Inflammatory Effects of Bothrops Phospholipases A2: Mechanisms Involved in Biosynthesis of Lipid Mediators and Lipid Accumulation
Abstract
1. Introduction
1.1. Phospholipases A2
1.2. Inflammation—General Concepts and Signaling Pathways
2. Inflammatory Effects of sPLA2 from Bothrops spp. Venoms
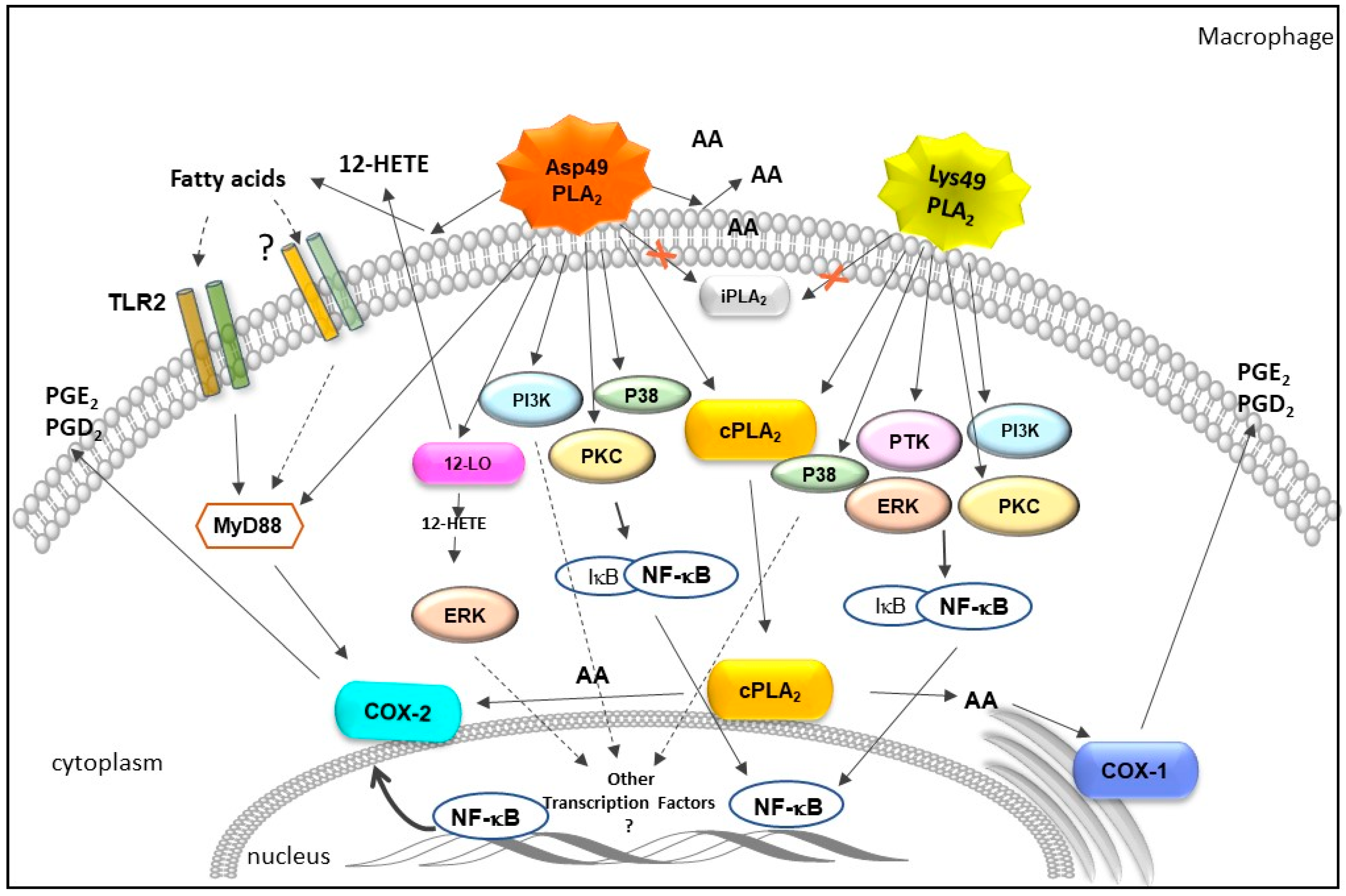
2.1. Bothrops svPLA2s Induce Inflammatory Events and Activate Defense Functions in Leukocytes
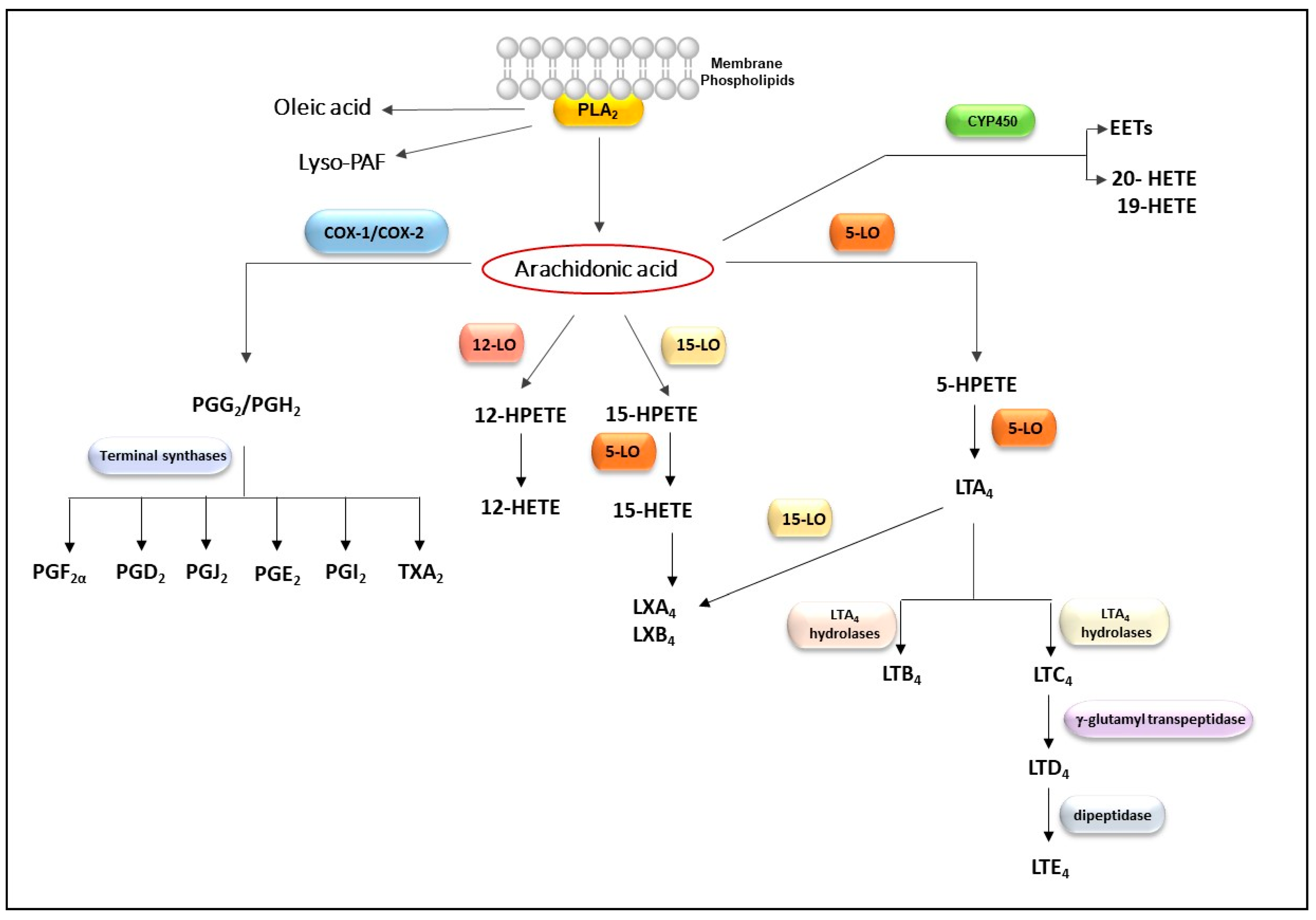
2.2. Influence of Bothrops svPLA2s on Pathways of Arachidonic Acid Metabolism
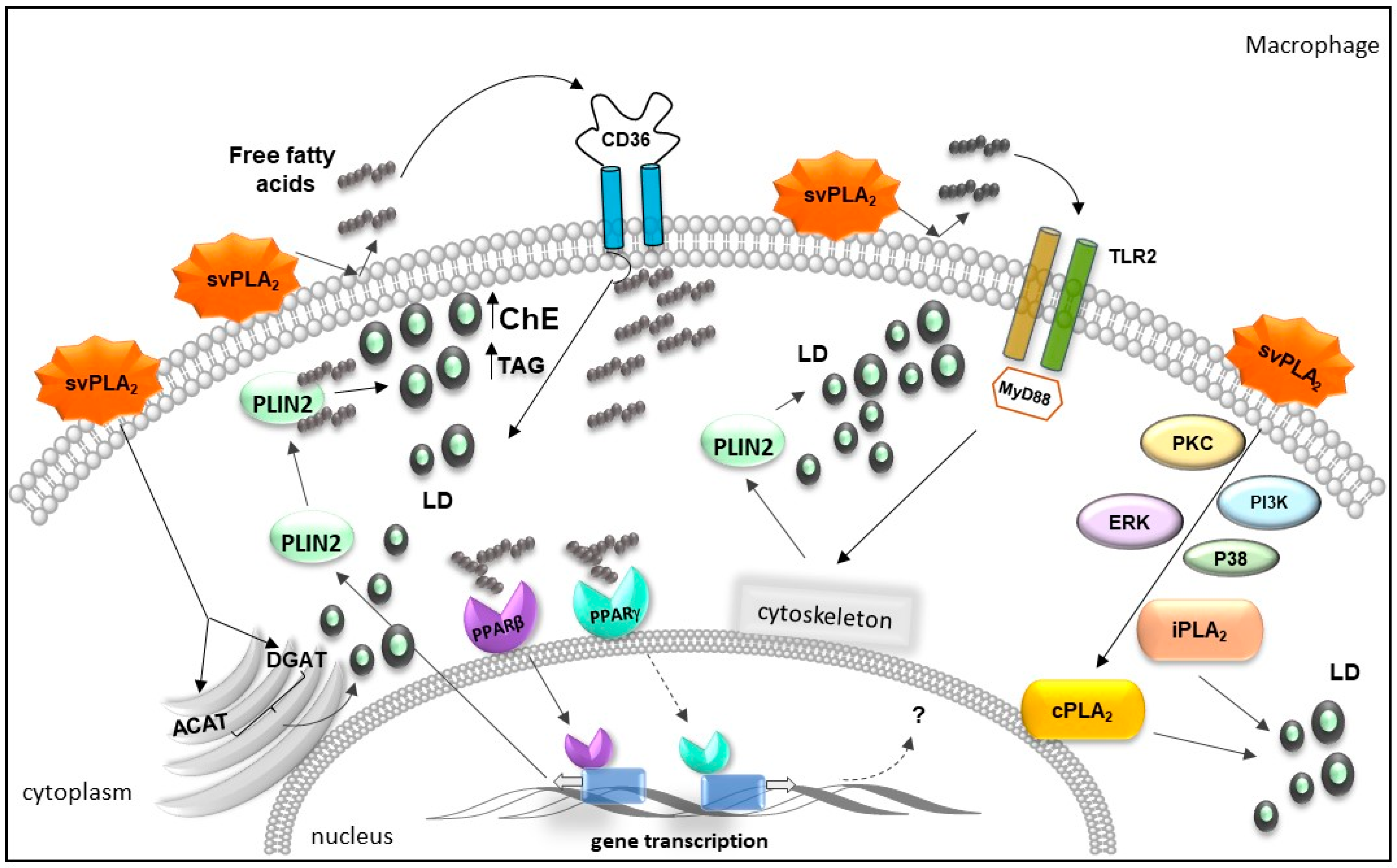
2.3. Bothrops svPLA2s Trigger Lipid Accumulation in Immunocompetent Cells
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teixeira, C.F.P.; Cury, Y.; Oga, S.; Jancar, S. Hyperalgesia induced by Bothrops jararaca venom in rats: Role of eicosanoids and platelet activating factor (PAF). Toxicon 1994, 32, 419–426. [Google Scholar] [CrossRef]
- Teixeira, C.; Cury, Y.; Moreira, V.; Picolo, G.; Chaves, F. Inflammation induced by Bothrops asper venom. Toxicon 2009, 54, 67–76. [Google Scholar] [CrossRef]
- Mamede, C.C.N.; de Sousa Simamoto, B.B.; da Cunha Pereira, D.F.; de Oliveira Costa, J.; Ribeiro, M.S.M.; de Oliveira, F. Edema, hyperalgesia and myonecrosis induced by Brazilian bothropic venoms: Overview of the last decade. Toxicon 2020, 187, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Chaves, F.; Díaz, C.; Escalante, T. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon 2009, 54, 958–975. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde Guia de Vigilância Epidemiológica; Ministério da Saúde: Brasília, Brazil, 2019; ISBN 9788533416321.
