Human Biomonitoring of T-2 Toxin, T-2 Toxin-3-Glucoside and Their Metabolites in Urine through High-Resolution Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
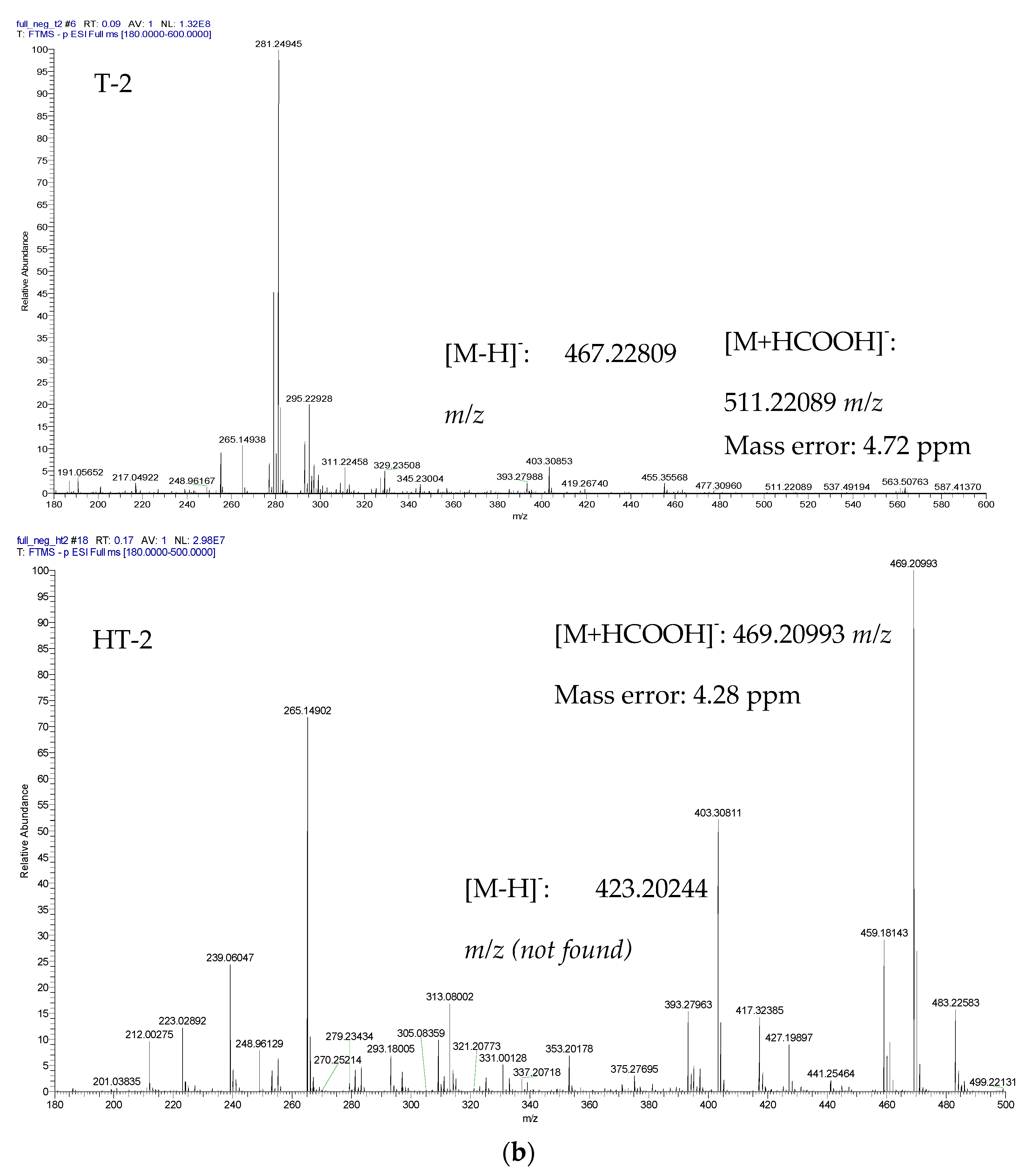
2.1. Optimization of Q-Orbitrap HRMS Parameters
2.2. Method Validation
2.3. Urinary Levels of T-2 and HT-2
2.4. Retrospective Analysis of Urine Samples
3. Conclusions
4. Material and Methods
4.1. Chemicals, Reagents and Materials
4.2. Sampling
4.3. Extraction Proczedure
4.4. UHPLC-Q-Orbitrap HRMS Analysis
4.5. Method Validation
4.6. Quality Control/Quality Assurance
4.7. Creatinine Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senatore, M.T.; Ward, T.J.; Cappelletti, E.; Beccari, G.; McCormick, S.P.; Busman, M.; Laraba, I.; O’Donnell, K.; Prodi, A. Species diversity and mycotoxin production by members of the Fusarium tricinctum species complex associated with Fusarium head blight of wheat and barley in Italy. Int. J. Food Microbiol. 2021, 358, 109298. [Google Scholar] [CrossRef]
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020, 137, 111161. [Google Scholar] [CrossRef]
- Carballo, D.; Tolosa, J.; Ferrer, E.; Berrada, H. Dietary exposure assessment to mycotoxins through total diet studies. A review. Food Chem. Toxicol. 2019, 128, 8–20. [Google Scholar] [CrossRef]
- Narváez, A.; Castaldo, L.; Izzo, L.; Pallarés, N.; Rodríguez-Carrasco, Y.; Ritieni, A. Deoxynivalenol contamination in cereal-based foodstuffs from Spain: Systematic review and meta-analysis approach for exposure assessment. Food Control. 2021, 132, 108521. [Google Scholar] [CrossRef]
- Pérez, R.; Domenech, E.; Coscollà, C.; Yusà, V. Human Biomonitoring of food contaminants in Spanish children: Design, sampling and lessons learned. Int. J. Hyg. Environ. Health 2017, 220, 1242–1251. [Google Scholar] [CrossRef]
- Carballo, D.; Pallarés, N.; Ferrer, E.; Barba, F.J.; Berrada, H. Assessment of Human Exposure to Deoxynivalenol, Ochratoxin A, Zearalenone and Their Metabolites Biomarker in Urine Samples Using LC-ESI-qTOF. Toxins 2021, 13, 530. [Google Scholar] [CrossRef]
- Ali, N.; Degen, G.H. Biomonitoring of zearalenone and its main metabolites in urines of Bangladeshi adults. Food Chem. Toxicol. 2019, 130, 276–283. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Izzo, L.; Gaspari, A.; Graziani, G.; Mañes, J.; Ritieni, A. Urinary levels of enniatin B and its phase I metabolites: First human pilot biomonitoring study. Food Chem. Toxicol. 2018, 118, 454–459. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Narváez, A.; Izzo, L.; Gaspari, A.; Graziani, G.; Ritieni, A. Biomonitoring of Enniatin B1 and Its Phase I Metabolites in Human Urine: First Large-Scale Study. Toxins 2020, 12, 415. [Google Scholar] [CrossRef]
