Effect of Botulinum Toxin Injection and Extracorporeal Shock Wave Therapy on Nerve Regeneration in Rats with Experimentally Induced Sciatic Nerve Injury
Abstract
1. Introduction
2. Results
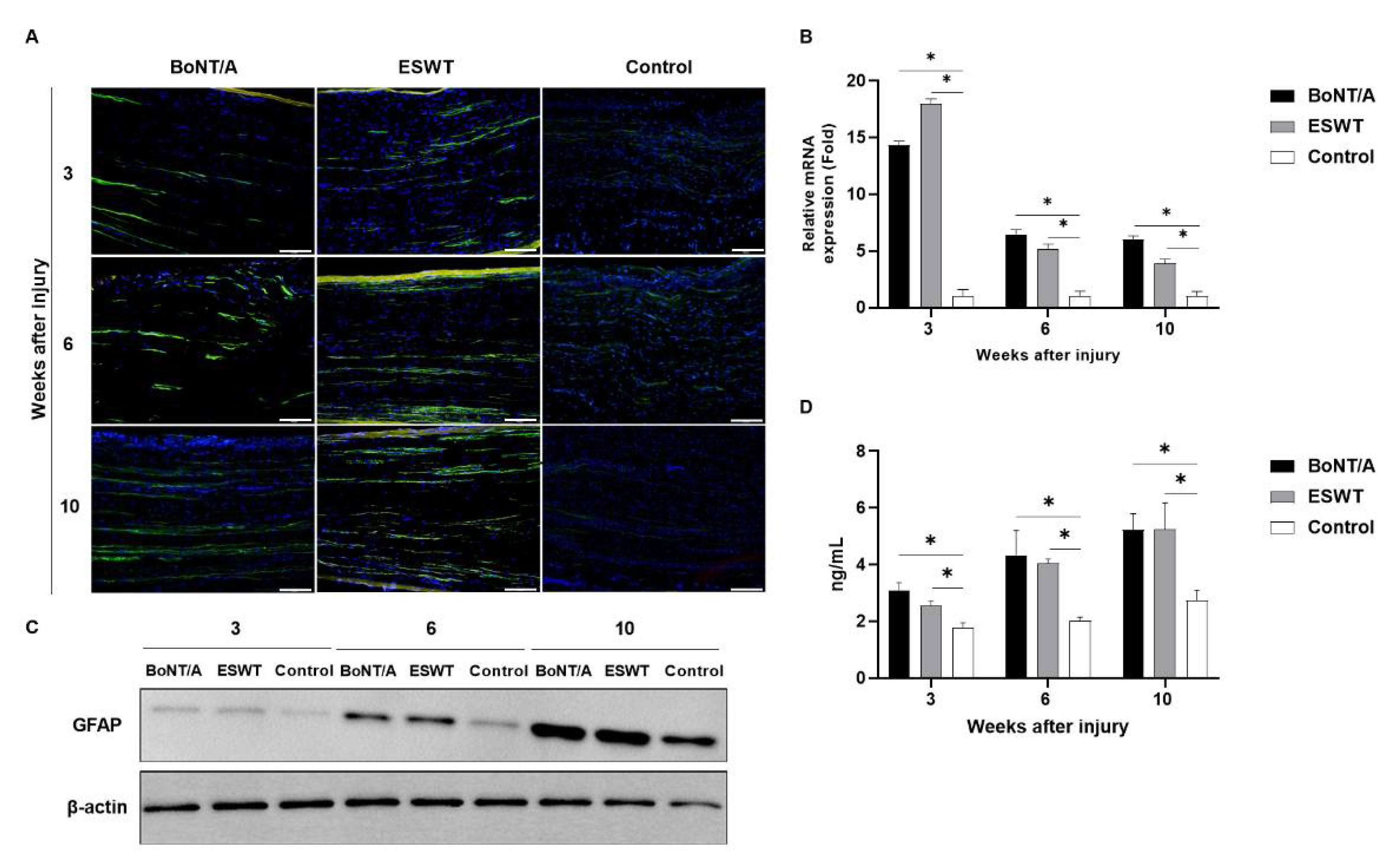
2.1. Effect of BoNT/A and ESWT on Schwann Cell Activity of Injured Sciatic Nerve (GFAP, S100β)
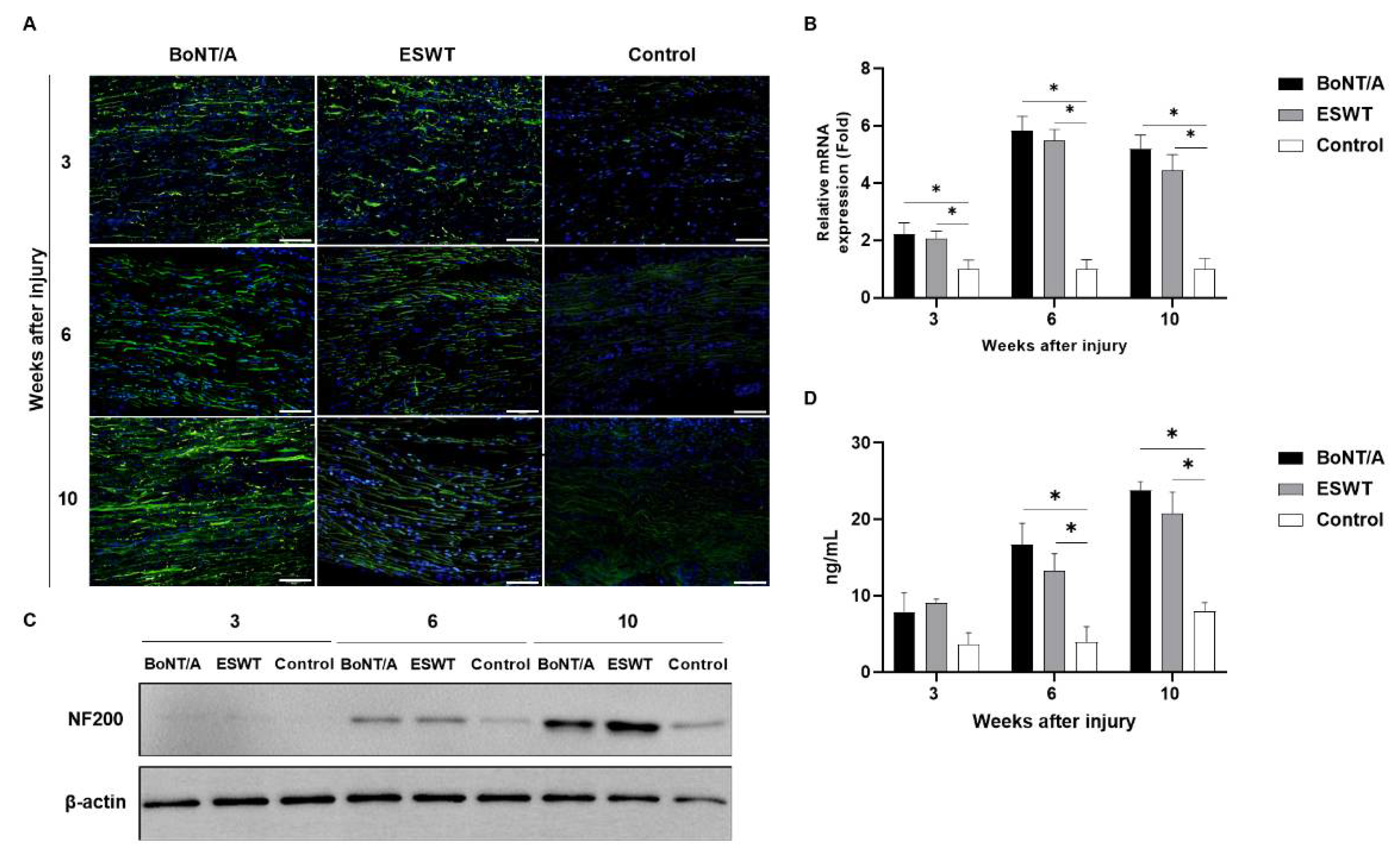
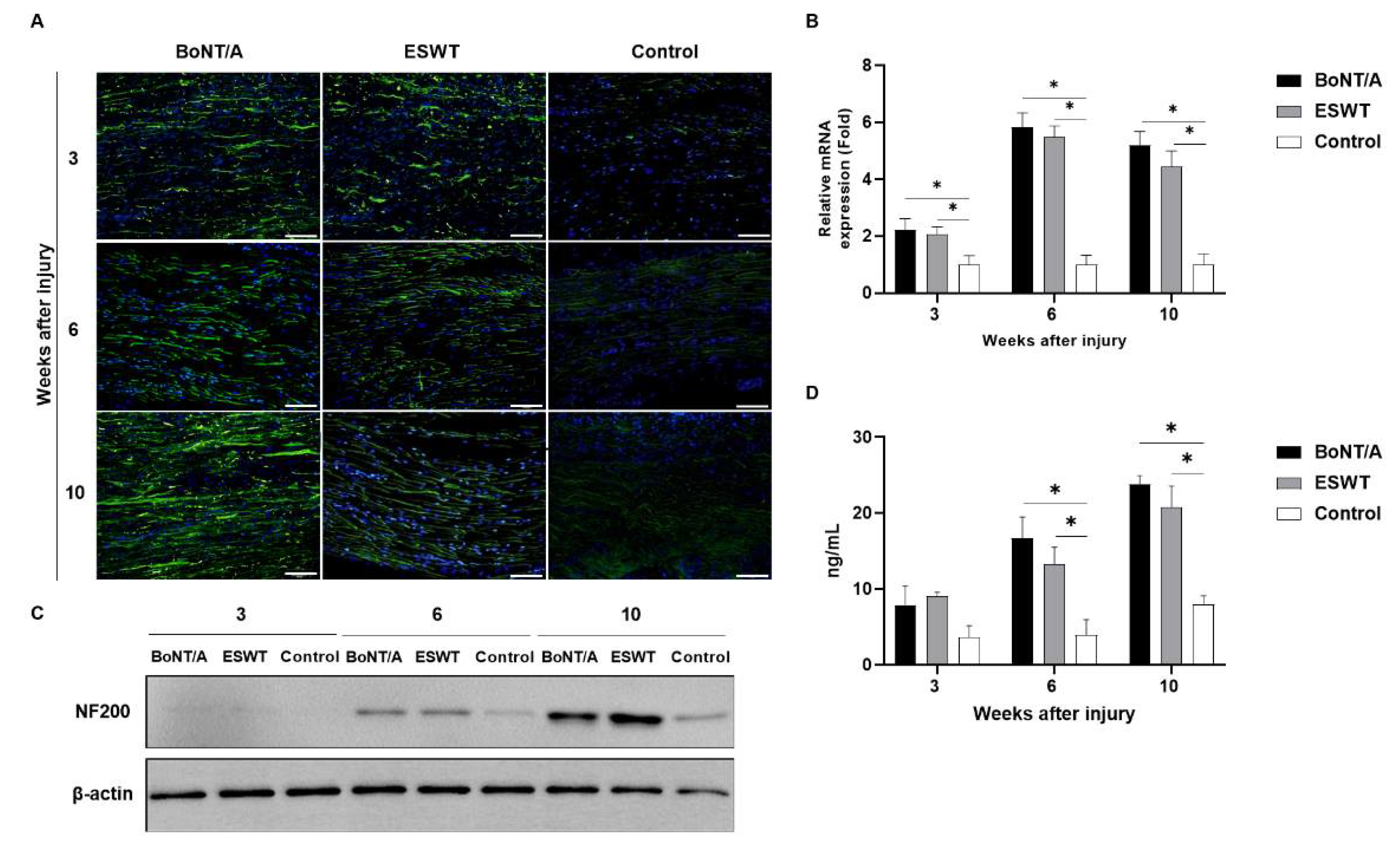
2.2. Effect of BoNT/A and ESWT on Axon Regeneration of Injured Sciatic Nerve (GAP43, ATF3, NF200)
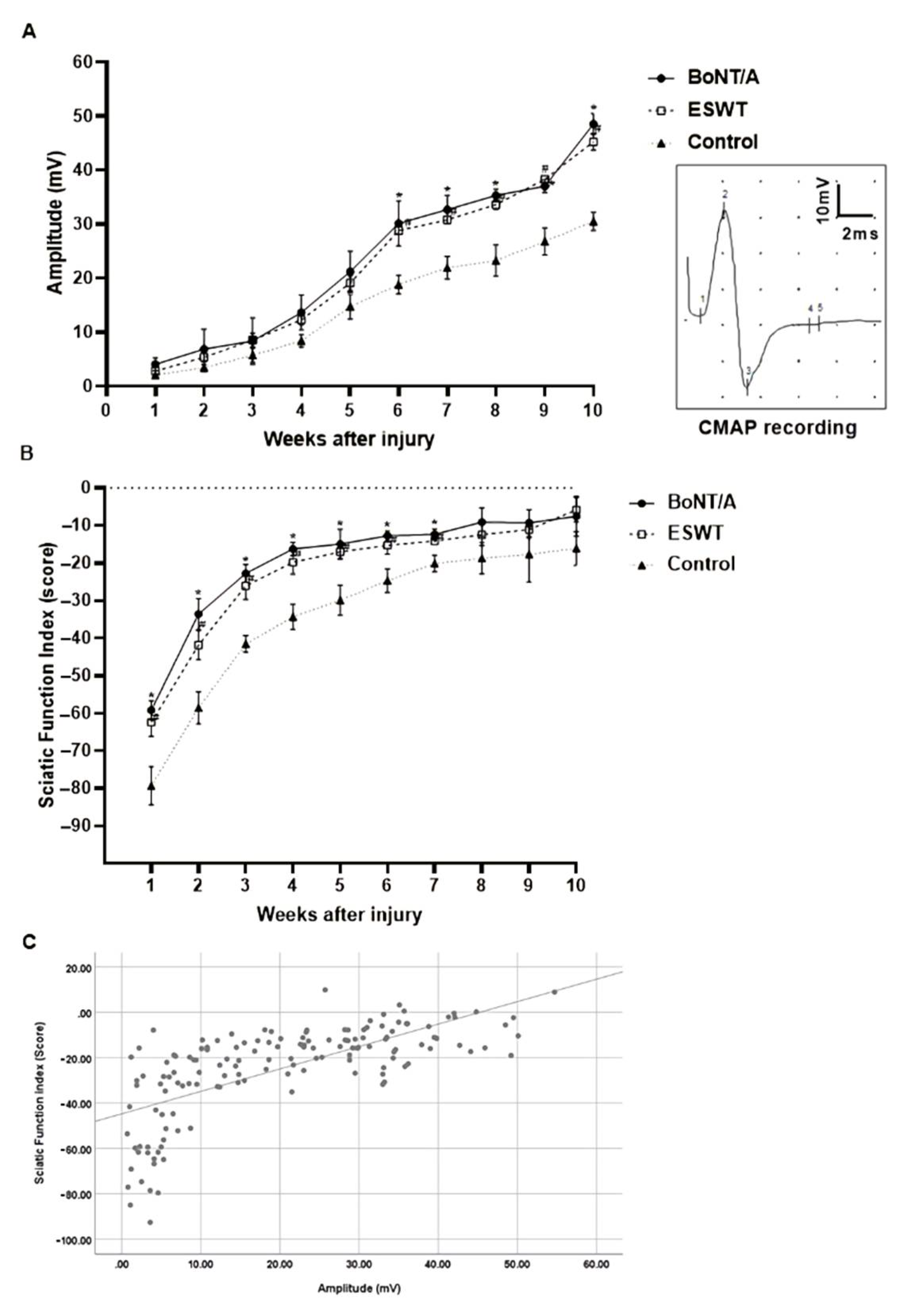
2.3. Effects of BoNT/A and ESWT on Functional Recovery after Sciatic Nerve Injury
2.3.1. Compound Muscle Action Potential (CMAP) Amplitude
2.3.2. Sciatic Function Index
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Surgical Procedure
5.2. Experimental Groups
5.2.1. Pharmacological Treatment
5.2.2. Extracorporeal Shock Wave Therapy
5.3. Neurophysiology Test
5.4. Behavioral Tests
5.5. Immunostaining of Sciatic Nerve
5.6. ELISA
5.7. Real-Time PCR
5.8. Western Blot
5.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATF3 | Activating transcription factor 3 |
| BoNT/A | Botulinum neurotoxin A |
| CI | Confidence intervals |
| CMAP | Compound muscle action potential |
| ESWT | Extracorporeal shock wave therapy |
| GAP43 | Growth associated protein 43 |
| GFAP | Glial fibrillary acid protein |
| MD | Mean difference |
| NF200 | Neurofilament 200 |
| SMD | Standardized mean difference |
References
- Novak, C.B.; Anastakis, D.J.; Beaton, D.E.; Katz, J. Patient-reported outcome after peripheral nerve injury. J. Hand Surg. Am. 2009, 34, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Kouyoumdjian, J.A. Peripheral nerve injuries: A retrospective survey of 456 cases. Muscle Nerve 2006, 34, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Odorico, S.K.; Shulzhenko, N.O.; Zeng, W.; Dingle, A.M.; Francis, D.O.; Poore, S.O. Effect of nimodipine and botulinum toxin A on peripheral nerve regeneration in rats: A pilot study. J. Surg. Res. 2021, 264, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Terzis, J.K.; Sun, D.D.; Thanos, P.K. Historical and basic science review: Past, present, and future of nerve repair. J. Reconstr. Microsurg. 