- Echeverría, S.; Leiguez, E.; Guijas, C.; do Nascimento, N.G.; Acosta, O.; Teixeira, C.; Leiva, L.C.; Rodríguez, J.P. Evaluation of pro-inflammatory events induced by Bothrops alternatus snake venom. Chem. Biol. Interact. 2018, 281, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R.J.B.; Monteiro, H.S.A.; Gonçalves-Machado, L.; Guarnieri, M.C.; Ximenes, R.M.; Borges-Nojosa, D.M.; Luna, K.P.D.O.; Zingali, R.B.; Corrêa-Netto, C.; Gutiérrez, J.M.; et al. Venomics and antivenomics of Bothrops erythromelas from five geographic populations within the Caatinga ecoregion of northeastern Brazil. J. Proteom. 2015, 114, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, C.A.; Carvalho, P.C.; Junqueira-de-Azevedo, I.L.M.; Teixeira-Ferreira, A.; Junqueira, M.; Perales, J.; Neves-Ferreira, A.G.C.; Valente, R.H. An in-depth snake venom proteopeptidome characterization: Benchmarking Bothrops jararaca. J. Proteom. 2017, 151, 214–231. [Google Scholar] [CrossRef]
- Sousa, L.F.; Portes-Junior, J.A.; Nicolau, C.A.; Bernardoni, J.L.; Nishiyama, M.Y., Jr.; Amazonas, D.R.; Freitas-de-Sousa, L.A.; Mourão, R.H.; Chalkidis, H.M.; Valente, R.H.; et al. Functional proteomic analyses of Bothrops atrox venom reveals phenotypes associated with habitat variation in the Amazon. J. Proteom. 2017, 159, 32–46. [Google Scholar] [CrossRef] [PubMed]
- De Farias, I.B.; de Morais-Zani, K.; Serino-Silva, C.; Sant’Anna, S.S.; da Rocha, M.M.; Grego, K.F.; Andrade-Silva, D.; Serrano, S.M.T.; Tanaka-Azevedo, A.M. Functional and proteomic comparison of Bothrops jararaca venom from captive specimens and the Brazilian Bothropic Reference Venom. J. Proteom. 2018, 174, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Mora-Obando, D.; Salazar-Valenzuela, D.; Pla, D.; Lomonte, B.; Guerrero-Vargas, J.A.; Ayerbe, S.; Gibbs, H.L.; Calvete, J.J. Venom variation in Bothrops asper lineages from North-Western South America. J. Proteom. 2020, 229, 103945. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 2013, 62, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Bickler, P.E. Amplification of Snake Venom Toxicity by Endogenous Signaling Pathways. Toxins 2020, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Hiu, J.J.; Yap, M.K.K. Cytotoxicity of snake venom enzymatic toxins: Phospholipase A2 and L-amino acid oxidase. Biochem. Soc. Trans. 2020, 48, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Chaves, F.; Bolaños, R.; Cerdas, L.; Rojas, E.; Arroyo, O.; Portilla, E. Neutralizacion de los efectos locales del veneno de Bothrops asper por un antiveneno polivalente. Toxicon 1981, 19, 493–500. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rojas, G.; Bogarín, G.; Lomonte, B. Evaluation of the neutralizing ability of antivenoms for the treatment of snake bite envenoming in Central America. Envenomings Treat. 1996, 223–231. [Google Scholar]
- Gutiérrez, J.; Avila, C.; Rojas, E.; Cerdas, L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon 1988, 26, 411–413. [Google Scholar] [CrossRef]
- Cardoso, J.L.; Fan, H.W.; França, F.O.; Jorge, M.T.; Leite, R.P.; Nishioka, S.A.; Avila, A.; Sano-Martins, I.S.; Tomy, S.C.; Santoro, M.L. Randomized comparative trial of three antivenoms in the treatment of envenoming by lance-headed vipers (Bothrops jararaca) in São Paulo, Brazil. Q. J. Med. 1993, 86, 315–325. [Google Scholar] [PubMed]
- Lewin, M.; Samuel, S.; Merkel, J.; Bickler, P. Varespladib (LY315920) appears to be a potent, broad-spectrum, inhibitor of snake venom phospholipase A2 and a possible pre-referral treatment for envenomation. Toxins 2016, 8, 248. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, D.; Xiao, H.; Xiong, S.; Huang, C. Exploration of the inhibitory potential of varespladib for snakebite envenomation. Molecules 2018, 23, 391. [Google Scholar] [CrossRef] [PubMed]
- Schaloske, R.H.; Dennis, E.A. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2006, 1761, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Six, D.A.; Dennis, E.A. The expanding superfamily of phospholipase A2 enzymes: Classification and characterization. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2000, 1488, 1–19. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef]
- Murakami, M.; Sato, H.; Taketomi, Y. Updating Phospholipase A2 Biology. Biomolecules 2020, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Fujita, M.; Nakanishi, H.; Miyata, H.; Ikawa, M.; Maeda, Y.; Murakami, Y.; Kinoshita, T. PGAP6, a GPI-specific phospholipase A2, has narrow substrate specificity against GPI-anchored proteins. J. Biol. Chem. 2020, 295, 14501–14509. [Google Scholar] [CrossRef]
- Aloulou, A.; Rahier, R.; Arhab, Y.; Noiriel, A.; Abousalham, A. Phospholipases: An Overview. In Methods in Molecular Biology; Sandoval, G., Ed.; Springer: New York, NY, USA, 2018; Volume 1835, pp. 69–105. ISBN 978-1-4939-8671-2. [Google Scholar]
- Quach, N.D.; Arnold, R.D.; Cummings, B.S. Secretory phospholipase A2 enzymes as pharmacological targets for treatment of disease. Biochem. Pharmacol. 2014, 90, 338–348. [Google Scholar] [CrossRef]
- Harris, J.B.; Scott-Davey, T. Secreted phospholipases A2 of snake venoms: Effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins 2013, 5, 2533–2571. [Google Scholar] [CrossRef] [PubMed]
- Triggiani, M.; Granata, F.; Frattini, A.; Marone, G. Activation of human inflammatory cells by secreted phospholipases A2. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2006, 1761, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Valentin, E.; Lambeau, G. What can venom phospholipases A2 tell us about the functional diversity of mammalian secreted phospholipases A2? Biochimie 2000, 82, 815–831. [Google Scholar] [CrossRef]
- Kudo, I.; Murakami, M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002, 68–69, 3–58. [Google Scholar] [CrossRef]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Lambeau, G.; Barhanin, J.; Schweitz, H.; Qar, J.; Lazdunski, M. Identification and properties of very high affinity brain membrane-binding sites for a neurotoxic phospholipase from the taipan venom. J. Biol. Chem. 1989, 264, 11503–11510. [Google Scholar] [CrossRef]
- Lambeau, G.; Schmid-Alliana, A.; Lazdunski, M.; Barhanin, J. Identification and purification of a very high affinity binding protein for toxic phospholipases A2 in skeletal muscle. J. Biol. Chem. 1990, 265, 9526–9532. [Google Scholar] [CrossRef]
- Silliman, C.C.; Moore, E.E.; Zallen, G.; Gonzalez, R.; Johnson, J.L.; Elzi, D.J.; Meng, X.; Hanasaki, K.; Ishizaki, J.; Arita, H.; et al. Presence of the M-type sPLA 2 receptor on neutrophils and its role in elastase release and adhesion. Am. J. Physiol. Physiol. 2002, 283, C1102–C1113. [Google Scholar] [CrossRef] [PubMed]
- Granata, F.; Petraroli, A.; Boilard, E.; Bezzine, S.; Bollinger, J.; Del Vecchio, L.; Gelb, M.H.; Lambeau, G.; Marone, G.; Triggiani, M. Activation of Cytokine Production by Secreted Phospholipase A 2 in Human Lung Macrophages Expressing the M-Type Receptor. J. Immunol. 2005, 174, 464–474. [Google Scholar] [CrossRef]
- Hanasaki, K.; Arita, H. Phospholipase A2 receptor: A regulator of biological functions of secretory phospholipase A2. Prostaglandins Other Lipid Mediat. 2002, 68–69, 71–82. [Google Scholar] [CrossRef]
- Ezekowitz, R.A.B.; Stahl, P.D. The structure and function of vertebrate mannose lectin-like proteins. J. Cell Sci. 1988, 1988, 121–133. [Google Scholar] [CrossRef]
- Taylor, M.E.; Conary, J.T.; Lennartz, M.R.; Stahl, P.D.; Drickamer, K. Primary structure of the mannose receptor contains multiple motifs resembling carbohydrate-recognition domains. J. Biol. Chem. 1990, 265, 12156–12162. [Google Scholar] [CrossRef]
- Gantzel, R.H.; Kjær, M.B.; Laursen, T.L.; Kazankov, K.; George, J.; Møller, H.J.; Grønbæk, H. Macrophage Activation Markers, Soluble CD163 and Mannose Receptor, in Liver Fibrosis. Front. Med. 2021, 7, 615599. [Google Scholar] [CrossRef]
- Gordon, S.; Clarke, S.; Greaves, D.; Doyle, A. Molecular immunobiology of macrophages: Recent progress. Curr. Opin. Immunol. 1995, 7, 24–33. [Google Scholar] [CrossRef]
- Stoy, N. Macrophage Biology and Pathobiology in the Evolution of Immune Responses: A Functional Analysis. Pathobiology 2001, 69, 179–211. [Google Scholar] [CrossRef]
- Hernández, M.; Burillo, S.L.; Crespo, M.S.; Nieto, M.L. Secretory Phospholipase A2 Activates the Cascade of Mitogen-activated Protein Kinases and Cytosolic Phospholipase A2 in the Human Astrocytoma Cell Line 1321N1. J. Biol. Chem. 1998, 273, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, E.; Handa, N.; Hanada, K.; Kajiyama, G.; Sugiyama, M. Activation of MAP kinase cascade induced by human pancreatic phospholipase A2 in a human pancreatic cancer cell line. FEBS Lett. 1997, 407, 343–346. [Google Scholar] [CrossRef]