- Wallin, S.; Gambacorta, L.; Kotova, N.; Lemming, E.W.; Nälsén, C.; Solfrizzo, M.; Olsen, M. Biomonitoring of concurrent mycotoxin exposure among adults in Sweden through urinary multi-biomarker analysis. Food Chem. Toxicol. 2015, 83, 133–139. [Google Scholar] [CrossRef]
- Van Der Fels-Klerx, H.J.; Stratakou, I. T-2 toxin and HT-2 toxin in grain and grain-based commodities in Europe: Occurrence, factors affecting occurrence, co-occurrence and toxicological effects. World Mycotoxin J. 2010, 3, 349–367. [Google Scholar] [CrossRef]
- Bouaziz, C.; Abid-Essefi, S.; Bouslimi, A.; El Golli, E.; Bacha, H. Cytotoxicity and related effects of T-2 toxin on cultured Vero cells. Toxicon 2006, 48, 343–352. [Google Scholar] [CrossRef]
- Wan, Q.; Wu, G.; He, Q.; Tang, H.; Wang, Y. The toxicity of acute exposure to T-2 toxin evaluated by the metabonomics technique. Mol. BioSyst. 2015, 11, 882–891. [Google Scholar] [CrossRef]
- Taroncher, M.; Rodríguez-Carrasco, Y.; Ruiz, M.-J. T-2 toxin and its metabolites: Characterization, cytotoxic mechanisms and adaptive cellular response in human hepatocarcinoma (HepG2) cells. Food Chem. Toxicol. 2020, 145, 111654. [Google Scholar] [CrossRef] [PubMed]
- Taroncher, M.; Rodríguez-Carrasco, Y.; Ruiz, M.-J. Interactions between T-2 toxin and its metabolites in HepG2 cells and in silico approach. Food Chem. Toxicol. 2020, 148, 111942. [Google Scholar] [CrossRef]
- Yang, S.; De Boevre, M.; Zhang, H.; De Ruyck, K.; Sun, F.; Zhang, J.; Jin, Y.; Li, Y.; Wang, Z.; Zhang, S.; et al. Metabolism of T-2 Toxin in Farm Animals and Human In Vitro and in Chickens In Vivo Using Ultra High-Performance Liquid Chromatography- Quadrupole/Time-of-Flight Hybrid Mass Spectrometry Along with Online Hydrogen/Deuterium Exchange Technique. J. Agric. Food Chem. 2017, 65, 7217–7227. [Google Scholar] [CrossRef]
- Broekaert, N.; Devreese, M.; De Baere, S.; De Backer, P.; Croubels, S. Modified Fusarium mycotoxins unmasked: From occurrence in cereals to animal and human excretion. Food Chem. Toxicol. 2015, 80, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Bryła, M.; Waśkiewicz, A.; Ksieniewicz-Woźniak, E.; Szymczyk, K.; Jędrzejczak, R. Modified Fusarium Mycotoxins in Cereals and Their Products—Metabolism, Occurrence, and Toxicity: An Updated Review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Van Poucke, C.; Wang, Z.; Zhang, S.; De Saeger, S.; De Boevre, M. Metabolic profile of the masked mycotoxin T-2 toxin-3-glucoside in rats (in vitro and in vivo) and humans (in vitro). World Mycotoxin J. 2017, 10, 349–362. [Google Scholar] [CrossRef]
- Gerding, J.; Cramer, B.; Humpf, H.-U. Determination of mycotoxin exposure in Germany using an LC-MS/MS multibiomarker approach. Mol. Nutr. Food Res. 2014, 58, 2358–2368. [Google Scholar] [CrossRef]
- De Ruyck, K.; Huybrechts, I.; Yang, S.; Arcella, D.; Claeys, L.; Abbeddou, S.; De Keyzer, W.; De Vries, J.; Ocke, M.; Ruprich, J.; et al. Mycotoxin exposure assessments in a multi-center European validation study by 24-hour dietary recall and biological fluid sampling. Environ. Int. 2020, 137, 105539. [Google Scholar] [CrossRef]
- Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. 2002. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32002D0657 (accessed on 2 December 2021).
- Ndaw, S.; Remy, A.; Jargot, D.; Antoine, G.; Denis, F.; Robert, A. Mycotoxins Exposure of French Grain Elevator Workers: Biomonitoring and Airborne Measurements. Toxins 2021, 13, 382. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Campillo, N.; López-García, I.; Hernández-Córdoba, M.; Viñas, P. High-Resolution mass spectrometry for the determination of mycotoxins in biological samples. A review. Microchem. J. 2021, 166, 106197. [Google Scholar] [CrossRef]
- Abia, W.A.; Warth, B.; Sulyok, M.; Krska, R.; Tchana, A.; Njobeh, P.B.; Turner, P.C.; Kouanfack, C.; Eyongetah, M.; Dutton, M.; et al. Bio-monitoring of mycotoxin exposure in Cameroon using a urinary multi-biomarker approach. Food Chem. Toxicol. 2013, 62, 927–934. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Mañes, J.; Berrada, H. Exposure assessment approach through mycotoxin/creatinine ratio evaluation in urine by GC–MS/MS. Food Chem. Toxicol. 2014, 72, 69–75. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Warth, B.; Ogara, I.M.; Abia, W.A.; Ezekiel, V.C.; Atehnkeng, J.; Sulyok, M.; Turner, P.C.; Tayo, G.O.; Krska, R.; et al. Mycotoxin exposure in rural residents in northern Nigeria: A pilot study using multi-urinary biomarkers. Environ. Int. 2014, 66, 138–145. [Google Scholar] [CrossRef]
- Warth, B.; Petchkongkaew, A.; Sulyok, M.; Krska, R. Utilising an LC-MS/MS-based multi-biomarker approach to assess mycotoxin exposure in the Bangkok metropolitan area and surrounding provinces. Food Addit. Contam. Part A 2014, 31, 2040–2046. [Google Scholar] [CrossRef]
- Heyndrickx, E.; Sioen, I.; Huybrechts, B.; Callebaut, A.; De Henauw, S.; De Saeger, S. Human biomonitoring of multiple mycotoxins in the Belgian population: Results of the BIOMYCO study. Environ. Int. 2015, 84, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Gerding, J.; Ali, N.; Schwartzbord, J.; Cramer, B.; Brown, D.L.; Degen, G.H.; Humpf, H.-U. A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany, and Haiti using a rapid and sensitive LC-MS/MS approach. Mycotoxin Res. 2015, 31, 127–136. [Google Scholar] [CrossRef]
- Fan, K.; Xu, J.; Jiang, K.; Liu, X.; Meng, J.; Di Mavungu, J.D.; Guo, W.; Zhang, Z.; Jing, J.; Li, H.; et al. Determination of multiple mycotoxins in paired plasma and urine samples to assess human exposure in Nanjing, China. Environ. Pollut. 2019, 248, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.W.; Currie, V.; Duncan, G.; Jackson, D. Multimycotoxin Exposure Assessment in UK Children Using Urinary Biomarkers—A Pilot Survey. J. Agric. Food Chem. 2019, 68, 351–357. [Google Scholar] [CrossRef]