1997, 13, 215–225. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, S.H. Effect of extracorporeal shock wave therapy on denervation atrophy and function caused by sciatic nerve injury. J. Phys. Ther. Sci. 2013, 25, 1067–1069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luvisetto, S. Botulinum toxin and neuronal regeneration after traumatic Injury of central and peripheral nervous system. Toxins 2020, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Cobianchi, S.; Jaramillo, J.; Luvisetto, S.; Pavone, F.; Navarro, X. Botulinum neurotoxin A promotes functional recovery after peripheral nerve injury by increasing regeneration of myelinated fibers. Neuroscience 2017, 359, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J. Extracorporeal shockwave therapy in musculoskeletal disorders. J. Orthop. Surg. Res. 2012, 7, 11. [Google Scholar] [CrossRef]
- Fiani, B.; Davati, C.; Griepp, D.W.; Lee, J.; Pennington, E.; Moawad, C.M. Enhanced spinal therapy: Extracorporeal shock wave therapy for the spine. Cureus 2020, 12, e11200. [Google Scholar] [CrossRef]
- Yang, E.; Lew, H.L.; Özçakar, L.; Wu, C.-H. Recent advances in the treatment of spasticity: Extracorporeal shock wave therapy. J. Clin. Med. 2021, 10, 4723. [Google Scholar] [CrossRef]
- de Oliveira, P.S.; Ziegelmann, M.J. Low-intensity shock wave therapy for the treatment of vasculogenic erectile dysfunction: A narrative review of technical considerations and treatment outcomes. Transl. Androl. Urol. 2021, 10, 2617. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yan, P.; Xiong, H.; Shuai, T.; Liu, J.; Zhu, L.; Lu, J.; Shi, X.; Yang, K.; Liu, J. Extracorporeal shock wave therapy for treating foot ulcers in adults with type 1 and type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Can. J. Diabetes 2020, 44, 196–204.e193. [Google Scholar] [CrossRef]
