given for each gene.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/toxins13120884/s1, Table S1: Identification of Fusarium species and enniatin genotype by PCR analyses, Table S2: Identification of Fusarium species using BLAST software, Figure S1: Bayesian inference phylogeny from tef-1α sequences of isolates from Equiseti clade, Figure S2: Bayesian inference phylogeny from rpb1 sequences of isolates from Equiseti clade, Figure S3: Bayesian inference phylogeny from rpb2 sequences of isolates from Equiseti clade, Figure S4: Bayesian inference phylogeny from cam sequences of isolates from Equiseti clade, Figure S5: Bayesian inference phylogeny from tub2 sequences of isolates from Equiseti clade, Figure S6: Bayesian inference phylogeny from top1 sequences of isolates from Equiseti clade, Figure S7: Bayesian inference phylogeny from pgk sequences of isolates from Equiseti clade, Figure S8: Bayesian inference phylogeny from lsu sequences of isolates from Equiseti clade.
Author Contributions
Conceptualization, M.Ż. and T.K.; methodology, M.Ż., T.M., J.W., T.K. and J.O.; validation, M.Ż. and T.M.; formal analysis, M.Ż. and T.M.; investigation, M.Ż., T.M., K.B., K.K., J.W., T.K., K.M. and J.O.; resources, T.K., J.O. and K.K.; data curation, M.Ż. and T.M.; writing—original draft preparation, M.Ż.; writing—review and editing, T.K.; visualization, M.Ż.; supervision, T.K.; project administration, T.K.; funding acquisition, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Development Program of the University of Warmia and Mazury in Olsztyn”, POWR.03.05.00-00-Z310/17, co-financed by the European Union under the European Social Fund from the Operational Program Knowledge Education Development. Maciej Żelechowski and Joanna Wyrębek are the recipients of a scholarship from the Programme Interdisciplinary Doctoral Studies in Biology and Biotechnology (POWR.03.05.00-00-Z310/17), which is funded by the European Social Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Assembled genomes of Fusarium spp. can be accessed at the NCBI bioproject under the accession number PRJNA730356.
Acknowledgments
We thank anonymous reviewer for his helpful comments to improve the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Pagano, M.C.; Miransari, M. The importance of soybean production worldwide. Abiotic Biot. Stress. Soybean Prod. 2016, 1, 1–26. [Google Scholar] [CrossRef]
- Clemente, T.E.; Cahoon, E.B. Soybean oil: Genetic approaches for modification of functionality and total content. Plant Physiol. 2009, 151, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Shea, Z.; Singer, W.M.; Zhang, B. Soybean production, versatility, and improvement. Legum. Crop. 2020. [Google Scholar] [CrossRef]
- Dorrance, A.E. Management of Phytophthora sojae of soybean: A review and future perspectives. Can. J. Plant Pathol. 2018, 40, 210–219. [Google Scholar] [CrossRef]
- Langenbach, C.; Campe, R.; Beyer, S.F.; Mueller, A.N.; Conrath, U. Fighting asian soybean rust. Front. Plant Sci. 2016, 7, 797. [Google Scholar] [CrossRef]
- Spampinato, C.P.; Scandiani, M.M.; Luque, A.G. Soybean sudden death syndrome: Fungal pathogenesis and plant response. Plant Pathol. 2021, 70, 3–12. [Google Scholar] [CrossRef]
- Cruz, D.R.; Leandro, L.F.S.; Munkvold, G.P. Effects of temperature and pH on Fusarium oxysporum and Soybean Seedling Disease. Plant Dis. 2019, 103, 3234–3243. [Google Scholar] [CrossRef] [PubMed]