- Kundu, G.C.; Mukherjee, A.B. Evidence that porcine pancreatic phospholipase A2 via its high affinity receptor stimulates extracellular matrix invasion by normal and cancer cells. J. Biol. Chem. 1997, 272, 2346–2353. [Google Scholar] [CrossRef]
- Triggiani, M.; Calabrese, C.; Granata, F.; Gentile, M.; Marone, G. Metabolism of Lipid Mediators in Human Eosinophils. In Human Eosinophils; KARGER: Basel, Switzerland, 2000; pp. 77–98. [Google Scholar]
- Hara, S.; Kudo, I.; Matsuta, K.; Miyamoto, T.; Inoue, K. Amino Acid Composition and NH2-Terminal Amino Acid Sequence of Human Phospholipase A2 Purified from Rheumatoid Synovial Fluid1. J. Biochem. 1988, 104, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Touqui, L.; Alaoui-El-Azher, M. Mammalian Secreted Phospholipases A2 and Their Pathophysiolo-gical Significance in Inflammatory Diseases. Curr. Mol. Med. 2001, 1, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.J.; Chapman, R.; Lin, Y.; Mmesi, J.; Bentham, A.; Tyreman, M.; Abraham, S.; Stevens, M.M. Point of care testing of phospholipase A2 group IIA for serological diagnosis of rheumatoid arthritis. Nanoscale 2016, 8, 4482–4485. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Ginsburg, I. A Novel Hypothetical Approach to Explain the Mechanisms of Pathogenicity of Rheumatic Arthritis. Mediterr. J. Rheumatol. 2021, 32, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Aufenanger, J.; Samman, M.; Quintel, M.; Fassbender, K.; Zimmer, W.; Bertsch, T. Pancreatic phospholipase A2 activity in acute pancreatitis: A prognostic marker for early identification of patients at risk. Clin. Chem. Lab. Med. 2002, 40, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.J.; Zhang, D.L.; Jiao, X.L.; Dong, C. Effect of phospholipase A2 silencing on acute experimental pancreatitis. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 3279–3284. [Google Scholar]
- Schröder, T.; Kivilaakso, E.; Kinnunen, P.K.J.; Lempinen, M. Serum Phospholipase A 2 in Human Acute Pancreatitis. Scand. J. Gastroenterol. 1980, 15, 633–636. [Google Scholar] [CrossRef]
- Vadas, P.; Pruzanski, W. Role of secretory phospholipases A2 in the pathobiology of disease. Lab. Investig. 1986, 55, 391–404. [Google Scholar] [PubMed]
- Ahmad, N.S.; Tan, T.L.; Arifin, K.T.; Ngah, W.Z.W.; Yusof, Y.A.M. High sPLA2-IIA level is associated with eicosanoid metabolism in patients with bacterial sepsis syndrome. PLoS ONE 2020, 15, e0230285. [Google Scholar] [CrossRef]
- Haapamäki, M.M.; Grönroos, J.M.; Nurmi, H.; Irjala, K.; Alanen, K.A.; Nevalainen, T.J. Phospholipase A2 in serum and colonic mucosa in ulcerative colitis. Scand. J. Clin. Lab. Investig. 1999, 59, 279–287. [Google Scholar] [CrossRef]
- Haapamäki, M.M.; Grönroos, J.M.; Nurmi, H.; Alanen, K.; Nevalainen, T.J. Gene Expression of Group Ii Phospholipase A2 in Intestine in Crohn’s Disease. Am. J. Gastroenterol. 1999, 94, 713–720. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Arumugam, T.V.; Shiels, I.A.; Newman, M.L.; Ross, P.A.; Reid, R.C.; Fairlie, D.P.; Taylor, S.M. A potent and selective inhibitor of group IIa secretory phospholipase A2 protects rats from TNBS-induced colitis. Int. Immunopharmacol. 2005, 5, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Stadel, J.M.; Hoyle, K.; Naclerio, R.M.; Roshak, A.; Chilton, F.H. Characterization of phospholipase A2 from human nasal lavage. Am. J. Respir. Cell Mol. Biol. 1994, 11, 108–113. [Google Scholar] [CrossRef]
- Kim, D.K.; Fukuda, T.; Thompson, B.T.; Cockrill, B.; Hales, C.; Bonventre, J.V. Bronchoalveolar lavage fluid phospholipase A2 activities are increased in human adult respiratory distress syndrome. Am. J. Physiol. Cell. Mol. Physiol. 1995, 269, L109–L118. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, E.; Htwe, Y.M.; Dudek, S.M. Secretory Phospholipase A2 Enzymes in Acute Lung Injury. Cell Biochem. Biophys. 2021, 79, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Granata, F.; Staiano, R.I.; Loffredo, S.; Petraroli, A.; Genovese, A.; Marone, G.; Triggiani, M. The role of mast cell-derived secreted phospholipases A2 in respiratory allergy. Biochimie 2010, 92, 588–593. [Google Scholar] [CrossRef]
- Sun, C.Q.; Zhong, C.Y.; Sun, W.W.; Xiao, H.; Zhu, P.; Lin, Y.Z.; Zhang, C.L.; Gao, H.; Song, Z.Y. Elevated Type II Secretory Phospholipase A2 Increases the Risk of Early Atherosclerosis in Patients with Newly Diagnosed Metabolic Syndrome. Sci. Rep. 2016, 6, 34929. [Google Scholar] [CrossRef] [PubMed]
- Menschikowski, M.; Hagelgans, A.; Siegert, G. Secretory phospholipase A2 of group IIA: Is it an offensive or a defensive player during atherosclerosis and other inflammatory diseases? Prostaglandins Other Lipid Mediat. 2006, 79, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Yao, L.; Oetinger, M.; Cort, L.; Blankenhorn, E.P.; Greenstein, J.I. Secreted phospholipase A2 activity in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Neuroinflamm. 2006, 3, 26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mirtti, T.; Laine, V.J.O.; Hiekkanen, H.; Hurme, S.; Rowe, O.; Nevalainen, T.J.; Kallajoki, M.; Alanen, K. Group IIA phospholipase A2 as a prognostic marker in prostate cancer: Relevance to clinicopathological variables and disease-specific mortality. Apmis 2009, 117, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Liu, Y.; Scott, K.F.; Levin, L.; Gaitonde, K.; Bracken, R.B.; Burke, B.; Zhai, Q.J.; Wang, J.; Oleksowicz, L.; et al. Secretory phospholipase A2-IIa is involved in prostate cancer progression and may potentially serve as a biomarker for prostate cancer. Carcinogenesis 2010, 31, 1948–1955. [Google Scholar] [CrossRef]
- Wang, M.; Hao, F.Y.; Wang, J.G.; Xiao, W. Group IIa secretory phospholipase A2 (sPLA2IIa) and progression in patients with lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2648–2654. [Google Scholar] [PubMed]
- Chen, J.; Ye, L.; Sun, Y.; Takada, Y. A Concise Update on the Relevance of Secretory Phospholipase A2 Group IIA and its Inhibitors with Cancer. Med. Chem. 2017, 13, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.; Pfeilschifter, J.; Märki, F.; van den Bosch, H. Interleukin-1β, tumor necrosis factor and forskolin stimulate the synthesis and secretion of group II phospholipase A2 in rat mesangial cells. Biochem. Biophys. Res. Commun. 1991, 174, 268–275. [Google Scholar] [CrossRef]
- Divchev, D.; Schieffer, B. The secretory phospholipase A2 group IIA: A missing link between inflammation, activated renin-angiotensin system, and atherogenesis? Vasc. Health Risk Manag. 2008, 4, 597–604. [Google Scholar] [CrossRef]
- Leistad, L.; Feuerherm, A.J.; Faxvaag, A.; Johansen, B. Multiple phospholipase A2 enzymes participate in the inflammatory process in osteoarthritic cartilage. Scand. J. Rheumatol. 2011, 40, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Lambeau, G.; Scholz-Pedretti, K.; Gelb, M.H.; Janssen, M.J.W.; Edwards, S.H.; Wilton, D.C.; Pfeilschifter, J.; Kaszkin, M. Potentiation of Tumor Necrosis Factor α-induced Secreted Phospholipase A2 (sPLA2)-IIA Expression in Mesangial Cells by an Autocrine Loop Involving sPLA2 and Peroxisome Proliferator-activated Receptor α Activation. J. Biol. Chem. 2003, 278, 29799–29812. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Shang, M.; Jian, Y.; Wang, C.; Bardeesi, A.S.A.; Li, Z.; Chen, T.; Zhao, L.; Zhou, L.; et al. Secreted phospholipase A2 of Clonorchis sinensis activates hepatic stellate cells through a pathway involving JNK signalling. Parasites Vectors 2017, 10, 147. [Google Scholar] [CrossRef]
- Sarate, R.M.; Chovatiya, G.L.; Ravi, V.; Khade, B.; Gupta, S.; Waghmare, S.K. sPLA 2 -IIA Overexpression in Mice Epidermis Depletes Hair Follicle Stem Cells and Induces Differentiation Mediated Through Enhanced JNK/c-Jun Activation. Stem Cells 2016, 34, 2407–2417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baek, S.-H.; Lim, J.-H.; Park, D.-W.; Kim, S.-Y.; Lee, Y.-H.; Kim, J.-R.; Kim, J.-H. Group IIA secretory phospholipase A2 stimulates inducible nitric oxide synthase expression via ERK and NF-κB in macrophages. Eur. J. Immunol. 2001, 31, 2709–2717. [Google Scholar] [CrossRef]
- Beck, G.C.; Yard, B.A.; Schulte, J.; Haak, M.; van Ackern, K.; van der Woude, F.J.; Kaszkin, M. Secreted phospholipases A2 induce the expression of chemokines in microvascular endothelium. Biochem. Biophys. Res. Commun. 2003, 300, 731–737. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Barton, G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef]