- Li, H.; Liu, M. Cardiac shock wave therapy: An alternative non-invasive therapy for refractory angina. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5402–5410. [Google Scholar] [PubMed]
- Aicher, A.; Heeschen, C.; Sasaki, K.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: A new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation 2006, 114, 2823–2830. [Google Scholar] [CrossRef] [PubMed]
- Murata, R.; Ohtori, S.; Ochiai, N.; Takahashi, N.; Saisu, T.; Moriya, H.; Takahashi, K.; Wada, Y. Extracorporeal shockwaves induce the expression of ATF3 and GAP-43 in rat dorsal root ganglion neurons. Auton Neurosci. 2006, 128, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Romeo, P.; d’Agostino, M.C.; Lazzerini, A.; Sansone, V.C. Extracorporeal shock wave therapy in pillar pain after carpal tunnel release: A preliminary study. Ultrasound Med. Biol. 2011, 37, 1603–1608. [Google Scholar] [CrossRef]
- Navarro, X.; Udina, E. Methods and protocols in peripheral nerve regeneration experimental research: Part III—Electrophysiological evaluation. Int. Rev. Neurobiol. 2009, 87, 105–126. [Google Scholar]
- Marinelli, S.; Luvisetto, S.; Cobianchi, S.; Makuch, W.; Obara, I.; Mezzaroma, E.; Caruso, M.; Straface, E.; Przewlocka, B.; Pavone, F. Botulinum neurotoxin type A counteracts neuropathic pain and facilitates functional recovery after peripheral nerve injury in animal models. Neuroscience 2010, 171, 316–328. [Google Scholar] [CrossRef]
- Baptista, A.F.; Gomes, J.R.; Oliveira, J.T.; Santos, S.M.; Vannier-Santos, M.A.; Martinez, A.M. A new approach to assess function after sciatic nerve lesion in the mouse—Adaptation of the sciatic static index. J. Neurosci. Methods 2007, 161, 259–264. [Google Scholar] [CrossRef]