- Niknejad, F.; Escrivá, L.; Rad, K.A.; Khoshnia, M.; Barba, F.J.; Berrada, H. Biomonitoring of Multiple Mycotoxins in Urine by GC–MS/MS: A Pilot Study on Patients with Esophageal Cancer in Golestan Province, Northeastern Iran. Toxins 2021, 13, 243. [Google Scholar] [CrossRef]
- Duringer, J.; Mazumder, R.; Palmer, V.; Craig, A.M.; Spencer, P. Case-Control Study of Nodding Syndrome in Acholiland: Urinary Multi-Mycotoxin Screening. Toxins 2021, 13, 313. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Leggieri, M.C.; Battilani, P.; Pietri, A. Co-occurrence of type A and B trichothecenes and zearalenone in wheat grown in northern Italy over the years 2009–2011. Food Addit. Contam. Part B 2014, 7, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrasco, Y.; Molto, J.C.; Berrada, H.; Mañes, J. A survey of trichothecenes, zearalenone and patulin in milled grain-based products using GC–MS/MS. Food Chem. 2014, 146, 212–219. [Google Scholar] [CrossRef]
- FAOSTAT; FAO. Food Balance Sheets. 2009. Available online: https://www.fao.org/faostat/en/#data/FBS (accessed on 17 October 2021).
- Schelstraete, W.; Devreese, M.; Croubels, S. Comparative toxicokinetics of Fusarium mycotoxins in pigs and humans. Food Chem. Toxicol. 2020, 137, 111140. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- EFSA. Human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, e04972. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Cao, X.; Hu, D.; Wang, Z.; Wang, Y.; Shen, J.; Zhang, S. Metabolic Pathways of T-2 Toxin in in Vivo and in Vitro Systems of Wistar Rats. J. Agric. Food Chem. 2013, 61, 9734–9743. [Google Scholar] [CrossRef]
- Knupp, C.A.; Corley, D.G.; Tempesta, M.S.; Swanson, S.P. Isolation and characterization of 4’-hydroxy T-2 toxin, a new metabolite of the trichothecene mycotoxin T-2. Drug Metab Dispos 1987, 15, 816. [Google Scholar]
- Wu, Q.; Dohnal, V.; Kuca, K.; Yuan, Z. Trichothecenes: Structure-Toxic Activity Relationships. Curr. Drug Metab. 2013, 14, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Qin, Z.; Kuca, K.; You, L.; Zhao, Y.; Liu, A.; Musilek, K.; Chrienova, Z.; Nepovimova, E.; Oleksak, P.; et al. An update on T-2 toxin and its modified forms: Metabolism, immunotoxicity mechanism, and human exposure assessment. Arch. Toxicol. 2020, 94, 3645–3669. [Google Scholar] [CrossRef] [PubMed]


| Analyte | Retention Time (min) | Molecular Formula | Adduct Ion | Exact Mass (m/z) | Observed Mass (m/z) | Mass Error (ppm) |
|---|---|---|---|---|---|---|
| Parent mycotoxin | ||||||
| T-2 | 4.82 | C24H34O9 | [M + NH4]+ | 484.25411 | 484.25504 | 1.92 |
| Phase I metabolites—Hydrolyzed group | ||||||
| 4-deAc-NEO a | - | C17H24O7 | [M + NH4]+ | 358.18602 | - | - |
| [M + H]+ | 341.15950 | - | - | |||
| HT-2 | 4.79 | C22H32O8 | [M + NH4]+ | 442.24354 | 442.24390 | 0.81 |
