Fatty Acid Modification of the Anticancer Peptide LVTX-9 to Enhance Its Cytotoxicity against Malignant Melanoma Cells
Abstract
:1. Introduction
2. Results
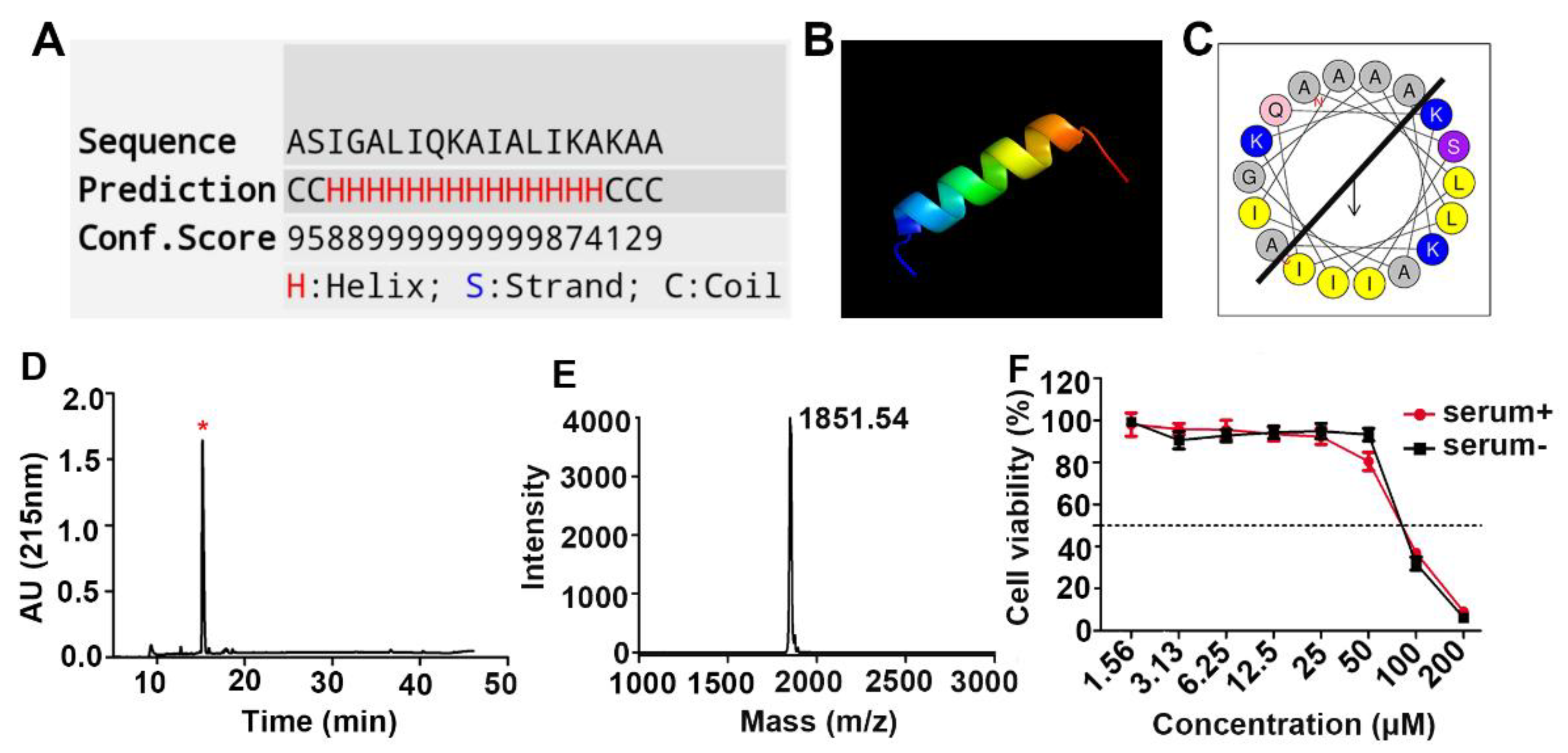
2.1. Design, Synthesis, and Cytotoxicity Characterization of the Anticancer Peptide LVTX-9
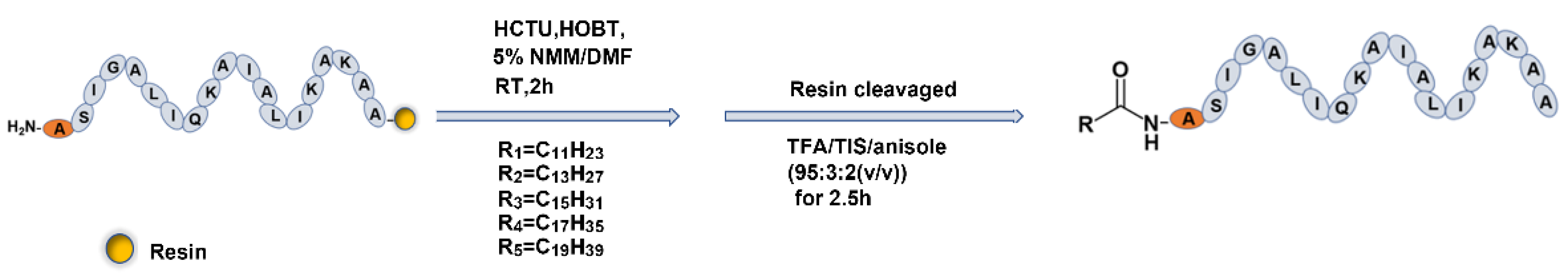
2.2. Fatty Acid Modification of Anticancer Peptide LVTX-9
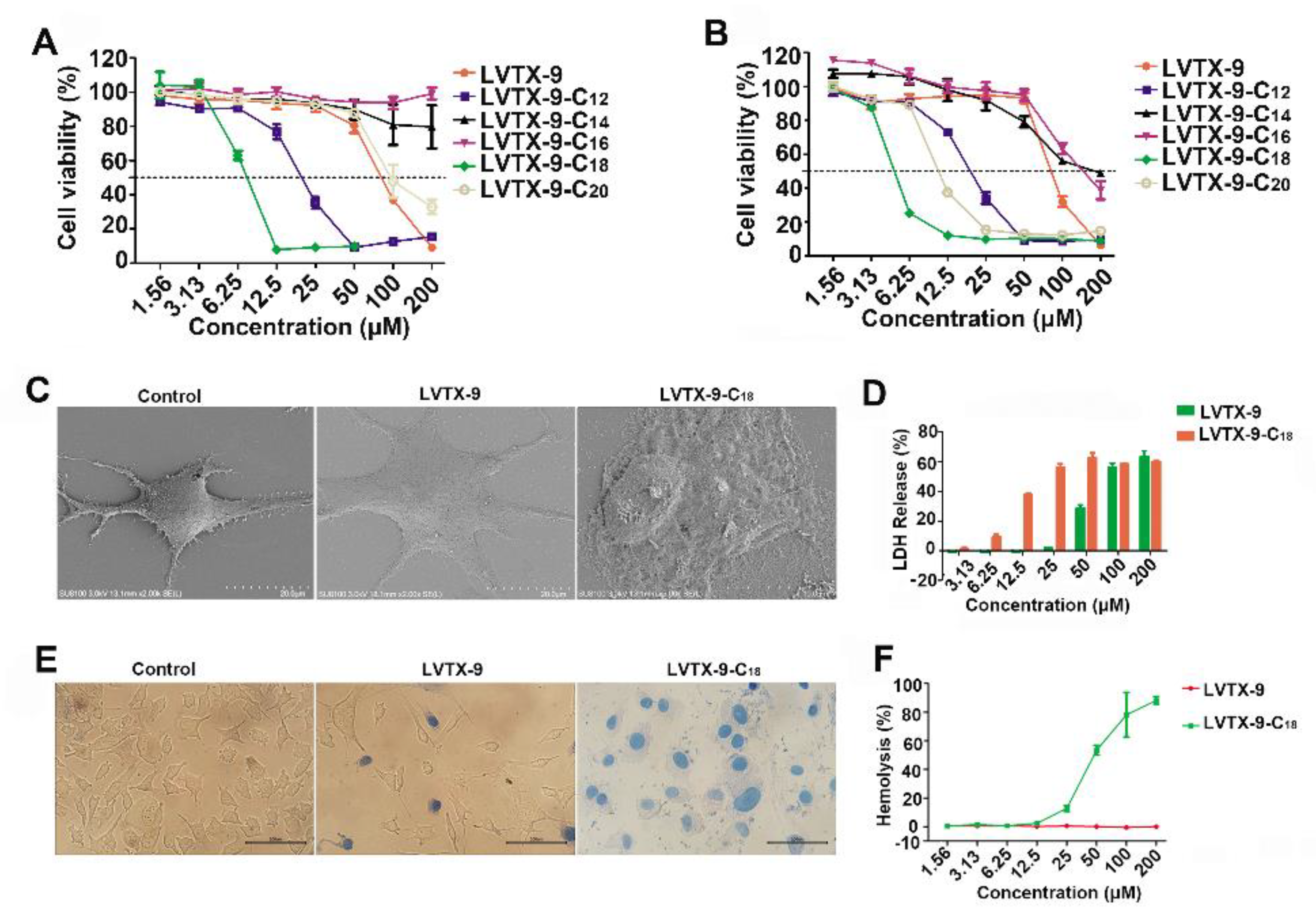
2.3. Cytotoxicity of Lipopeptides
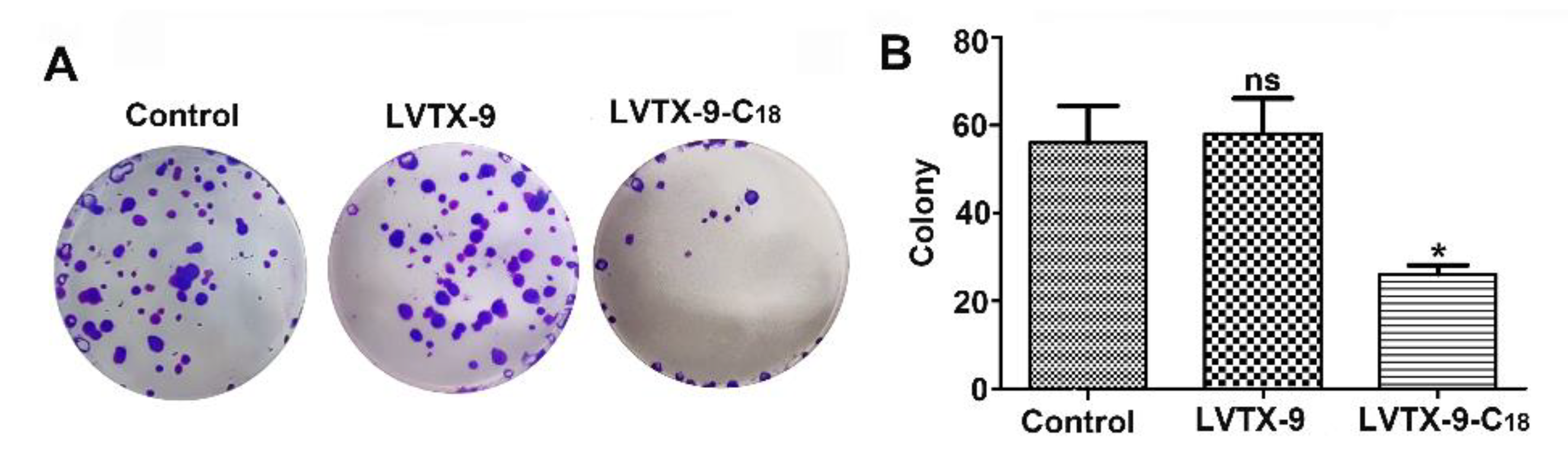
2.4. Anti-Proliferative Effect of LVTX-9 and LVTX-9-C18
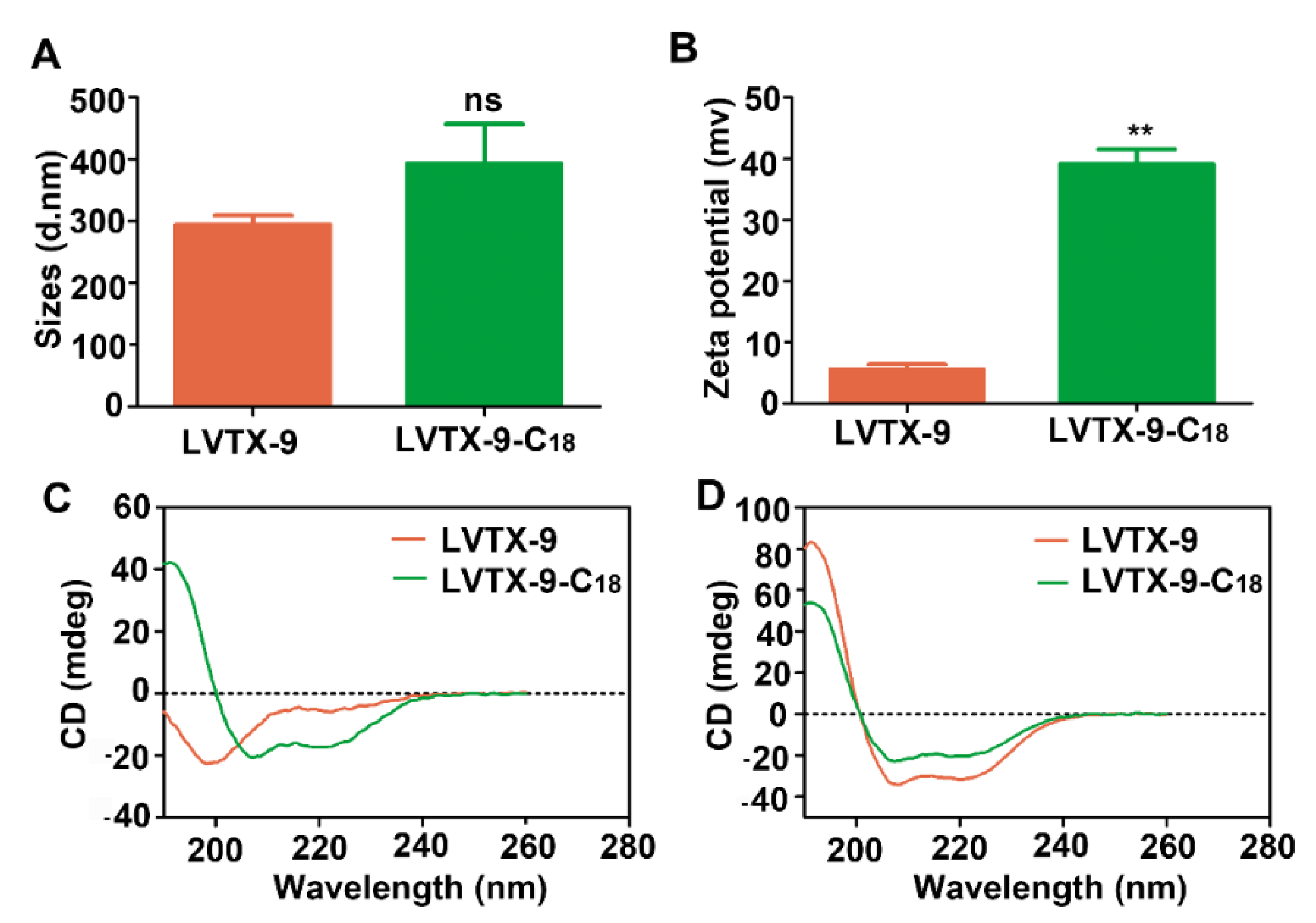
2.5. Physicochemical Characteristics of LVTX-9 and LVTX-9-C18
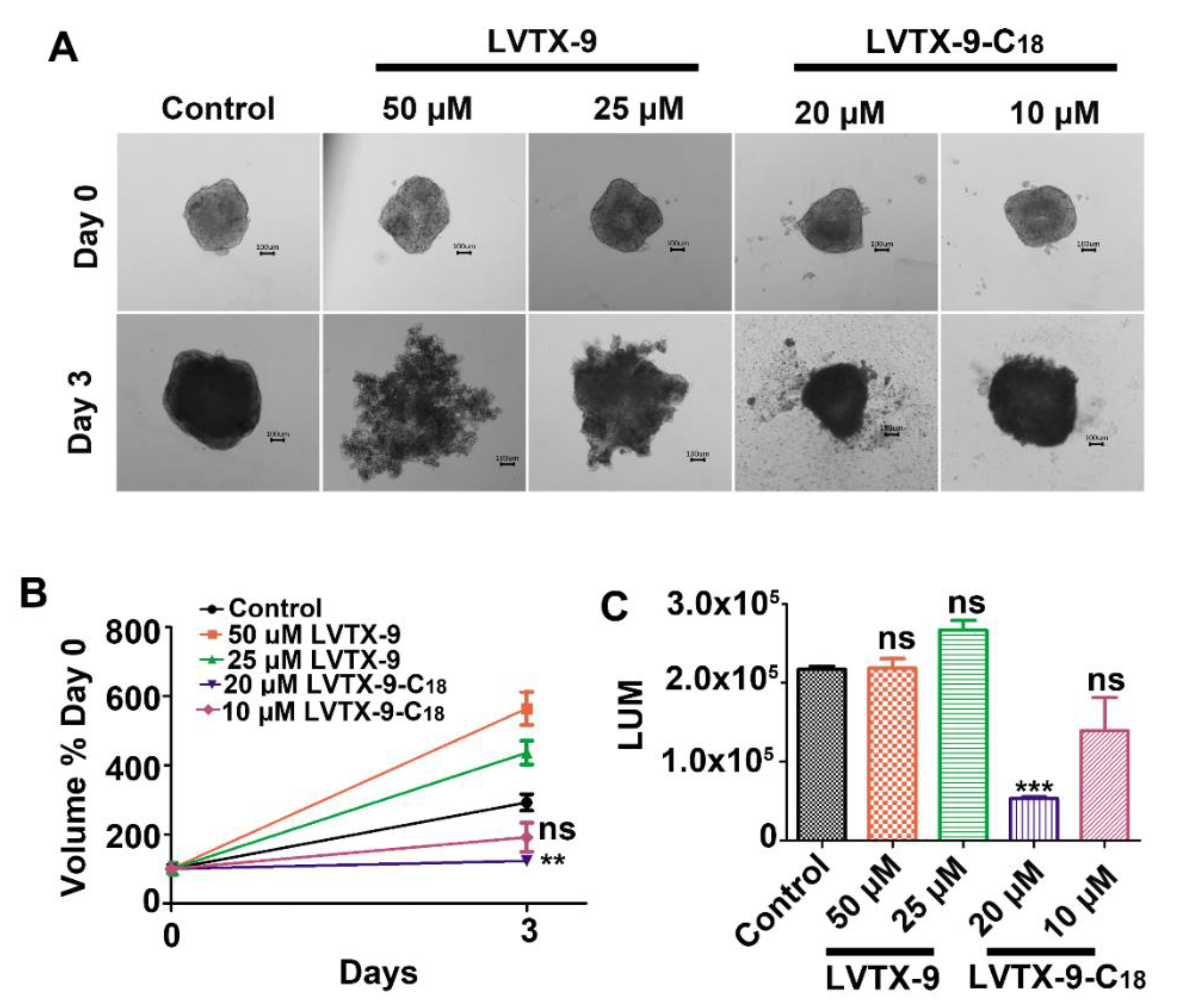
2.6. LVTX-9-C18 Exhibits Cytotoxicity in Relation to B16-F10 Tumor Spheroids
3. Discussion and Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Cell Cultures
4.3. Synthesis, Purification, and Identification of LVTX-9 and Five Lipopeptides
4.4. Cell Viability Assays
4.5. Scanning Electron Microscopy
4.6. Physical Characterization of Peptides
4.7. LDH Leakage Assays
4.8. Colony Formation Assay
4.9. Trypan Blue Staining
4.10. Determination of Hemolytic Activity
4.11. Toxicity in 3D Tumor Spheroids
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, D.; Armugam, A.; Jeyaseelan, K. Snake venom components and their applications in biomedicine. Cell. Mol. Life Sci. 2006, 63, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Primon-Barros, M.; Macedo, A.J. Animal Venom Peptides: Potential for New Antimicrobial Agents. Curr. Top. Med. Chem. 2016, 17, 1119–1156. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Hou, X.; Wang, L.; Zhang, Y.; Xi, X.; Wang, H.; Zhou, M.; Duan, J.; Wei, M.; Chen, T.; et al. AaeAP1 and AaeAP2: Novel antimicrobial peptides from the venom of the scorpion, Androctonus aeneas: Structural characterisation, molecular cloning of biosynthetic precursor-encoding cDNAs and engineering of analogues with enhanced antimicrobial and anticancer activities. Toxins 2015, 7, 219–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, J.; Chua, P.J.; Bay, B.H.; Gopalakrishnakone, P. Scorpion venoms as a potential source of novel cancer therapeutic compounds. Exp. Biol. Med. 2014, 239, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef]
- Han, T.S.; Teichert, R.W.; Olivera, B.M.; Bulaj, G. Conus venoms—A rich source of peptide-based therapeutics. Curr. Pharm. Des. 2008, 14, 2462–2479. [Google Scholar] [CrossRef] [PubMed]
- Akef, H.M. Anticancer, antimicrobial, and analgesic activities of spider venoms. Toxicol. Res. 2018, 7, 381–395. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Deng, M.; Xiang, J.; Ma, H.; Hu, W.; Zhao, Y.; Li, D.W.; Liang, S. A novel spider peptide toxin suppresses tumor growth through dual signaling pathways. Curr. Mol. Med. 2012, 12, 1350–1360. [Google Scholar] [CrossRef]