- Cobianchi, S.; Marinelli, S.; Florenzano, F.; Pavone, F.; Luvisetto, S. Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience 2010, 168, 273–287. [Google Scholar] [CrossRef]
- Nakazato-Imasato, E.; Kurebayashi, Y. Pharmacological characteristics of the hind paw weight bearing difference induced by chronic constriction injury of the sciatic nerve in rats. Life Sci. 2009, 84, 622–626. [Google Scholar] [CrossRef]
- Gupta, R.; Rummler, L.S.; Palispis, W.; Truong, L.; Chao, T.; Rowshan, K.; Mozaffar, T.; Steward, O. Local down-regulation of myelin-associated glycoprotein permits axonal sprouting with chronic nerve compression injury. Exp. Neurol. 2006, 200, 418–429. [Google Scholar] [CrossRef] [PubMed]
- de la Hoz, C.L.; Oliveira, A.L.; Queiroz Lde, S.; Langone, F. Wallerian degeneration in C57BL/6J and A/J mice: Differences in time course of neurofilament and myelin breakdown, macrophage recruitment and iNOS expression. J. Anat. 2003, 203, 567–578. [Google Scholar] [CrossRef]
- Bhatheja, K.; Field, J. Schwann cells: Origins and role in axonal maintenance and regeneration. Int. J. Biochem. Cell Biol. 2006, 38, 1995–1999. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Koob, J.W.; Liu, D.Z.; Tong, A.Y.; Hunter, D.A.; Parsadanian, A.; Mackinnon, S.E.; Myckatyn, T.M. A double-transgenic mouse used to track migrating schwann cells and regenerating axons following engraftment of injured nerves. Exp. Neurol. 2007, 207, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Triolo, D.; Dina, G.; Lorenzetti, I.; Malaguti, M.; Morana, P.; Del Carro, U.; Comi, G.; Messing, A.; Quattrini, A.; Previtali, S.C. Loss of glial fibrillary acidic protein (GFAP) impairs schwann cell proliferation and delays nerve regeneration after damage. J. Cell Sci. 2006, 119, 3981–3993. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, S.; Vacca, V.; Ricordy, R.; Uggenti, C.; Tata, A.M.; Luvisetto, S.; Pavone, F. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves schwann cells and astrocytes. PLoS ONE 2012, 7, e47977. [Google Scholar] [CrossRef]