- Nirenberg, H. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Section Liseola. Mitt. Biol. Bundesanst. Land Forstwirtsch. 1976, 169, 1–117. [Google Scholar]
- Sherbakoff, C.D. Fusaria of potatoes. Mem. Cornell Univ. Agric. Exp. Station 1915, 6, 87–270. [Google Scholar]
- Saccardo, P.A. Sylloge Hyphomycetum. Sylloge Fung. 1886, 4, 1–807. [Google Scholar]
- Berkeley, M.J. Notices of North American fungi. Grevillea 1875, 3, 97–112. [Google Scholar]
- Schwabe, S.H. Flora Anhaltina; Apud Georigum Reimerum: Berlin, Germany, 1839; Volume 2, pp. 1–425. [Google Scholar]
- Gutleb, A.C.; Caloni, F.; Giraud, F.; Cortinovis, C.; Pizzo, F.; Hoffmann, L.; Bohn, T.; Pasquali, M. Detection of multiple mycotoxin occurrences in soy animal feed by traditional mycological identification combined with molecular species identification. Toxicol. Rep. 2015, 2, 275–279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ivić, D.; Domijan, A.-M.; Peraica, M.; Milicević, T.; Cvjetković, B. Fusarium spp. contamination of wheat, maize, soybean, and pea grain in Croatia. Arh. Hig. Rada Toksikol. 2009, 60, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Chiotta, M.L.; Alaniz Zanon, M.S.; Palazzini, J.M.; Alberione, E.; Barros, G.G.; Chulze, S.N. Fusarium graminearum species complex occurrence on soybean and F. graminearum sensu stricto inoculum maintenance on residues in soybean-wheat rotation under field conditions. J. Appl. Microbiol. 2021, 130, 208–216. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Fate of Fusarium mycotoxins during processing of Nigerian traditional infant foods (ogi and soybean powder). Food Res. Int. 2019, 116, 408–418. [Google Scholar] [CrossRef]
- Naeem, M.; Li, H.; Yan, L.; Raza, M.A.; Gong, G.; Chen, H.; Yang, C.; Zhang, M.; Shang, J.; Liu, T.; et al. Characterization and pathogenicity of Fusarium species associated with soybean pods in maize/soybean strip intercropping. Pathogen 2019, 8, 245. [Google Scholar] [CrossRef]
- Lu, D.Y.; Qiu, D.J.; Wang, M.S.; Xu, P.J.; Ma, P.G.; Shi, D.J.; Bao, P.Z. Species diversity and toxigenic potential of Fusarium incarnatum-equiseti species complex isolates from rice and soybean in China. Plant Dis. 2021, 105, 9. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Beyer, M.; Pasquali, M.; Jenny, E.; Musa, T.; Bucheli, T.D.; Wettstein, F.E.; Forrer, H.R. An eight-year survey of wheat shows distinctive effects of cropping factors on different Fusarium species and associated mycotoxins. Eur. J. Agron. 2019, 105, 62–77. [Google Scholar] [CrossRef]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Badeck, F.W.; Lattanzio, V.M.T.; Pascale, M.; Terzi, V. Occurrence of Fusarium langsethiae and T-2 and HT-2 Toxins in Italian Malting Barley. Toxins 2016, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Castañares, E.; Martínez, M.; Cristos, D.; Rojas, D.; Lara, B.; Stenglein, S.; Dinolfo, M.I. Fusarium species and mycotoxin contamination in maize in Buenos Aires province, Argentina. Eur. J. Plant Pathol. 2019, 155, 1265–1275. [Google Scholar] [CrossRef]
- Saccardo, P.A. Supplementum Universale, Pars. III. Sylloge Fung. 1895, 11, 1–753. [Google Scholar]
- Kristensen, R.; Torp, M.; Kosiak, B.; Holst-Jensen, A. Phylogeny and toxigenic potential is correlated in Fusarium species as revealed by partial translation elongation factor 1 alpha gene sequences. Mycol. Res. 2005, 109, 173–186. [Google Scholar] [CrossRef]
- Stielow, J.; Lévesque, C.; Seifert, K.; Meyer, W.; Irinyi, L.; Smits, D.; Renfurm, R.; Verkley, G.; Groenewald, M.; Chaduli, D.; et al. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 2015, 35, 242–263. [Google Scholar] [CrossRef] [PubMed]