- Duffy, C.; Sorolla, A.; Wang, E.; Golden, E.; Woodward, E.; Davern, K.; Ho, D.; Johnstone, E.; Pfleger, K.; Redfern, A.; et al. Honeybee venom and melittin suppress growth factor receptor activation in HER2-enriched and triple-negative breast cancer. NPJ Precis. Oncol. 2020, 4, 24. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, J.; Yan, Y.; Chen, B.; Liu, B.; Jian, C.; Zhu, B.; Liang, S.; Zeng, Y.; Liu, Z. Arginine modification of lycosin-I to improve inhibitory activity against cancer cells. Org. Biomol. Chem. 2017, 15, 9379–9388. [Google Scholar] [CrossRef]
- Shuai, Q.; Cai, Y.; Zhao, G.; Sun, X. Cell-Penetrating Peptide Modified PEG-PLA Micelles for Efficient PTX Delivery. Int. J. Mol. Sci. 2020, 21, 1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, M.J.; Bentley, M.D.; Harris, J.M. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 2002, 54, 459–476. [Google Scholar] [CrossRef]
- Jian, C.; Zhang, P.; Ma, J.; Jian, S.; Zhang, Q.; Liu, B.; Liang, S.; Liu, M.; Zeng, Y.; Liu, Z. The Roles of Fatty-Acid Modification in the Activity of the Anticancer Peptide R-Lycosin-I. Mol. Pharm. 2018, 15, 4612–4620. [Google Scholar] [CrossRef]
- Matemu, A.O.; Katayama, S.; Kayahara, H.; Murasawa, H.; Nakamura, S. Improving surface functional properties of tofu whey-derived peptides by chemical modification with fatty acids. J. Food Sci. 2012, 77, C333–C339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, J.; Zhang, Q.; Jian, S.; Sun, X.; Liu, B.; Nie, L.; Liu, M.; Liang, S.; Zeng, Y.; et al. Monosaccharide Analogues of Anticancer Peptide R-Lycosin-I: Role of Monosaccharide Conjugation in Complexation and the Potential of Lung Cancer Targeting and Therapy. J. Med. Chem. 2019, 62, 7857–7873. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.E.; Leonard, J.N. Stabilization of exosome-targeting peptides via engineered glycosylation. J. Biol. Chem. 2015, 290, 8166–8172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehto, T.; Vasconcelos, L.; Margus, H.; Figueroa, R.; Pooga, M.; Hällbrink, M.; Langel, Ü. Saturated Fatty Acid Analogues of Cell-Penetrating Peptide PepFect14: Role of Fatty Acid Modification in Complexation and Delivery of Splice-Correcting Oligonucleotides. Bioconjugate Chem. 2017, 28, 782–792. [Google Scholar] [CrossRef]
- Deng, X.; Qiu, Q.; Ma, K.; Wang, X.; Huang, W.; Qian, H. Aliphatic acid-conjugated antimicrobial peptides--potential agents with anti-tumor, multidrug resistance-reversing activity and enhanced stability. Org. Biomol. Chem. 2015, 13, 7673–7680. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, T.; Gou, S.; He, Y.; Zhu, N.; Zhu, Y.; Wang, L.; Liu, H.; Zhang, Y.; Yao, J.; et al. Design and synthesis of new N-terminal fatty acid modified-antimicrobial peptide analogues with potent in vitro biological activity. Eur. J. Med. Chem. 2019, 182, 111636. [Google Scholar] [CrossRef]
- Zhang, P.; Jian, C.; Jian, S.; Zhang, Q.; Sun, X.; Nie, L.; Liu, B.; Li, F.; Li, J.; Liu, M.; et al. Position Effect of Fatty Acid Modification on the Cytotoxicity and Antimetastasis Potential of the Cytotoxic Peptide Lycosin-I. J. Med. Chem. 2019, 62, 11108–11118. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, C.; Tan, H.; Wang, H.; Jiang, Y.; Liang, S.; Zhang, F.; Liu, Z. A survey of the venom of the spider Lycosa vittata by biochemical, pharmacological and transcriptomic analyses. Toxicon Off. J. Int. Soc. Toxinol. 2015, 107, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Yan, Y.; Wang, J.; Dong, X.; Zhang, G.; Zeng, Y.; Liu, Z. An Anti-Cancer Peptide LVTX-8 Inhibits the Proliferation and Migration of Lung Tumor Cells by Regulating Causal Genes’ Expression in p53-Related Pathways. Toxins 2020, 12, 367. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, Q.; Yan, Q.; Hao, X.; Chen, Y. Alpha-helical cationic anticancer peptides: A promising candidate for novel anticancer drugs. Mini Rev. Med. Chem. 2015, 15, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, M.; Ericsson, A.C. Antimicrobial Peptides: Potential Application in Liver Cancer. Front. Microbiol. 2019, 10, 1257. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, Y.; Zhang, W.; Chen, J.; Yang, X.; Ma, P.; Zhang, B.; Liu, B.; Ni, J.; Wang, R. Cell penetrating peptide TAT can kill cancer cells via membrane disruption after attachment of camptothecin. Peptides 2015, 63, 143–149. [Google Scholar] [CrossRef]
- Kaspar, A.A.; Reichert, J.M. Future directions for peptide therapeutics development. Drug Discov. Today 2013, 18, 807–817. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B. Animal Venoms have Potential to Treat Cancer. Curr. Top. Med. Chem. 2018, 18, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Zha, R.H.; Sur, S.; Stupp, S.I. Self-assembly of cytotoxic peptide amphiphiles into supramolecular membranes for cancer therapy. Adv. Healthc. Mater. 2013, 2, 126–133. [Google Scholar] [CrossRef]
- Johnstone, S.A.; Gelmon, K.; Mayer, L.D.; Hancock, R.E.; Bally, M.B. In vitro characterization of the anticancer activity of membrane-active cationic peptides. I. Peptide-mediated cytotoxicity and peptide-enhanced cytotoxic activity of doxorubicin against wild-type and p-glycoprotein over-expressing tumor cell lines. Anti Cancer Drug Des. 2000, 15, 151–160. [Google Scholar]
- Chen, C.; Yang, C.; Chen, Y.; Wang, F.; Mu, Q.; Zhang, J.; Li, Z.; Pan, F.; Xu, H.; Lu, J.R. Surface Physical Activity and Hydrophobicity of Designed Helical Peptide Amphiphiles Control Their Bioactivity and Cell Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 26501–26510. [Google Scholar] [CrossRef]
- Utsugi, T.; Schroit, A.J.; Connor, J.; Bucana, C.D.; Fidler, I.J. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991, 51, 3062–3066. [Google Scholar] [PubMed]
- Lee, T.H.; Hall, K.N.; Aguilar, M.I. Antimicrobial Peptide Structure and Mechanism of Action: A Focus on the Role of Membrane Structure. Curr. Top. Med. Chem. 2016, 16, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, L.; Li, G.; Zhai, N.; Jiang, H.; Chen, Y. Role of helicity of α-helical antimicrobial peptides to improve specificity. Protein Cell 2014, 5, 631–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G. Improved methods for classification, prediction, and design of antimicrobial peptides. Methods Mol. Biol. 2015, 1268, 43–66. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, R.M.; Sordat, B.; Bamat, J.; Gabbert, H.; Bourrat, B.; Mueller-Klieser, W. Oxygenation and differentiation in multicellular spheroids of human colon carcinoma. Cancer Res. 1986, 46, 5320–5329. [Google Scholar]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Vinci, M.; Gowan, S.; Boxall, F.; Patterson, L.; Zimmermann, M.; Court, W.; Lomas, C.; Mendiola, M.; Hardisson, D.; Eccles, S.A. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Mende, F.; Seitz, O. 9-Fluorenylmethoxycarbonyl-based solid-phase synthesis of peptide α-thioesters. Angew. Chem. 2011, 50, 1232–1240. [Google Scholar] [CrossRef]
- Tan, H.; Ding, X.; Meng, S.; Liu, C.; Wang, H.; Xia, L.; Liu, Z.; Liang, S. Antimicrobial potential of lycosin-I, a cationic and amphiphilic peptide from the venom of the spider Lycosa singorensis. Curr. Mol. Med. 2013, 13, 900–910. [Google Scholar] [CrossRef] [PubMed]
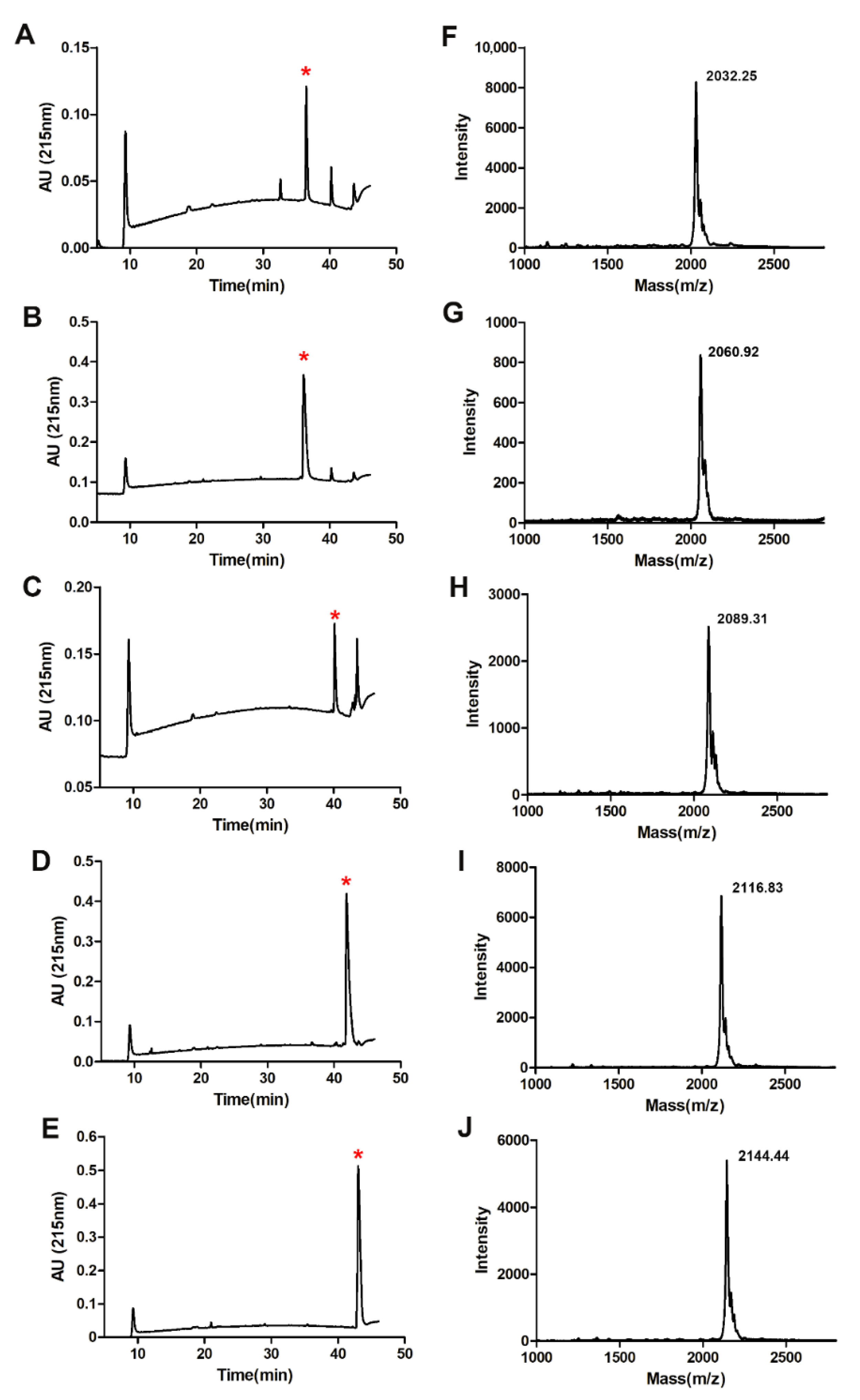
| Peptides | Sequence | Mw (Da) | TR a (min) |
|---|---|---|---|
| LVTX-9 | NH2-ASIGALIQKAIALIKAKAA-CONH2 | 1851.28 | 15.21 |
| LVTX-9-C12 | CH3-(CH2)10-CONHASIGALIQKAIALIKAKAA-CONH2 | 2033.64 | 36.52 |
| LVTX-9-C14 | CH3-(CH2)12-CONHASIGALIQKAIALIKAKAA-CONH2 | 2061.65 | 36.15 |
| LVTX-9-C16 | CH3-(CH2)14-CONHASIGALIQKAIALIKAKAA-CONH2 | 2089.70 | 40.21 |