- Kruger, P.; Saffarzadeh, M.; Weber, A.N.R.; Rieber, N.; Radsak, M.; von Bernuth, H.; Benarafa, C.; Roos, D.; Skokowa, J.; Hartl, D. Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury. PLOS Pathog. 2015, 11, e1004651. [Google Scholar] [CrossRef] [PubMed]
- Bonecchi, R. Chemokines and chemokine receptors: An overview. Front. Biosci. 2009, 14, 540. [Google Scholar] [CrossRef]
- Branco, A.C.C.C.; Yoshikawa, F.S.Y.; Pietrobon, A.J.; Sato, M.N. Role of Histamine in Modulating the Immune Response and Inflammation. Mediat. Inflamm. 2018, 2018, 9524075. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Gilroy, D.W. Lipid Mediators in Inflammation. Microbiol. Spectr. 2016, 4, 4–6. [Google Scholar] [CrossRef]
- Krieglstein, C. Adhesion molecules and their role in vascular disease. Am. J. Hypertens. 2001, 14, S44–S54. [Google Scholar] [CrossRef]
- Kameritsch, P.; Renkawitz, J. Principles of Leukocyte Migration Strategies. Trends Cell Biol. 2020, 30, 818–832. [Google Scholar] [CrossRef]
- Rosales, C.; Uribe-Querol, E. Phagocytosis: A Fundamental Process in Immunity. Biomed Res. Int. 2017, 2017, 9042851. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef]
- Collin, M.; Ehlers, M. The carbohydrate switch between pathogenic and immunosuppressive antigen-specific antibodies. Exp. Dermatol. 2013, 22, 511–514. [Google Scholar] [CrossRef]
- Medzhitov, R. TLR-mediated innate immune recognition. Semin. Immunol. 2007, 19, 1–2. [Google Scholar] [CrossRef]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef]
- Motta, V.; Soares, F.; Sun, T.; Philpott, D.J. NOD-Like Receptors: Versatile Cytosolic Sentinels. Physiol. Rev. 2015, 95, 149–178. [Google Scholar] [CrossRef]
- Corridoni, D.; Simmons, A. Innate immune receptors for cross-presentation: The expanding role of NLRs. Mol. Immunol. 2019, 113, 6–10. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A. Localisation and trafficking of Toll-like receptors: An important mode of regulation. Curr. Opin. Immunol. 2010, 22, 20–27. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Vanaja, S.K.; Fitzgerald, K.A. Regulation of inflammasome signaling. Nat. Immunol. 2012, 13, 333–342. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.-D. Inflammasome activation and assembly at a glance. J. Cell Sci. 2017, 130, 3955–3963. [Google Scholar] [CrossRef]
- Lin, Y.-Z.; Yao, S.; Veach, R.A.; Torgerson, T.R.; Hawiger, J. Inhibition of Nuclear Translocation of Transcription Factor NF-κB by a Synthetic Peptide Containing a Cell Membrane-permeable Motif and Nuclear Localization Sequence. J. Biol. Chem. 1995, 270, 14255–14258. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Kwiatkowska, K.; Sobota, A. Signaling pathways in phagocytosis. Bioessays 1999, 21, 422–431. [Google Scholar] [CrossRef]
- Kitaura, J.; Eto, K.; Kinoshita, T.; Kawakami, Y.; Leitges, M.; Lowell, C.A.; Kawakami, T. Regulation of Highly Cytokinergic IgE-Induced Mast Cell Adhesion by Src, Syk, Tec, and Protein Kinase C Family Kinases. J. Immunol. 2005, 174, 4495–4504. [Google Scholar] [CrossRef]
- Yang, Q.; Langston, J.C.; Tang, Y.; Kiani, M.F.; Kilpatrick, L.E. The role of tyrosine phosphorylation of protein kinase C delta in infection and inflammation. Int. J. Mol. Sci. 2019, 20, 1498. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Stephenson, J.D.; Shepherd, V.L. Purification of the human alveolar macrophage mannose receptor. Biochem. Biophys. Res. Commun. 1987, 148, 883–889. [Google Scholar] [CrossRef]
- Ezekowitz, R.A.; Sastry, K.; Bailly, P.; Warner, A. Molecular characterization of the human macrophage mannose receptor: Demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J. Exp. Med. 1990, 172, 1785–1794. [Google Scholar] [CrossRef]
- Greenberg, S. Modular components of phagocytosis. J. Leukoc. Biol. 1999, 66, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Fernández, N.; Alonso, S.; Valera, I.; Vigo, A.G.; Renedo, M.; Barbolla, L.; Crespo, M.S. Mannose-Containing Molecular Patterns Are Strong Inducers of Cyclooxygenase-2 Expression and Prostaglandin E 2 Production in Human Macrophages. J. Immunol. 2005, 174, 8154–8162. [Google Scholar] [CrossRef]
- McNally, A.K.; DeFife, K.M.; Anderson, J.M. Interleukin-4-induced macrophage fusion is prevented by inhibitors of mannose receptor activity. Am. J. Pathol. 1996, 149, 975–985. [Google Scholar]
- Newton, R.; Holden, N. Inhibitors of p38 Mitogen-Activated Protein Kinase. BioDrugs 2003, 17, 113–129. [Google Scholar] [CrossRef]
- Janssen, W.J.; Henson, P.M. Cellular Regulation of the Inflammatory Response. Toxicol. Pathol. 2012, 40, 166–173. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Feehan, K.T.; Gilroy, D.W. Is Resolution the End of Inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Chan, Y.M. Accelerated Evolution and Molecular Surface of Venom Phospholipase A2 Enzymes. J. Mol. Evol. 1999, 48, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Krizaj, I.; Bieber, A.L.; Ritonja, A.; Gubensek, F. The primary structure of ammodytin L, a myotoxic phospholipase A2 homologue from Vipera ammodytes venom. Eur. J. Biochem. 1991, 202, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, B.; Tessari, M.; de Haas, G.H.; Verheij, H.M.; Boelens, R.; Kaptein, R. Solution structure of porcine pancreatic phospholipase A2. EMBO J. 1995, 14, 4123–4131. [Google Scholar] [CrossRef]
- Díaz, C.; Gutiérrez, J.; Lomonte, B.; Gené, J. The effect of myotoxins isolated from Bothrops snake venoms on multilamellar liposomes: Relationship to phospholipase A2, anticoagulant and myotoxic activities. Biochim. Biophys. Acta-Biomembr. 1991, 1070, 455–460. [Google Scholar] [CrossRef]
- Lomonte, B.; Gutiérrez, J.M. Phospholipases A2 from viperidae snake venoms: How do they induce skeletal muscle damage? Acta Chim. Slov. 2011, 58, 647–659. [Google Scholar]
- Zuliani, J.P.; Fernandes, C.M.; Zamuner, S.R.; Gutiérrez, J.M.; Teixeira, C.F.P. Inflammatory events induced by Lys-49 and Asp-49 phospholipases A2 isolated from Bothrops asper snake venom: Role of catalytic activity. Toxicon 2005, 45, 335–346. [Google Scholar] [CrossRef]
- Teixeira, C.F.P.; Landucci, E.C.T.; Antunes, E.; Chacur, M.; Cury, Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon 2003, 42, 947–962. [Google Scholar] [CrossRef]
- Landucci, E.C.T.; De Castro, R.C.; Toyama, M.; Giglio, J.R.; Marangoni, S.; De Nucci, G.; Antunes, E. Inflammatory oedema induced by the Lys-49 phospholipase A2 homologue piratoxin-I in the rat and rabbit. Effect of polyanions and p-bromophenacyl bromide. Biochem. Pharmacol. 2000, 59, 1289–1294. [Google Scholar] [CrossRef]
- Chaves, F.; León, G.; Alvarado, V.H.; Gutiérrez, J.M. Pharmacological modulation of edema induced by Lys-49 and Asp-49 myotoxic phospholipases A2 isolated from the venom of the snake Bothrops asper (Terciopelo). Toxicon 1998, 36, 1861–1869. [Google Scholar] [CrossRef]
- Daniele, J.J.; Bianco, I.D.; Fidelio, G.D. Kinetic and Pharmacological Characterization of Phospholipases A2 from Bothrops neuwiedii Venom. Arch. Biochem. Biophys. 1995, 318, 65–70. [Google Scholar] [CrossRef]
- Landucci, E.C.; Castro, R.C.; Pereira, M.F.; Cintra, A.C.; Giglio, J.R.; Marangoni, S.; Oliveira, B.; Cirino, G.; Antunes, E.; De Nucci, G. Mast cell degranulation induced by two phospholipase A2 homologues: Dissociation between enzymatic and biological activities. Eur. J. Pharmacol. 1998, 343, 257–263. [Google Scholar] [CrossRef]
- Ketelhut, D.F.; Homem de Mello, M.; Veronese, E.L.; Esmeraldino, L.; Murakami, M.; Arni, R.; Giglio, J.; Cintra, A.C.; Sampaio, S. Isolation, characterization and biological activity of acidic phospholipase A2 isoforms from Bothrops jararacussu snake venom. Biochimie 2003, 85, 983–991. [Google Scholar] [CrossRef]
- Cogo, J.C.; Lilla, S.; Souza, G.H.M.F.; Hyslop, S.; de Nucci, G. Purification, sequencing and structural analysis of two acidic phospholipases A2 from the venom of Bothrops insularis (jararaca ilhoa). Biochimie 2006, 88, 1947–1959. [Google Scholar] [CrossRef]
- de Castro, R.; Landucci, E.C.; Toyama, M.; Giglio, J.; Marangoni, S.; De Nucci, G.; Antunes, E. Leucocyte recruitment induced by type II phospholipases A2 into the rat pleural cavity. Toxicon 2000, 38, 1773–1785. [Google Scholar] [CrossRef]