- Luvisetto, S.; Marinelli, S.; Cobianchi, S.; Pavone, F. Anti-allodynic efficacy of botulinum neurotoxin A in a model of neuropathic pain. Neuroscience 2007, 145, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Djedovic, G.; Kamelger, F.S.; Jeschke, J.; Piza-Katzer, H. Effect of extracorporeal shock wave treatment on deep partial-thickness burn injury in rats: A pilot study. Plast Surg. Int. 2014, 2014, 495967. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Fukumoto, Y.; Shimokawa, H. Extracorporeal shock wave therapy as a new and non-invasive angiogenic strategy. Tohoku J. Exp. Med. 2009, 219, 1–9. [Google Scholar] [CrossRef]
- Mittermayr, R.; Antonic, V.; Hartinger, J.; Kaufmann, H.; Redl, H.; Teot, L.; Stojadinovic, A.; Schaden, W. Extracorporeal shock wave therapy (ESWT) for wound healing: Technology, mechanisms, and clinical efficacy. Wound Repair Regen. 2012, 20, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, Y.; Jiang, Z.; Han, L. Effect of low-energy extracorporeal shock wave on vascular regeneration after spinal cord injury and the recovery of motor function. Neuropsychiatr. Dis. Treat. 2016, 12, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Foldager, C.B.; Kearney, C.; Spector, M. Clinical application of extracorporeal shock wave therapy in orthopedics: Focused versus unfocused shock waves. Ultrasound Med. Biol. 2012, 38, 1673–1680. [Google Scholar] [CrossRef]
- Cifu, D.X. Braddom’s Physical Medicine and Rehabilitation, 5 ed.; Elsevier: Philadelphia, PA, USA, 2015. [Google Scholar]
- Lee, J.H.; Kim, S.G. Effects of extracorporeal shock wave therapy on functional recovery and neurotrophin-3 expression in the spinal cord after crushed sciatic nerve injury in rats. Ultrasound Med. Biol. 2015, 41, 790–796. [Google Scholar] [CrossRef]