| LVTX-9-C18 | CH3-(CH2)16-CONHASIGALIQKAIALIKAKAA-CONH2 | 2117.76 | 41.89 |
| LVTX-9-C20 | CH3-(CH2)18-CONHASIGALIQKAIALIKAKAA-CONH2 | 2145.81 | 43.06 |
| IC50 (μM) | |||||
|---|---|---|---|---|---|
| Peptides | L-929 | A549 | 4T1 | HepG2 | Hela |
| LVTX-9 | >100 | 51.7 ± 4.3 | >100 | >100 | >100 |
| LVTX-9-C18 | 3.6 ± 1.0 | 6.2 ± 1.4 | 7.6 ± 1.3 | 7.0 ± 1.0 | 4.7 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Wu, S.; Chen, N.; Zhu, J.; Zhao, X.; Zhang, P.; Zeng, Y.; Liu, Z. Fatty Acid Modification of the Anticancer Peptide LVTX-9 to Enhance Its Cytotoxicity against Malignant Melanoma Cells. Toxins 2021, 13, 867. https://doi.org/10.3390/toxins13120867
Li F, Wu S, Chen N, Zhu J, Zhao X, Zhang P, Zeng Y, Liu Z. Fatty Acid Modification of the Anticancer Peptide LVTX-9 to Enhance Its Cytotoxicity against Malignant Melanoma Cells. Toxins. 2021; 13(12):867. https://doi.org/10.3390/toxins13120867
Chicago/Turabian StyleLi, Fengjiao, Saizhi Wu, Ninglin Chen, Jingyu Zhu, Xinxin Zhao, Peng Zhang, Youlin Zeng, and Zhonghua Liu. 2021. "Fatty Acid Modification of the Anticancer Peptide LVTX-9 to Enhance Its Cytotoxicity against Malignant Melanoma Cells" Toxins 13, no. 12: 867. https://doi.org/10.3390/toxins13120867
APA StyleLi, F., Wu, S., Chen, N., Zhu, J., Zhao, X., Zhang, P., Zeng, Y., & Liu, Z. (2021). Fatty Acid Modification of the Anticancer Peptide LVTX-9 to Enhance Its Cytotoxicity against Malignant Melanoma Cells. Toxins, 13(12), 867. https://doi.org/10.3390/toxins13120867