| NEO | 4.25 | C19H26O8 | [M + NH4]+ | 400.19669 | 400.19659 | −0.25 |
| Phase I metabolites—Hydroxylated group | ||||||
| T-2 triol | 4.7 | C20H30O7 | [M + H]+ | 383.20642 | 383.20662 | 0.52 |
| 3′-OH-T-2 | 4.78 | C24H34O10 | [M + NH4]+ | 500.24902 | 500.24926 | 0.48 |
| Phase II metabolites—Conjugated group | ||||||
| T-2-3-GlcA | 4.69 | C30H42O15 | [M + H]+ | 643.25964 | 643.25974 | 0.16 |
| HT-2-3-GlcA | 4.67 | C28H40O14 | [M + H]+ | 601.24908 | 601.24998 | 1.5 |
| HT-2-4-GlcA | 4.39 | C28H40O14 | [M + H]+ | 601.24908 | 601.24998 | 1.5 |
| Parent mycotoxin | ||||||
| T-2-3-Glc | 4.38 | C30H44O14 | [M + NH4]+ | 646.30693 | 646.30793 | 1.55 |
| Phase I metabolites—Hydrolyzed group | ||||||
| HT-2-3-Glc | 4.59 | C28H42O13 | [M + NH4]+ | 604.29636 | 604.29616 | −0.33 |
| NEO-3-Glc | 3.85 | C25H36O13 | [M + NH4]+ | 562.24941 | 562.24897 | −0.78 |
| Phase I metabolites—Hydroxylated group | ||||||
| 3′-OH-T-2-3-Glc | 3.91 | C30H44O15 | [M + NH4]+ | 662.30184 | 662.30121 | −0.95 |
| 4′-OH-T-2-3-Glc | 4.04 | C30H44O15 | [M + NH4]+ | 662.30184 | 662.30121 | −0.95 |
| Analyte | Linearity (r2) | SSE (%) | Recovery (%) | Precision (%) [RSDr, (RSDR)] | |||||
|---|---|---|---|---|---|---|---|---|---|
| 5 ng/mL | 1 ng/mL | 0.5 ng/mL | 5 ng/mL | 1 ng/mL | 0.5 ng/mL | LOQ (ng/mL) | |||
| HT-2 | 0.9901 | 102 | 116 | 86 | 85 | 11 (9) | 5 (6) | 12 (17) | 0.4 |
| T-2 | 0.9944 | 98 | 116 | 87 | 79 | 8 (7) | 11 (17) | 19 (14) | 0.2 |
| Sample Treatment | Analytical Method | LOQ a (ng/mL) | Reference | |
|---|---|---|---|---|
| T-2 | HT-2 | |||
| Dilute and shoot | UHPLC-Q-TRAP-MS/MS | 0.5 | 4 | Gerding et al. [20] |
| SALLE | UPLC-QqQ-MS/MS | 0.013 | 0.036 | De Ruyck et al. [21] |
| Clean-up | UHPLC-HR-Q-Orbitrap-MS/MS | 1 | 0.5 | Ndaw et al. [23] |
| LLE | HPLC-Q-TRAP-MS/MS | 6.7 | 67 | Abia et al. [25] |
| QuEChERS | GC-QqQ-MS/MS | 1 | 2 | Rodríguez-Carrasco et al. [26] |
| Dilute and shoot | UHPLC-Q-TRAP-MS/MS | 1 | 40 | Ezekiel et al. [27] |
| LLE | HPLC-Q-TRAP-MS/MS | 1 | 40 | Warth et al. [28] |
| Filter and shoot | UHPLC-QqQ-MS/MS | 0.03 | 0.5 | Heyndrickx et al. [29] |
| LLE | UHPLC-QqQ-MS/MS | 0.1 | 0.84 | |
| LLE | HPLC-Q-TRAP-MS/MS | 0.2 | 9 | Gerding et al. [30] |
| LLE | UHPLC-Q-TRAP-MS/MS | 0.1 | 0.5 | Fan et al. [31] |
| IA-SPE | UHPLC-QqQ-MS/MS | 0.013 | 0.031 | Gratz et al. [32] |
| DLLME | GC-QqQ-MS/MS | 1 | 2 | Niknejad et al. [33] |
| Dilute and shoot | HPLC-Q-TRAP-MS/MS | 10 | 5 | Duringer et al. [34] |
| SALLE + clean-up | UHPLC-HR-Q-Orbitrap-MS/MS | 0.2 | 0.4 | Present study |
| Provenance | Cohort (age) | Samples (n) | LOQ (ng/mL)a | Prevalence (%) | Range of Concentration (ng/mg Crea) | Mean (ng/mg Crea) | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T-2 | HT-2 | T-2 | HT-2 | T-2 | HT-2 | T-2 | HT-2 | ||||
| Germany | Adults (20–30) | 101 | 0.5 | 4 | 1 | nd | <LOQ | nd | na | nd | Gerding et al. [20] |