- Kanashiro, M.M.; Rita de Cássia, M.E.; Petretski, J.H.; Prates, M.V.; Alves, E.W.; Machado, O.L.; da Silva, W.D.; Kipnis, T.L. Biochemical and biological properties of phospholipases A2 from Bothrops atrox snake venom. Biochem. Pharmacol. 2002, 64, 1179–1186. [Google Scholar] [CrossRef]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Maurer, M. Mast cells—Key effector cells in immune responses. Trends Immunol. 2007, 28, 234–241. [Google Scholar] [CrossRef]
- Chacur, M.; Longo, I.; Picolo, G.; Gutiérrez, J.M.; Lomonte, B.; Guerra, J.L.; Teixeira, C.F.P.; Cury, Y. Hyperalgesia induced by Asp49 and Lys49 phospholipases A2 from Bothrops asper snake venom: Pharmacological mediation and molecular determinants. Toxicon 2003, 41, 667–678. [Google Scholar] [CrossRef]
- Gambero, A.; Landucci, E.C.T.; Toyama, M.H.; Marangoni, S.; Giglio, J.R.; Nader, H.B.; Dietrich, C.P.; De Nucci, G.; Antunes, E. Human neutrophil migration in vitro induced by secretory phospholipases A2: A role for cell surface glycosaminoglycans. Biochem. Pharmacol. 2002, 63, 65–72. [Google Scholar] [CrossRef]
- Gambero, A.; Thomazzi, S.M.; Cintra, A.C.O.; Landucci, E.C.T.; De Nucci, G.; Antunes, E. Signalling pathways regulating human neutrophil migration induced by secretory phospholipases A2. Toxicon 2004, 44, 473–481. [Google Scholar] [CrossRef]
- Zuliani, J.P.; Gutiérrez, J.M.; Teixeira, C. Signaling pathways involved in zymosan phagocytosis induced by two secreted phospholipases A2 isolated from Bothrops asper snake venom in macrophages. Int. J. Biol. Macromol. 2018, 113, 575–582. [Google Scholar] [CrossRef]
- de Freitas Oliveira, C.; da Silva Lopes, D.; Mendes, M.M.; Homsi-Brandeburgo, M.I.; Hamaguchi, A.; de Alcântara, T.M.; Clissa, P.B.; de Melo Rodrigues, V. Insights of local tissue damage and regeneration induced by BnSP-7, a myotoxin isolated from Bothrops (neuwiedi) pauloensis snake venom. Toxicon 2009, 53, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Menaldo, D.L.; Bernardes, C.P.; Zoccal, K.F.; Jacob-Ferreira, A.L.; Costa, T.R.; Del Lama, M.P.F.M.; Naal, R.M.Z.G.; Frantz, F.G.; Faccioli, L.H.; Sampaio, S.V. Immune cells and mediators involved in the inflammatory responses induced by a P-I metalloprotease and a phospholipase A2 from Bothrops atrox venom. Mol. Immunol. 2017, 85, 238–247. [Google Scholar] [CrossRef]
- Cedro, R.C.A.; Menaldo, D.L.; Costa, T.R.; Zoccal, K.F.; Sartim, M.A.; Santos-Filho, N.A.; Faccioli, L.H.; Sampaio, S.V. Cytotoxic and inflammatory potential of a phospholipase A2 from Bothrops jararaca snake venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Arend, W.P.; Gabay, C. Cytokines in the rheumatic diseases. Rheum. Dis. Clin. N. Am. 2004, 30, 41–67. [Google Scholar] [CrossRef]
- David, B.A.; Kubes, P. Exploring the complex role of chemokines and chemoattractants in vivo on leukocyte dynamics. Immunol. Rev. 2019, 289, 9–30. [Google Scholar] [CrossRef]
- de Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Corasolla Carregari, V.; Stuani Floriano, R.; Rodrigues-Simioni, L.; Winck, F.V.; Baldasso, P.A.; Ponce-Soto, L.A.; Marangoni, S. Biochemical, Pharmacological, and Structural Characterization of New Basic Bbil-TX from Bothriopsis bilineata Snake Venom. Biomed Res. Int. 2013, 2013, 612649. [Google Scholar] [CrossRef]
- Moura, A.A.D.; Kayano, A.M.; Oliveira, G.A.; Setúbal, S.S.; Ribeiro, J.G.; Barros, N.B.; Nicolete, R.; Moura, L.A.; Fuly, A.L.; Nomizo, A.; et al. Purification and Biochemical Characterization of Three Myotoxins from Bothrops mattogrossensis Snake Venom with Toxicity against Leishmania and Tumor Cells. Biomed Res. Int. 2014, 2014, 195356. [Google Scholar] [CrossRef]
- Boeno, C.N.; Paloschi, M.V.; Lopes, J.A.; Pires, W.L.; Setúbal, S.D.S.; Evangelista, J.R.; Soares, A.M.; Zuliani, J.P. Inflammasome Activation Induced by a Snake Venom Lys49-Phospholipase A2 Homologue. Toxins 2019, 12, 22. [Google Scholar] [CrossRef]
- Ranéia e Silva, P.A.; de Lima, D.S.; Mesquita Luiz, J.P.; Câmara, N.O.S.; Alves-Filho, J.C.F.; Pontillo, A.; Bortoluci, K.R.; Faquim-Mauro, E.L. Inflammatory effect of Bothropstoxin-I from Bothrops jararacussu venom mediated by NLRP3 inflammasome involves ATP and P2X7 receptor. Clin. Sci. 2021, 135, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Setúbal, S.S.; Pontes, A.S.; Furtado, J.L.; Xavier, C.V.; Silva, F.L.; Kayano, A.M.; Izidoro, L.F.M.; Soares, A.M.; Calderon, L.A.; Stábeli, R.G.; et al. Action of two phospholipases A2 purified from Bothrops alternatus snake venom on macrophages. Biochemistry 2013, 78, 194–203. [Google Scholar] [CrossRef]
- Rueda, A.Q.; Rodríguez, I.G.; Arantes, E.C.; Setúbal, S.S.; Calderon, L.D.A.; Zuliani, J.P.; Stábeli, R.G.; Soares, A.M. Biochemical Characterization, Action on Macrophages, and Superoxide Anion Production of Four Basic Phospholipases A 2 from Panamanian Bothrops asper Snake Venom. Biomed Res. Int. 2013, 2013, 789689. [Google Scholar] [CrossRef]
- Zuliani, J.P.; Gutiérrez, J.M.; e Silva, L.L.C.; Sampaio, S.C.; Lomonte, B.; de Fátima Pereira Teixeira, C. Activation of cellular functions in macrophages by venom secretory Asp-49 and Lys-49 phospholipases A2. Toxicon 2005, 46, 523–532. [Google Scholar] [CrossRef]
- Furtado, J.L.; Oliveira, G.A.; Pontes, A.S.; Setúbal, S.D.S.; Xavier, C.V.; Lacouth-Silva, F.; Lima, B.F.; Zaqueo, K.D.; Kayano, A.M.; Calderon, L.A.; et al. Activation of J77A.1 macrophages by three phospholipases A2 isolated from Bothrops atrox snake venom. Biomed Res. Int. 2014, 2014, 683123. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Zychlinsky, A. Beneficial suicide: Why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007, 5, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, F.V.S.; Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef]
- Setúbal, S.D.S.; Pontes, A.S.; Nery, N.M.; Rego, C.M.A.; Santana, H.M.; de Lima, A.M.; Boeno, C.N.; Paloschi, M.V.; Soares, A.M.; Zuliani, J.P. Human neutrophils functionality under effect of an Asp49 phospholipase A2 isolated from Bothrops atrox venom. Toxicon X 2020, 6, 100032. [Google Scholar] [CrossRef]
- Bon, C.; Choumet, V.; Delot, E.; Faure, G.; Robbe-Vincent, A.; Saliou, B. Different Evolution of Phospholipase A 2 Neurotoxins (Beta-Neurotoxins) from Elapidae and Viperidae Snakes. Ann. N. Y. Acad. Sci. 1994, 710, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Morbiato, L.; Caccin, P.; Rigoni, M.; Montecucco, C. Presynaptic enzymatic neurotoxins. J. Neurochem. 2006, 97, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, S.C.; Brigatte, P.; Sousa-E-Silva, M.C.C.; Dos-Santos, E.C.; Rangel-Santos, A.C.; Curi, R.; Cury, Y. Contribution of crotoxin for the inhibitory effect of Crotalus durissus terrificus snake venom on macrophage function. Toxicon 2003, 41, 899–907. [Google Scholar] [CrossRef]
- Sampaio, S.C.; Rangel-Santos, A.C.; Peres, C.M.; Curi, R.; Cury, Y. Inhibitory effect of phospholipase A2 isolated from Crotalus durissus terrificus venom on macrophage function. Toxicon 2005, 45, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.P.; Favoretto, B.C.; Clissa, P.B.; Sampaio, S.C.; Faquim-Mauro, E.L. Crotoxin Isolated from Crotalus durissus terrificus Venom Modulates the Functional Activity of Dendritic Cells via Formyl Peptide Receptors. J. Immunol. Res. 2018, 2018, 7873257. [Google Scholar] [CrossRef]
- Giannotti, K.C.; Leiguez, E.; De Carvalho, A.E.Z.; Nascimento, N.G.; Matsubara, M.H.; Fortes-Dias, C.L.; Moreira, V.; Teixeira, C. A snake venom group IIA PLA2 with immunomodulatory activity induces formation of lipid droplets containing 15-d-PGJ2 in macrophages. Sci. Rep. 2017, 7, 4098. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.; De Castro Souto, P.C.M.; Ramirez Vinolo, M.A.; Lomonte, B.; María Gutiérrez, J.; Curi, R.; Teixeira, C. A catalytically-inactive snake venom Lys49 phospholipase A2 homolog induces expression of cyclooxygenase-2 and production of prostaglandins through selected signaling pathways in macrophages. Eur. J. Pharmacol. 2013, 708, 68–79. [Google Scholar] [CrossRef]
- Moreira, V.; Gutiérrez, J.M.; Amaral, R.B.; Zamunér, S.R.; de Fátima Pereira Teixeira, C. Effects of Bothrops asper snake venom on the expression of cyclooxygenases and production of prostaglandins by peritoneal leukocytes in vivo, and by isolated neutrophils and macrophages in vitro. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 107–114. [Google Scholar] [CrossRef]
- Moreira, V.; Lomonte, B.; Vinolo, M.A.R.; Curi, R.; Gutiérrez, J.M.; Teixeira, C. An asp49 phospholipase A2 from snake venom induces cyclooxygenase-2 expression and prostaglandin E2 production via activation of NF- κ B, p38MAPK, and PKC in macrophages. Mediat. Inflamm. 2014. [Google Scholar] [CrossRef]