- Geiser, D.M.; Al-Hatmi, A.M.S.; Aoki, T.; Arie, T.; Balmas, V.; Barnes, I.; Bergstrom, G.C.; Bhattacharyya, M.K.; Blomquist, C.L.; Bowden, R.L.; et al. Phylogenomic analysis of a 55.1-kb 19-gene dataset resolves a monophyletic Fusarium that includes the Fusarium solani Species Complex. Phytopathology 2021, 111, 7–1064. [Google Scholar] [CrossRef]
- Xia, J.W.; Sandoval-Denis, M.; Crous, P.W.; Zhang, X.G.; Lombard, L. Numbers to names–restyling the Fusarium incarnatum-equiseti species complex. Pers. Mol. Phylogeny Evol. Fungi 2019, 43, 186. [Google Scholar] [CrossRef]
- Kulik, T.; Abarenkov, K.; Buśko, M.; Bilska, K.; Diepeningen, A.D. van Ostrowska-Kołodziejczak, A.; Krawczyk, K.; Brankovics, B.; Stenglein, S.; Sawicki, J.; et al. ToxGen: An improved reference database for the identification of type B-trichothecene genotypes in Fusarium. PeerJ 2017, 5, e2992. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Groenewald, J.Z.; Slippers, B.; Wingfield, M.J. Global food and fibre security threatened by current inefficiencies in fungal identification. Philos. Trans. Soc. Biol. Sci. 2016, 371, 20160024. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.F.; Hwang, S.F.; Conner, R.L.; Ahmed, H.U.; Zhou, Q.; Fu, H.; Turnbull, G.D.; Nyandoro, R.; Strelkov, S.E.; McLaren, D.L.; et al. Effects of Fusarium avenaceum and Rhizoctonia solani on the growth of soybean in saline soils. Can. J. Plant. 2018, 99, 128–137. [Google Scholar] [CrossRef]
- Żelechowski, M.; Olszewski, J.; Kulik, T. A preliminary survey of cultured Fusaria from symptomatic legume grains in north-eastern Poland. Toxins 2019, 11, 569. [Google Scholar] [CrossRef]
- Stèpień, L.; Jestoi, M.; Chełkowski, J. Cyclic hexadepsipeptides in wheat field samples and esyn1 gene divergence among enniatin producing Fusarium avenaceum strains. World Mycotoxin. J. 2013, 6, 399–409. [Google Scholar] [CrossRef]
- Bilska, K.; Jurczak, S.; Kulik, T.; Ropelewska, E.; Olszewski, J.; Żelechowski, M.; Zapotoczny, P. Species composition and trichothecene genotype profiling of Fusarium field isolates recovered from wheat in Poland. Toxins 2018, 10, 325. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Gueidan, C.; Crous, P.W.; Geiser, D.M. Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum-F. equiseti and F. chlamydosporum species complexes within the United States. J. Clin. Microbiol. 2009, 47, 3851–3861. [Google Scholar] [CrossRef]
- O’Donnell, K.; Rooney, A.P.; Proctor, R.H.; Brown, D.W.; McCormick, S.P.; Ward, T.J.; Frandsen, R.J.N.; Lysøe, E.; Rehner, S.A.; Aoki, T.; et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013, 52, 20–31. [Google Scholar] [CrossRef]
- Wang, M.M.; Chen, Q.; Diao, Y.Z.; Duan, W.J.; Cai, L. Fusarium incarnatum-equiseti complex from China. Persoonia 2019, 43, 70–89. [Google Scholar] [CrossRef]
- Hartman, G.L.; McCormick, S.P.; O’Donnell, K. Trichothecene-producing Fusarium species isolated from soybean roots in Ethiopia and Ghana and their pathogenicity on soybean. Plant Dis. 2019, 103, 2070–2075. [Google Scholar] [CrossRef]