| Belgium, Czech Republic, Netherlands, and Norway | Adults (45–65) | 188 | 0.013 | 0.036 | 21.8 | 6.4 | <LOQ—0.77 | <LOQ—4.60 | 0.05 c,d | 0.48 c,d | De Ruyck et al. [21] |
| France | Adults, grain elevator workers (19–56) | 18 | 1 | 0.5 | 4 | 4 | <LOQ—2.73 | <LOQ—3.29 | na | na | Ndaw et al. [23] |
| Spain | Children (8–14) | 16 | nd | 6.2 | nd | 12.6 b | nd | 12.6 | Rodríguez-Carrasco et al. [26] | ||
| Young adults (18–28) | 16 | nd | nd | nd | nd | nd | nd | ||||
| Adults (>28) | 22 | nd | 13.6 | nd | 15.8 b | nd | 14.3 | ||||
| Total | 54 | 1 | 2 | nd | 7.4 | nd | 15.8 b | nd | na | ||
| China | Adults (18–66) | 260 | 0.1 | 0.5 | 2.3 | nd | 0.392–4.23 | nd | 1.75 | nd | Fan et al. [31] |
| United Kingdom | Children (2–6) | 21 | 0.013 | 0.031 | 5 | 5 | 0.03 | 6.13 | 0.03 | 6.13 | Gratz et al. [32] |
| Iran | Adults, esophageal cancer patients (50–92) | 17 | 6 | 18 | na | na | 44.7 | 29.09 | Niknejad et al. [33] | ||
| Adults, control group (20–46) | 10 | 1 | 2 | nd | 10 | nd | na | nd | 16.81 | ||
| Uganda | Children, nodding syndrome patients (5–18) | 50 | 74 | nd | 0–288 c | nd | 29 c | nd | Duringer et al. [34] | ||
| Children, control group (5–18) | 50 | 70 | nd | 0–425 c | nd | 49 c | nd | ||||
| Total | 100 | 10 | 5 | 72 | nd | 0–425 c | nd | 39 c | nd | ||
| Italy | Children, teenagers and adults (≤30) | 94 | 20 | 32 | 0.42–2.37 | 0.44–2.32 | 1.26 | 1.19 | Present study | ||
| Adults (31–59) | 72 | 19 | 28 | 0.33–6.54 | 0.46–2.75 | 1.48 | 1.48 | ||||
| Elderly (≥60) | 134 | 22 | 30 | 0.22–2.51 | 0.44–2.39 | 1.4 | 1.13 | ||||
| Total | 300 | 0.2 | 0.4 | 21 | 30 | 0.22–6.54 | 0.44–2.75 | 1.34 | 1.23 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narváez, A.; Izzo, L.; Pallarés, N.; Castaldo, L.; Rodríguez-Carrasco, Y.; Ritieni, A. Human Biomonitoring of T-2 Toxin, T-2 Toxin-3-Glucoside and Their Metabolites in Urine through High-Resolution Mass Spectrometry. Toxins 2021, 13, 869. https://doi.org/10.3390/toxins13120869
Narváez A, Izzo L, Pallarés N, Castaldo L, Rodríguez-Carrasco Y, Ritieni A. Human Biomonitoring of T-2 Toxin, T-2 Toxin-3-Glucoside and Their Metabolites in Urine through High-Resolution Mass Spectrometry. Toxins. 2021; 13(12):869. https://doi.org/10.3390/toxins13120869
Chicago/Turabian StyleNarváez, Alfonso, Luana Izzo, Noelia Pallarés, Luigi Castaldo, Yelko Rodríguez-Carrasco, and Alberto Ritieni. 2021. "Human Biomonitoring of T-2 Toxin, T-2 Toxin-3-Glucoside and Their Metabolites in Urine through High-Resolution Mass Spectrometry" Toxins 13, no. 12: 869. https://doi.org/10.3390/toxins13120869
APA StyleNarváez, A., Izzo, L., Pallarés, N., Castaldo, L., Rodríguez-Carrasco, Y., & Ritieni, A. (2021). Human Biomonitoring of T-2 Toxin, T-2 Toxin-3-Glucoside and Their Metabolites in Urine through High-Resolution Mass Spectrometry. Toxins, 13(12), 869. https://doi.org/10.3390/toxins13120869