- Moreira, V.; Gutiérrez, J.M.; Amaral, R.B.; Lomonte, B.; Purgatto, E.; Teixeira, C. A phospholipase A2 from Bothrops asper snake venom activates neutrophils in culture: Expression of cyclooxygenase-2 and PGE2 biosynthesis. Toxicon 2011, 57, 288–296. [Google Scholar] [CrossRef]
- Moreira, V.; Gutiérrez, J.M.; Lomonte, B.; Vinolo, M.A.R.; Curi, R.; Lambeau, G.; Teixeira, C. 12-HETE is a regulator of PGE2 production via COX-2 expression induced by a snake venom group IIA phospholipase A2 in isolated peritoneal macrophages. Chem. Biol. Interact. 2020, 317, 108903. [Google Scholar] [CrossRef] [PubMed]
- Cristina Giannotti, K.; Leiguez, E.; Moreira, V.; Nascimento, N.G.; Lomonte, B.; Gutiérrez, J.M.; Lopes De Melo, R.; Teixeira, C. A Lys49 phospholipase A2, isolated from Bothrops asper snake venom, induces lipid droplet formation in macrophages which depends on distinct signaling pathways and the C-terminal region. Biomed Res. Int. 2013, 2013, 807982. [Google Scholar] [CrossRef]
- Giannotti, K.C.; Weinert, S.; Viana, M.N.; Leiguez, E.; Araujo, T.L.S.; Laurindo, F.R.M.; Lomonte, B.; Braun-Dullaeus, R.; Teixeira, C. A secreted phospholipase A2 induces formation of smooth muscle foam cells which transdifferentiate to macrophage-like state. Molecules 2019, 24, 3244. [Google Scholar] [CrossRef]
- Leiguez, E.; Motta, P.; Maia Marques, R.; Lomonte, B.; Sampaio, S.V.; Teixeira, C. A Representative GIIA Phospholipase A2 Activates Preadipocytes to Produce Inflammatory Mediators Implicated in Obesity Development. Biomolecules 2020, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Leiguez, E.; Giannotti, K.C.; Do Nascimento Viana, M.; Matsubara, M.H.; Fernandes, C.M.; Gutiérrez, J.M.; Lomonte, B.; Teixeira, C. A snake venom-secreted phospholipase A2 induces foam cell formation depending on the activation of factors involved in lipid homeostasis. Mediat. Inflamm. 2018, 2018, 2547918. [Google Scholar] [CrossRef]
- Murakami, M.; Nakatani, Y.; Atsumi, G.I.; Inoue, K.; Kudo, I. Regulatory functions of phospholipase A2. Crit. Rev. Immunol. 2017, 37, 121–180. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, T.; Ogawa, Y.; Uozumi, N.; Kume, K.; Izumi, T.; Shimizu, T. cDNA Cloning and Mutagenesis Study of Leukotriene B4 12-Hydroxydehydrogenase. Adv. Exp. Med. Biol. 1997, 151–156. [Google Scholar] [CrossRef]
- Sarau, H.M.; Foley, J.J.; Schmidt, D.B.; Martin, L.D.; Webb, E.F.; Tzimas, M.N.; Breton, J.J.; Chabot-Fletcher, M.; Underwood, D.C.; Hay, D.W.P.; et al. In vitro and in vivo pharmacological characterization of SB 201993, an eicosanoid-like LTB4receptor antagonist with anti-inflammatory activity. Prostaglandins Leukot. Essent. Fat. Acids 1999, 61, 55–64. [Google Scholar] [CrossRef]
- Serhan, C.N.; Takano, T.; Maddox, J.F. Aspirin-Triggered 15-Epi-Lipoxin A4 and Stable Analogs of Lipoxin A4 are Potent Inhibitors of Acute Inflammation. Adv. Exp. Med. Biol. 1999, 133–149. [Google Scholar] [CrossRef]
- Chen, J.-K.; Wang, D.-W.; Falck, J.R.; Capdevila, J.; Harris, R.C. Transfection of an Active Cytochrome P450 Arachidonic Acid Epoxygenase Indicates That 14,15-Epoxyeicosatrienoic Acid Functions as an Intracellular Second Messenger in Response to Epidermal Growth Factor. J. Biol. Chem. 1999, 274, 4764–4769. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosanoids. Essays Biochem. 2020, 64, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, B.; Dahlén, S.-E.; Lindgren, J.Å.; Rouzer, C.A.; Serhan, C.N. Leukotrienes and Lipoxins: Structures, Biosynthesis, and Biological Effects. Science 1987, 237, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Rocca, B.; FitzGerald, G.A. Cyclooxygenases and prostaglandins: Shaping up the immune response. Int. Immunopharmacol. 2002, 2, 603–630. [Google Scholar] [CrossRef]
- O’Neill, G.P.; Ford-Hutchinson, A.W. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett. 1993, 330, 157–160. [Google Scholar] [CrossRef]
- Merlie, J.P.; Fagan, D.; Mudd, J.; Needleman, P. Isolation and characterization of the complementary DNA for sheep seminal vesicle prostaglandin endoperoxide synthase (cyclooxygenase). J. Biol. Chem. 1988, 263, 3550–3553. [Google Scholar] [CrossRef]
- Funk, C.D.; Funk, L.B.; Kennedy, M.E.; Pong, A.S.; Fitzgerald, G.A. Human platelet/erythroleukemia cell prostaglandin G/H synthase: cDNA cloning, expression, and gene chromosomal assignment. FASEB J. 1991, 5, 2304–2312. [Google Scholar] [CrossRef]
- Pannunzio, A.; Coluccia, M. Cyclooxygenase-1 (COX-1) and COX-1 inhibitors in cancer: A review of oncology and medicinal chemistry literature. Pharmaceuticals 2018, 11, 101. [Google Scholar] [CrossRef]
- Pruzanski, W.; Stefanski, E.; Vadas, P.; Kennedy, B.P.; van den Bosch, H. Regulation of the cellular expression of secretory and cytosolic phospholipases A2, and cyclooxygenase-2 by peptide growth factors. Biochim. Biophys. Acta-Mol. Cell Res. 1998, 1403, 47–56. [Google Scholar] [CrossRef][Green Version]
- Martínez-Colón, G.J.; Moore, B.B. Prostaglandin E2 as a Regulator of Immunity to Pathogens. Pharmacol. Ther. 2018, 185, 135–146. [Google Scholar] [CrossRef]
- Minghetti, L. Cyclooxygenase-2 (COX-2) in Inflammatory and Degenerative Brain Diseases. J. Neuropathol. Exp. Neurol. 2004, 63, 901–910. [Google Scholar] [CrossRef]
- Zidar, N.; Odar, K.; Glavac, D.; Jerse, M.; Zupanc, T.; Stajer, D. Cyclooxygenase in normal human tissues—Is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J. Cell. Mol. Med. 2009, 13, 3753–3763. [Google Scholar] [CrossRef]
- Moreira, V.; Gutiérrez, J.M.; Soares, A.M.; Zamunér, S.R.; Purgatto, E.; de Fátima Pereira Teixeira, C. Secretory phospholipases A2 isolated from Bothrops asper and from Crotalus durissus terrificus snake venoms induce distinct mechanisms for biosynthesis of prostaglandins E2 and D2 and expression of cyclooxygenases. Toxicon 2008, 52, 428–439. [Google Scholar] [CrossRef]
- Gerritsen, M.E. Physiological and pathophysiological roles of eicosanoids in the microcirculation. Cardiovasc. Res. 1996, 32, 720–732. [Google Scholar] [CrossRef]
- Kida, T.; Sawada, K.; Kobayashi, K.; Hori, M.; Ozaki, H.; Murata, T. Diverse effects of prostaglandin e2on vascular contractility. Heart Vessel 2014, 29, 390–395. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Lomonte, B.; Chaves, F.; Moreno, E.; Cerdas, L. Pharmacological activities of a toxic phospholipase a isolated from the venom of the snake Bothrops asper. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1986, 84, 159–164. [Google Scholar] [CrossRef]
- Moreira, V.; Zamuner, S.R.; Wallace, J.L.; de Fátima PereiraTeixeira, C. Bothrops jararaca and Crotalus durissus terrificus venoms elicit distinct responses regarding to production of prostaglandins E2 and D2, and expression of cyclooxygenases. Toxicon 2007, 49, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Evans, H.J. The role of enzymatic activity in inhibition of the extrinsic tenase complex by phospholipase A2 isoenzymes from Naja nigricollis venom. Toxicon 1995, 33, 1585–1590. [Google Scholar] [CrossRef]
- Thommesen, L.; Sjursen, W.; Gåsvik, K.; Hanssen, W.; Brekke, O.L.; Skattebøl, L.; Holmeide, A.K.; Espevik, T.; Johansen, B.; Laegreid, A. Selective inhibitors of cytosolic or secretory phospholipase A2 block TNF-induced activation of transcription factor nuclear factor-kappa B and expression of ICAM-1. J. Immunol. 1998, 161, 3421–3430. [Google Scholar] [PubMed]
- Anthonsen, M.W.; Solhaug, A.; Johansen, B. Functional Coupling between Secretory and Cytosolic Phospholipase A2 Modulates Tumor Necrosis Factor-α- and Interleukin-1β-induced NF-κB Activation. J. Biol. Chem. 2001, 276, 30527–30536. [Google Scholar] [CrossRef]
- Balsinde, J.; Balboa, M.A.; Dennis, E.A. Functional coupling between secretory phospholipase A2 and cyclooxygenase-2 and its regulation by cytosolic group IV phospholipase A2. Proc. Natl. Acad. Sci. USA 1998, 95, 7951–7956. [Google Scholar] [CrossRef]
- Peng, Z.; Chang, Y.; Fan, J.; Ji, W.; Su, C. Phospholipase A2 superfamily in cancer. Cancer Lett. 2021, 497, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Naraba, H.; Murakami, M.; Matsumoto, H.; Shimbara, S.; Ueno, A.; Kudo, I.; Oh-ishi, S. Segregated coupling of phospholipases A2, cyclooxygenases, and terminal prostanoid synthases in different phases of prostanoid biosynthesis in rat peritoneal macrophages. J. Immunol. 1998, 160, 2974–2982. [Google Scholar] [PubMed]
- Kuwata, H.; Nakatani, Y.; Murakami, M.; Kudo, I. Cytosolic Phospholipase A2 Is Required for Cytokine-induced Expression of Type IIA Secretory Phospholipase A2 That Mediates Optimal Cyclooxygenase-2-dependent Delayed Prostaglandin E2 Generation in Rat 3Y1 Fibroblasts. J. Biol. Chem. 1998, 273, 1733–1740. [Google Scholar] [CrossRef]