- Davis, T.A.; Stojadinovic, A.; Anam, K.; Amare, M.; Naik, S.; Peoples, G.E.; Tadaki, D.; Elster, E.A. Extracorporeal shock wave therapy suppresses the early proinflammatory immune response to a severe cutaneous burn injury. Int. Wound J. 2009, 6, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hausner, T.; Pajer, K.; Halat, G.; Hopf, R.; Schmidhammer, R.; Redl, H.; Nogradi, A. Improved rate of peripheral nerve regeneration induced by extracorporeal shock wave treatment in the rat. Exp. Neurol. 2012, 236, 363–370. [Google Scholar] [CrossRef]
- Sagir, D.; Bereket, C.; Onger, M.E.; Bakhit, N.; Keskin, M.; Ozkan, E. Efficacy of extracorporeal shockwaves therapy on peripheral nerve regeneration. J. Craniofac. Surg. 2019, 30, 2635–2639. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zeng, B.; Chai, Y.; Luo, C.; Li, X. Improvement of blood flow, expression of nitric oxide, and vascular endothelial growth factor by low-energy shockwave therapy in random-pattern skin flap model. Ann. Plast Surg. 2008, 61, 646–653. [Google Scholar] [CrossRef]
- Hausdorf, J.; Sievers, B.; Schmitt-Sody, M.; Jansson, V.; Maier, M.; Mayer-Wagner, S. Stimulation of bone growth factor synthesis in human osteoblasts and fibroblasts after extracorporeal shock wave application. Arch. Orthop. Trauma Surg. 2011, 131, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.M.; Hercher, D.; Stainer, M.; Hopf, R.; Teuschl, A.H.; Schmidhammer, R.; Redl, H. Extracorporeal shockwave treatment: A novel tool to improve schwann cell isolation and culture. Cytotherapy 2016, 18, 760–770. [Google Scholar] [CrossRef]
- Dubovy, P. Wallerian degeneration and peripheral nerve conditions for both axonal regeneration and neuropathic pain induction. Ann. Anat. 2011, 193, 267–275. [Google Scholar] [CrossRef]