- Maryani, N.; Sandoval-Denis, M.; Lombard, L.; Crous, P.W.; Kema, G.H.J. New endemic Fusarium species hitch-hiking with pathogenic Fusarium strains causing Panama disease in small-holder banana plots in Indonesia. Persoonia 2019, 43, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Moretti, A.; De Saeger, S.; Han, Z.; Di Mavungu, J.D.; Soares, C.M.G.; Proctor, R.H.; Venâncio, A.; Lima, N.; Stea, G.; et al. A polyphasic approach for characterization of a collection of cereal isolates of the Fusarium incarnatum-equiseti species complex. Int. J. Food Microbiol. 2016, 234, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.E.; Proctor, R.H. Molecular biology of Fusarium mycotoxins. Int. J. Food Microbiol. 2007, 119, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Tralamazza, S.M.; Rocha, L.O.; Oggenfuss, U.; Corrêa, B.; Croll, D.; Rose, L. Complex evolutionary origins of specialized metabolite gene cluster diversity among the plant pathogenic fungi of the Fusarium graminearum species complex. Genome Biol. Evol. 2019, 11, 3106–3122. [Google Scholar] [CrossRef] [PubMed]
- Jestoi, M.J.; Rokka, M.; Yli-Mattila, T.; Parikka, P.; Rizzo, A.; Peltonen, K. Presence and concentrations of the Fusarium-related mycotoxins beauvericin, enniatins and moniliformin in finnish grain samples. Food Addit. Contam. 2007, 21, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Barros, G.; Zanon, M.S.A.; Palazzini, J.M.; Haidukowski, M.; Pascale, M.; Chulze, S. Trichothecenes and zearalenone production by Fusarium equiseti and Fusarium semitectum species isolated from Argentinean soybean. Food Addit. Contam. Part Chem. Anal. Control. Expo. Risk Assess. 2012, 29, 1436–1442. [Google Scholar] [CrossRef]
- Liu, C.M.; Kachur, S.; Dwan, M.G.; Abraham, A.G.; Aziz, M.; Hsueh, P.-R.; Huang, Y.-T.; Busch, J.D.; Lamit, L.J.; Gehring, C.A.; et al. FungiQuant: A broad-coverage fungal quantitative real-time PCR assay. BMC Microbiol. 2012, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Waalwijk, C.; van der Heide, R.; de Vries, I.; van der Lee, T.; Schoen, C.; Costrel-de Corainville, G.; Häuser-Hahn, I.; Kastelein, P.; Köhl, J.; Lonnet, P.; et al. Quantitative detection of Fusarium species in wheat using TaqMan. Eur. J. Plant Pathol. 2004, 110, 481–494. [Google Scholar] [CrossRef]
- Bilska, K.; Kulik, T.; Ostrowska-Kołodziejczak, A.; Buśko, M.; Pasquali, M.; Beyer, M.; Baturo-Cieśniewska, A.; Juda, M.; Załuski, D.; Treder, K.; et al. Development of a highly sensitive FcMito qPCR assay for the quantification of the toxigenic fungal plant pathogen Fusarium culmorum. Toxins 2018, 10, 211. [Google Scholar] [CrossRef]
- Nicolaisen, M.; Suproniene, S.; Nielsen, L.K.; Lazzaro, I.; Spliid, N.H.; Justesen, A.F. Real-time PCR for quantification of eleven individual Fusarium species in cereals. J. Microbiol. Methods 2009, 76, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Kulik, T.; Ostrowska, A.; Buśko, M.; Pasquali, M.; Beyer, M.; Stenglein, S.; Załuski, D.; Sawicki, J.; Treder, K.; Perkowski, J. Development of an FgMito assay: A highly sensitive mitochondrial based qPCR assay for quantification of Fusarium graminearum sensu stricto. Int. J. Food Microbiol. 2015, 210, 16–23. [Google Scholar] [CrossRef]
- Kulik, T.; Jestoi, M.; Okorski, A. Development of TaqMan assays for the quantitative detection of Fusarium avenaceum/Fusarium tricinctum and Fusarium poae esyn1 genotypes from cereal grain. FEMS Microbiol. Lett. 2011, 314, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 August 2021).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.A.; Korobeynikov, A.; Lapidus, A.; Prjibelski, A.D.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J. Comput. Biol. 2013, 20, 714–737. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinf. Appl. Note 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).