- Murakami, M.; Shimbara, S.; Kambe, T.; Kuwata, H.; Winstead, M.V.; Tischfield, J.A.; Kudo, I. The Functions of Five Distinct Mammalian Phospholipase A2s in Regulating Arachidonic Acid Release. J. Biol. Chem. 1998, 273, 14411–14423. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Das, S.; Kim, Y.-J.; Cho, W.; Kudo, I. Perinuclear localization of cytosolic phospholipase A 2 α is important but not obligatory for coupling with cyclooxygenases. FEBS Lett. 2003, 546, 251–256. [Google Scholar] [CrossRef]
- Ghosh, M.; Stewart, A.; Tucker, D.E.; Bonventre, J.V.; Murphy, R.C.; Leslie, C.C. Role of Cytosolic Phospholipase A 2 in Prostaglandin E 2 Production by Lung Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2004, 30, 91–100. [Google Scholar] [CrossRef]
- Belich, M.P.; Salmerón, A.; Johnston, L.H.; Ley, S.C. TPL-2 kinase regulates the proteolysis of the NF-κB-inhibitory protein NF-κB1 p105. Nature 1999, 397, 363–368. [Google Scholar] [CrossRef]
- Kifor, O.; MacLeod, R.J.; Diaz, R.; Bai, M.; Yamaguchi, T.; Yao, T.; Kifor, I.; Brown, E.M. Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected HEK293 cells. Am. J. Physiol. Physiol. 2001, 280, F291–F302. [Google Scholar] [CrossRef]
- Aoki, K.; Zubkov, A.Y.; Parent, A.D.; Zhang, J.H. Mechanism of ATP-Induced [Ca2+] i Mobilization in Rat Basilar Smooth Muscle Cells. Stroke 2000, 31, 1377–1385. [Google Scholar] [CrossRef]
- Leiguez, E.; Giannotti, K.C.; Moreira, V.; Matsubara, M.H.; Gutíerrez, J.M.; Lomonte, B.; Rodriǵuez, J.P.; Balsinde, J.; Teixeira, C. Critical role of TLR2 and MyD88 for functional response of macrophages to a group IIA-secreted phospholipase A2from snake venom. PLoS ONE 2014, 9, e93741. [Google Scholar] [CrossRef]
- Soto, M.E.; Guarner-Lans, V.; Herrera-Morales, K.Y.; Pérez-Torres, I. Participation of arachidonic acid metabolism in the aortic aneurysm formation in patients with Marfan syndrome. Front. Physiol. 2018, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Rikitake, Y.; Hirata, K.; Kawashima, S.; Takeuchi, S.; Shimokawa, Y.; Kojima, Y.; Inoue, N.; Yokoyama, M. Signaling Mechanism Underlying COX-2 Induction by Lysophosphatidylcholine. Biochem. Biophys. Res. Commun. 2001, 281, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Fulford, M.; Tjandrawinata, R.R.; Li, C.-F.; Sayyah, S. Arachidonic acid, an omega-6 fatty acid, induces cytoplasmic phospholipase A 2 in prostate carcinoma cells. Carcinogenesis 2005, 26, 1520–1526. [Google Scholar] [CrossRef]
- Ruipérez, V.; Casas, J.; Balboa, M.A.; Balsinde, J. Group V Phospholipase A 2 -Derived Lysophosphatidylcholine Mediates Cyclooxygenase-2 Induction in Lipopolysaccharide-Stimulated Macrophages. J. Immunol. 2007, 179, 631–638. [Google Scholar] [CrossRef]
- Gubern, A.; Barceló-Torns, M.; Casas, J.; Barneda, D.; Masgrau, R.; Picatoste, F.; Balsinde, J.; Balboa, M.A.; Claro, E. Lipid droplet biogenesis induced by stress involves triacylglycerol synthesis that depends on Group VIA phospholipase A2. J. Biol. Chem. 2009, 284, 5697–5708. [Google Scholar] [CrossRef] [PubMed]
- Guijas, C.; Pérez-Chacón, G.; Astudillo, A.M.; Rubio, J.M.; Gil-de-Gómez, L.; Balboa, M.A.; Balsinde, J. Simultaneous activation of p38 and JNK by arachidonic acid stimulates the cytosolic phospholipase A2-dependent synthesis of lipid droplets in human monocytes. J. Lipid Res. 2012, 53, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Jarc, E.; Kump, A.; Malavašič, P.; Eichmann, T.O.; Zimmermann, R.; Petan, T. Lipid droplets induced by secreted phospholipase A2 and unsaturated fatty acids protect breast cancer cells from nutrient and lipotoxic stress. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2018, 1863, 247–265. [Google Scholar] [CrossRef]
- Arrese, E.L.; Saudale, F.Z.; Soulages, J.L. Lipid droplets as signaling platforms linking metabolic and cellular functions. Lipid Insights 2014, 7, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Onal, G.; Kutlu, O.; Gozuacik, D.; Dokmeci Emre, S. Lipid Droplets in Health and Disease. Lipids Health Dis. 2017, 16, 128. [Google Scholar] [CrossRef]
- Welte, M.A. Expanding roles for lipid droplets. Curr. Biol. 2015, 25, R470–R481. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Sánchez-Álvarez, M.; Fajardo, A.; Kapetanovic, R.; Steiner, B.; Dutra, F.; Moreira, L.; López, J.A.; Campo, R.; Marí, M.; et al. Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science 2020, 370, 6514. [Google Scholar] [CrossRef]
- Karagiannis, F.; Masouleh, S.K.; Wunderling, K.; Surendar, J.; Schmitt, V.; Kazakov, A.; Michla, M.; Hölzel, M.; Thiele, C.; Wilhelm, C. Lipid-Droplet Formation Drives Pathogenic Group 2 Innate Lymphoid Cells in Airway Inflammation. Immunity 2020, 52, 620–634.e6. [Google Scholar] [CrossRef] [PubMed]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Garces, F.; López, F.; Nĩo, C.; Fernandez, A.; Chacin, L.; Hurt-Camejo, E.; Camejo, G.; Apitz-Castro, R. High plasma phospholipase A 2 activity, inflammation markers, and LDL alterations in obesity with or without type 2 diabetes. Obesity 2010, 18, 2023–2029. [Google Scholar] [CrossRef]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef]
- Schaftenaar, F.; Frodermann, V.; Kuiper, J.; Lutgens, E. Atherosclerosis: The interplay between lipids and immune cells. Curr. Opin. Lipidol. 2016, 27, 209–215. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Leiguez, E.; Guijas, C.; Lomonte, B.; Gutiérrez, J.M.; Teixeira, C.; Balboa, M.A.; Balsinde, J. A lipidomic perspective of the action of group iia secreted phospholipase a2 on human monocytes: Lipid droplet biogenesis and activation of cytosolic phospholipase a2α. Biomolecules 2020, 10, 891. [Google Scholar] [CrossRef]
- Moujaber, O.; Stochaj, U. The Cytoskeleton as Regulator of Cell Signaling Pathways. Trends Biochem. Sci. 2020, 45, 96–107. [Google Scholar] [CrossRef]
- Leiguez, E.; Zuliani, J.P.; Cianciarullo, A.M.; Fernandes, C.M.; Gutierrez, J.M.; Teixeira, C. A group IIA-secreted phospholipase A2 from snake venom induces lipid body formation in macrophages: The roles of intracellular phospholipases A2 and distinct signaling pathways. J. Leukoc. Biol. 2011, 90, 155–166. [Google Scholar] [CrossRef]
- Yu, Y.H.; Liao, P.R.; Guo, C.J.; Chen, C.H.; Mochly-Rosen, D.; Chuang, L.M. PKC-ALDH2 pathway plays a novel role in adipocyte differentiation. PLoS ONE 2016, 11, e0161993. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Shen, W.J.; Yeo, H.L.; Wang, S.M. Signaling pathway of magnolol-stimulated lipolysis in sterol ester-loaded 3T3-L1 preadipocyes. J. Cell. Biochem. 2004, 91, 1021–1029. [Google Scholar] [CrossRef]
- Le Lay, S.; Hajduch, E.; Lindsay, M.R.; Le Lièpvre, X.; Thiele, C.; Ferré, P.; Parton, R.G.; Kurzchalia, T.; Simons, K.; Dugail, I. Cholesterol-induced caveolin targeting to lipid droplets in adipocytes: A role for caveolar endocytosis. Traffic 2006, 7, 549–561. [Google Scholar] [CrossRef]
- Than, N.G.; Sumegi, B.; Bellyei, S.; Berki, T.; Szekeres, G.; Janaky, T.; Szigeti, A.; Bohn, H.; Than, G.N. Lipid droplet and milk lipid globule membrane associated placental protein 17b (PP17b) is involved in apoptotic and differentiation processes of human epithelial cervical carcinoma cells. Eur. J. Biochem. 2003, 270, 1176–1188. [Google Scholar] [CrossRef]
- Zhong, W.; Fan, B.; Cong, H.; Wang, T.; Gu, J. Oleic acid-induced perilipin 5 expression and lipid droplets formation are regulated by the PI3K/PPARα pathway in HepG2 cells. Appl. Physiol. Nutr. Metab. 2019, 44, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, T.; Zhang, J.; Liu, X.; Li, Z.; Wang, G.; Song, Q.; Pang, D.; Ouyang, H.; Tang, X. Apolipoprotein CIII regulates lipoprotein-associated phospholipase A2 expression via the MAPK and NFκB pathways. Biol. Open 2015, 4, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-B.; Zou, Q.; Lv, X.; Zhou, R.L.; Niu, X.; Weng, C.; Chen, F.; Fan, Y.W.; Deng, Z.Y.; Li, J. 9t18:1 and 11t18:1 activate the MAPK pathway to regulate the expression of PLA2 and cause inflammation in HUVECs. Food Funct. 2020, 11, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.; Boström, P.; Ericson, J.; Rutberg, M.; Magnusson, B.; Marchesan, D.; Ruiz, M.; Asp, L.; Huang, P.; Frohman, M.A.; et al. PLD1 and ERK2 regulate cytosolic lipid droplet formation. J. Cell Sci. 2006, 119, 2246–2257. [Google Scholar] [CrossRef]