- Koussounadis, A.; Langdon, S.P.; Um, I.H.; Harrison, D.J.; Smith, V.A. Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci. Rep. 2015, 5, 10775. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ito, A.; Aoyama, T.; Nakahara, R.; Nakahata, A.; Ji, X.; Zhang, J.; Kawai, H.; Kuroki, H. Functional evaluation outcomes correlate with histomorphometric changes in the rat sciatic nerve crush injury model: A comparison between sciatic functional index and kinematic analysis. PLoS ONE 2018, 13, e0208985. [Google Scholar] [CrossRef]
- Lu, L.; Atchabahian, A.; Mackinnon, S.E.; Hunter, D.A. Nerve injection injury with botulinum toxin. Plast Reconstr. Surg. 1998, 101, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Hogan, Q.H. Pathophysiology of peripheral nerve injury during regional anesthesia. Reg. Anesth. Pain Med. 2008, 33, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Friessem, C.H.; Eitner, L.B.; Kaisler, M.; Maier, C.; Vollert, J.; Westermann, A.; Zahn, P.K.; Avila Gonzalez, C.A. Perineural injection of botulinum toxin-A in painful peripheral nerve injury—A case series: Pain relief, safety, sensory profile and sample size recommendation. Curr Med. Res. Opin. 2019, 35, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, D.; Lago, N.; Verdu, E.; Penkowa, M.; Carrasco, J.; Navarro, X.; Palmiter, R.D.; Hidalgo, J. Role of metallothioneins in peripheral nerve function and regeneration. Cell Mol. Life Sci. 2003, 60, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Khanijou, S.; Rubino, J.; Aoki, K.R. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain 2004, 107, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.R.; Mackinnon, S.E.; Hunter, D.A. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr. Surg. 1989, 83, 129–138. [Google Scholar] [CrossRef]
- Finocchiaro, A.; Marinelli, S.; De Angelis, F.; Vacca, V.; Luvisetto, S.; Pavone, F. Botulinum toxin B affects neuropathic pain but not functional recovery after peripheral nerve injury in a mouse model. Toxins 2018, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Linda, H.; Sköld, M.K.; Ochsmann, T. Activating transcription factor 3, a useful marker for regenerative response after nerve root injury. Front. Neurol. 2011, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Mahar, M.; Cavalli, V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 2018, 19, 323–337. [Google Scholar] [CrossRef] [PubMed]


| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| GFAP | 5′-AGT GGT ATC GGT CCA AGT TTG C-3′ | 5′-TGG CGG CGA TAG TCA TTA GC-3′ |
| S100β | 5′-GCC CTC ATT GAT GTC TTC C-3′ | 5′-TCC TTT AGT TTC TCG TCC TTC-3′ |
| GAP43 | 5′-AAG AAG GAG GGA GAT GGC TCT-3′ | 5′-GAG GAC GGC GAG TTA TCA GTG-3′ |
| ATF3 | 5′-CCT GCA GAA GGA GTC AGA GAA-3′ | 5′-CGT TCT GAG CCC GGA CGA TA-3′ |
| NF200 | 5′-GGA GGA GAG CCG TCA GGT AGA C-3′ | 5′-TTT CTG TAA TCA GCA GCG ATC TCA AT-3′ |
| GAPDH | 5′-GGC ACA GTC AAG GCT GAG AAT G-3′ | 5′-ATG GTG AAG ACG CCA GTA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.; Lim, D.; Kim, S.; Kim, T.; Kwon, B.S.; Nam, K. Effect of Botulinum Toxin Injection and Extracorporeal Shock Wave Therapy on Nerve Regeneration in Rats with Experimentally Induced Sciatic Nerve Injury. Toxins 2021, 13, 879. https://doi.org/10.3390/toxins13120879
Seo M, Lim D, Kim S, Kim T, Kwon BS, Nam K. Effect of Botulinum Toxin Injection and Extracorporeal Shock Wave Therapy on Nerve Regeneration in Rats with Experimentally Induced Sciatic Nerve Injury. Toxins. 2021; 13(12):879. https://doi.org/10.3390/toxins13120879
Chicago/Turabian StyleSeo, Minsu, Dongin Lim, Shengshu Kim, Taeyeon Kim, Bum Sun Kwon, and Kiyeun Nam. 2021. "Effect of Botulinum Toxin Injection and Extracorporeal Shock Wave Therapy on Nerve Regeneration in Rats with Experimentally Induced Sciatic Nerve Injury" Toxins 13, no. 12: 879. https://doi.org/10.3390/toxins13120879
APA StyleSeo, M., Lim, D., Kim, S., Kim, T., Kwon, B. S., & Nam, K. (2021). Effect of Botulinum Toxin Injection and Extracorporeal Shock Wave Therapy on Nerve Regeneration in Rats with Experimentally Induced Sciatic Nerve Injury. Toxins, 13(12), 879. https://doi.org/10.3390/toxins13120879