- Boström, P.; Andersson, L.; Li, L.; Perkins, R.; Højlund, K.; Borén, J.; Olofsson, S.O. The assembly of lipid droplets and its relation to cellular insulin sensitivity. Biochem. Soc. Trans. 2009, 37, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Reustle, A.; Torzewski, M. Role of p38 MAPK in atherosclerosis and aortic valve sclerosis. Int. J. Mol. Sci. 2018, 19, 3761. [Google Scholar] [CrossRef] [PubMed]
- Kavurma, M.M.; Rayner, K.J.; Karunakaran, D. The walking dead: Macrophage inflammation and death in atherosclerosis. Curr. Opin. Lipidol. 2017, 28, 91–98. [Google Scholar] [CrossRef]
- Chakraborti, S. Phospholipase A2 isoforms: A perspective. Cell. Signal. 2003, 15, 637–665. [Google Scholar] [CrossRef]
- Hooks, S.B.; Cummings, B.S. Role of Ca2+-independent phospholipase A2 in cell growth and signaling. Biochem. Pharmacol. 2008, 76, 1059–1067. [Google Scholar] [CrossRef]
- Kita, Y.; Shindou, H.; Shimizu, T. Cytosolic phospholipase A 2 and lysophospholipid acyltransferases. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2019, 1864, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, A. Obesity and Lipotoxicity; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Mei, C.-L.; He, P.; Cheng, B.; Liu, W.; Wang, Y.-F.; Wan, J.-J. Chlamydia pneumoniae induces macrophage-derived foam cell formation via PPAR α and PPAR γ-dependent pathways. Cell Biol. Int. 2009, 33, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef]
- Son, S.H.; Goo, Y.H.; Chang, B.H.; Paul, A. Perilipin 2 (PLIN2)-deficiency does not increase cholesterol-induced toxicity in macrophages. PLoS ONE 2012, 7, e33063. [Google Scholar] [CrossRef][Green Version]
- Turkish, A.; Sturley, S.L. Regulation of Triglyceride Metabolism. I. Eukaryotic neutral lipid synthesis: “Many ways to skin ACAT or a DGAT”. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, 953–957. [Google Scholar] [CrossRef]
- Yu, X.H.; Fu, Y.C.; Zhang, D.W.; Yin, K.; Tang, C.K. Foam cells in atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.L.S.; Barreto, E.D.A.; Fazolini, N.P.B.; Viola, J.P.B.; Bozza, P.T. Lipid droplets: Platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.S.G.; Soares, V.C.; Ferreira, A.C.; Sacramento, C.Q.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Teixeira, L.; Nunes da Silva, M.A.; Barreto, E.; Mattos, M.; et al. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog. 2020, 16, e1009127. [Google Scholar] [CrossRef]
- Jarc, E.; Petan, T. A twist of FATe: Lipid droplets and inflammatory lipid mediators. Biochimie 2020, 169, 69–87. [Google Scholar] [CrossRef]
- De Carvalho, A.E.Z.; Giannotti, K.; Junior, E.L.; Matsubara, M.; Dos Santos, M.C.; Fortes-Dias, C.L.; Teixeira, C. Crotalus durissus ruruima Snake Venom and a Phospholipase A2 Isolated from This Venom Elicit Macrophages to Form Lipid Droplets and Synthesize Inflammatory Lipid Mediators. J. Immunol. Res. 2019, 2019, 2745286. [Google Scholar] [CrossRef]
- Juge-Aubry, C.E.; Henrichot, E.; Meier, C.A. Adipose tissue: A regulator of inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Antoniades, C. The role of adipose tissue in cardiovascular health and disease. Nat. Rev. Cardiol. 2019, 16, 83–99. [Google Scholar] [CrossRef]
- La Cava, A.; Matarese, G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004, 4, 371–379. [Google Scholar] [CrossRef]
- Tsatsanis, C.; Zacharioudaki, V.; Androulidaki, A.; Dermitzaki, E.; Charalampopoulos, I.; Minas, V.; Gravanis, A.; Margioris, A.N. Adiponectin induces TNF-α and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem. Biophys. Res. Commun. 2005, 335, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Argueta, J.G.M.; Masuhiro, Y.; Kagishita, M.; Nonaka, K.; Saito, T.; Hanazawa, S.; Yamashita, Y. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005, 579, 6821–6826. [Google Scholar] [CrossRef]
- Martínez-Sánchez, N. There and back again: Leptin actions in white adipose tissue. Int. J. Mol. Sci. 2020, 21, 6039. [Google Scholar] [CrossRef] [PubMed]

| PLA2 | Origin | Basic or Acid | Type of PLA2 Variant | Inflammatory Activity/ Experimental Model | Refs. |
|---|---|---|---|---|---|
| Piratoxin-I | B. pirajai | Basic | Lys49 | Increase in vascular permeability (in vivo) Mast cell degranulation, neutrophil chemotaxis (in vitro) | [125,136] |
| P-1 | B. neuwiedii | Acidic | nd | Edema (in vivo) | [126] |
| P-2 | B. neuwiedii | Acidic | nd | Edema (in vivo) | [126] |
| SIIISPIIA | B. jararacussu | Acidic | Asp49 | Edema (in vivo) | [129] |
| SIIISPIIB | B. jararacussu | Acidic | Asp49 | Edema (in vivo) | [129] |
| SIIISPIIIA | B. jararacussu | Acidic | Asp49 | Edema (in vivo) | [129] |
| SIIISPIIIB | B. jararacussu | Acidic | Asp49 | Edema (in vivo) | [129] |
| BintTX-I | B. insularis | Acidic | Asp49 | Edema (in vivo) | [130] |
| Bothropstoxin-I (BthTX-I) | B. jararacussu | Basic | Lys49 | Edema, leukocyte migration, mast cell degranulation (in vivo) Neutrophil chemotaxis, activation of inflammasome (in vitro) | [128,131,136,147,148] |
| Bothropstoxin-II (BthTX-II) | B. jararacussu | Basic | Asp49 | Edema, leukocyte migration, mast cell degranulation (in vivo) Neutrophil chemotaxis (in vitro) | [128,131,136] |
| Myotoxin-II (MT-II) | B. asper | Basic | Lys49 | Increase in vascular permeability, leukocyte migration, release of mediators, hyperalgesia, eicosanoid production, COX-2 expression (in vivo) Phagocytosis, H2O2 production COX-2 expression, lipid droplet formation (in vitro) | [123,126,135,138,161,162,163,164,165,166,167] |
| Myotoxin-III (MT-III) | B. asper | Basic | Asp49 | Increase in vascular permeability, leukocyte migration, release of mediators, hyperalgesia, eicosanoid production; COX-2 expression (in vivo) Phagocytosis, H2O2 production, COX-2 expression, lipid droplet formation, preadipocyte activation (in vitro) | [123,126,135,138,162,163,164,165,166,168,169,170] |
| BnSP-7 | B. pauloensis | Basic | Lys49 | Edema (in vivo) | [139] |
| BatroxPLA2 | B. atrox | Acidic | Asp49 | Leukocyte chemotaxis, mediators release (in vivo) Mast cell degranulation (in vitro) | [140] |
| BJ-PLA2-I | B. jararaca | Acidic | Asp49 | Leukocyte migration, mediators release (in vivo) | [141] |
| Bbil-TX | B. bilineata | Basic | nd | Neutrophil migration, mediators release (in vivo) | [145] |
| BmaTX-I | B. mattogrossensis | Basic | Lys49 | Mediator release (in vitro) | [146] |
| BmaTX-II | B. mattogrossensis | Basic | Lys49 | Mediator release (in vitro) | [146] |
| BaltTX-I | B. alternatus | Basic | Lys49 | Phagocytosis, superoxide production (in vitro) | [149] |
| BaltTX-II | B. alternatus | Basic | Asp49 | Superoxide production (in vitro) | [149] |
| pMTX-II | B. asper | Basic | Lys49 | Phagocytosis, superoxide production (in vitro) | [150] |
| pMTX-III | B. asper | Basic | Asp49 | Phagocytosis, superoxide production (in vitro) | [150] |
| pMTX-IV | B. asper | Basic | Lys49 | Phagocytosis, superoxide production (in vitro) | [150] |
| BaTX-I | B. atrox | Basic | Lys49 | Superoxide production, lipid droplet formation (in vitro) | [155] |
| BaTX-II | B. atrox | Basic | Asp49 | Superoxide and H2O2 production, MPO release, NET formation, lipid droplet formation (in vitro) | [155] |
| BaPLA2 | B. atrox | Acidic | Asp49 | Superoxide production, lipid droplet formation (in vitro) | [140,155] |
| BaPLA2I | B. atrox | Basic | nd | Mast cell degranulation, edema (in vivo) | [132] |
| BaPLA2III | B. atrox | Neutral | nd | Mast cell Degranulation, edema (in vivo) | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, V.; Leiguez, E.; Janovits, P.M.; Maia-Marques, R.; Fernandes, C.M.; Teixeira, C. Inflammatory Effects of Bothrops Phospholipases A2: Mechanisms Involved in Biosynthesis of Lipid Mediators and Lipid Accumulation. Toxins 2021, 13, 868. https://doi.org/10.3390/toxins13120868
Moreira V, Leiguez E, Janovits PM, Maia-Marques R, Fernandes CM, Teixeira C. Inflammatory Effects of Bothrops Phospholipases A2: Mechanisms Involved in Biosynthesis of Lipid Mediators and Lipid Accumulation. Toxins. 2021; 13(12):868. https://doi.org/10.3390/toxins13120868
Chicago/Turabian StyleMoreira, Vanessa, Elbio Leiguez, Priscila Motta Janovits, Rodrigo Maia-Marques, Cristina Maria Fernandes, and Catarina Teixeira. 2021. "Inflammatory Effects of Bothrops Phospholipases A2: Mechanisms Involved in Biosynthesis of Lipid Mediators and Lipid Accumulation" Toxins 13, no. 12: 868. https://doi.org/10.3390/toxins13120868
APA StyleMoreira, V., Leiguez, E., Janovits, P. M., Maia-Marques, R., Fernandes, C. M., & Teixeira, C. (2021). Inflammatory Effects of Bothrops Phospholipases A2: Mechanisms Involved in Biosynthesis of Lipid Mediators and Lipid Accumulation. Toxins, 13(12), 868. https://doi.org/10.3390/toxins13120868





