Toxigenic Species Aspergillus parasiticus Originating from Maize Kernels Grown in Serbia
Abstract
:1. Introduction
2. Results
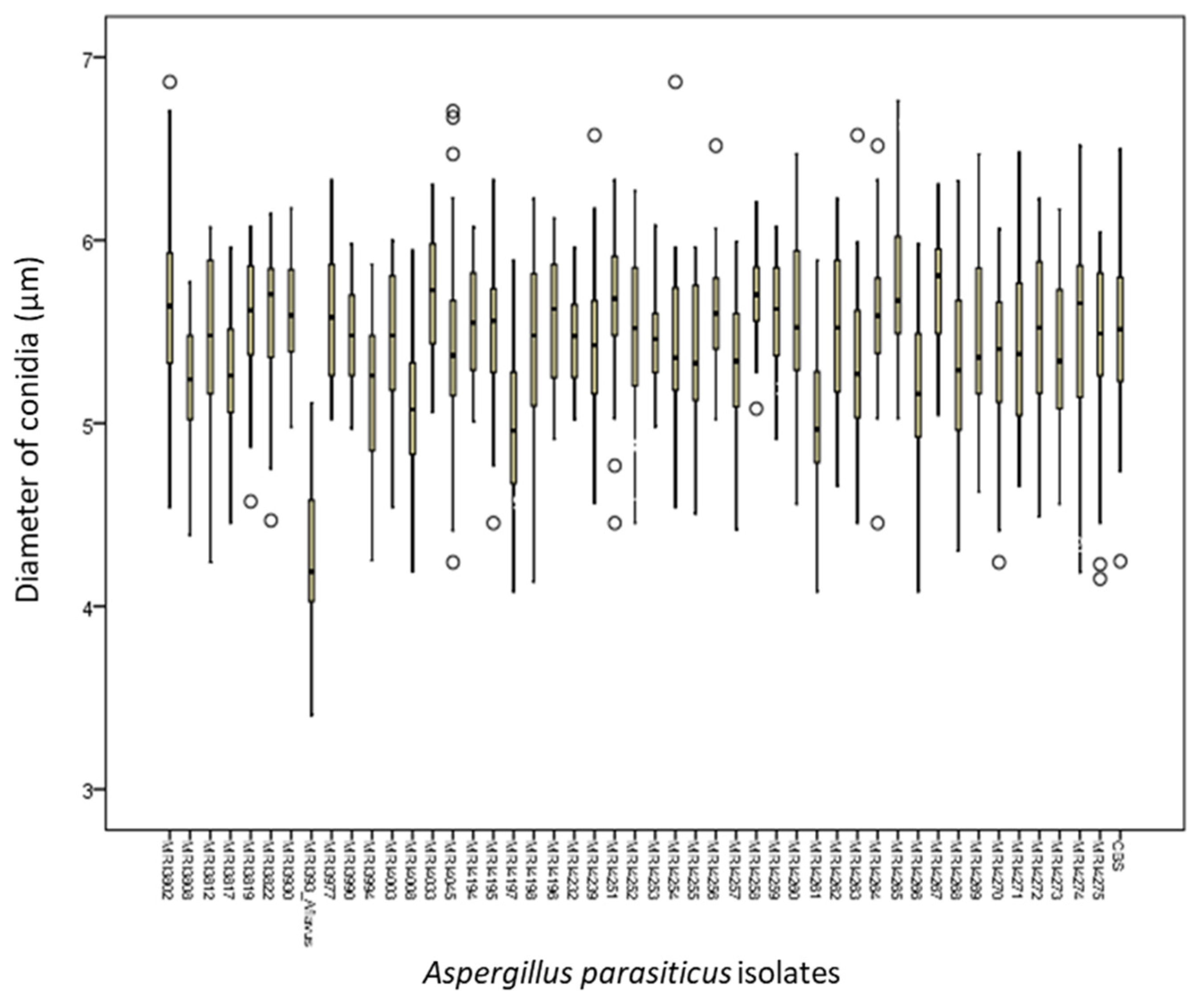
2.1. Macroscopic and Microscopic Morphological Characteristics of Identified Isolates
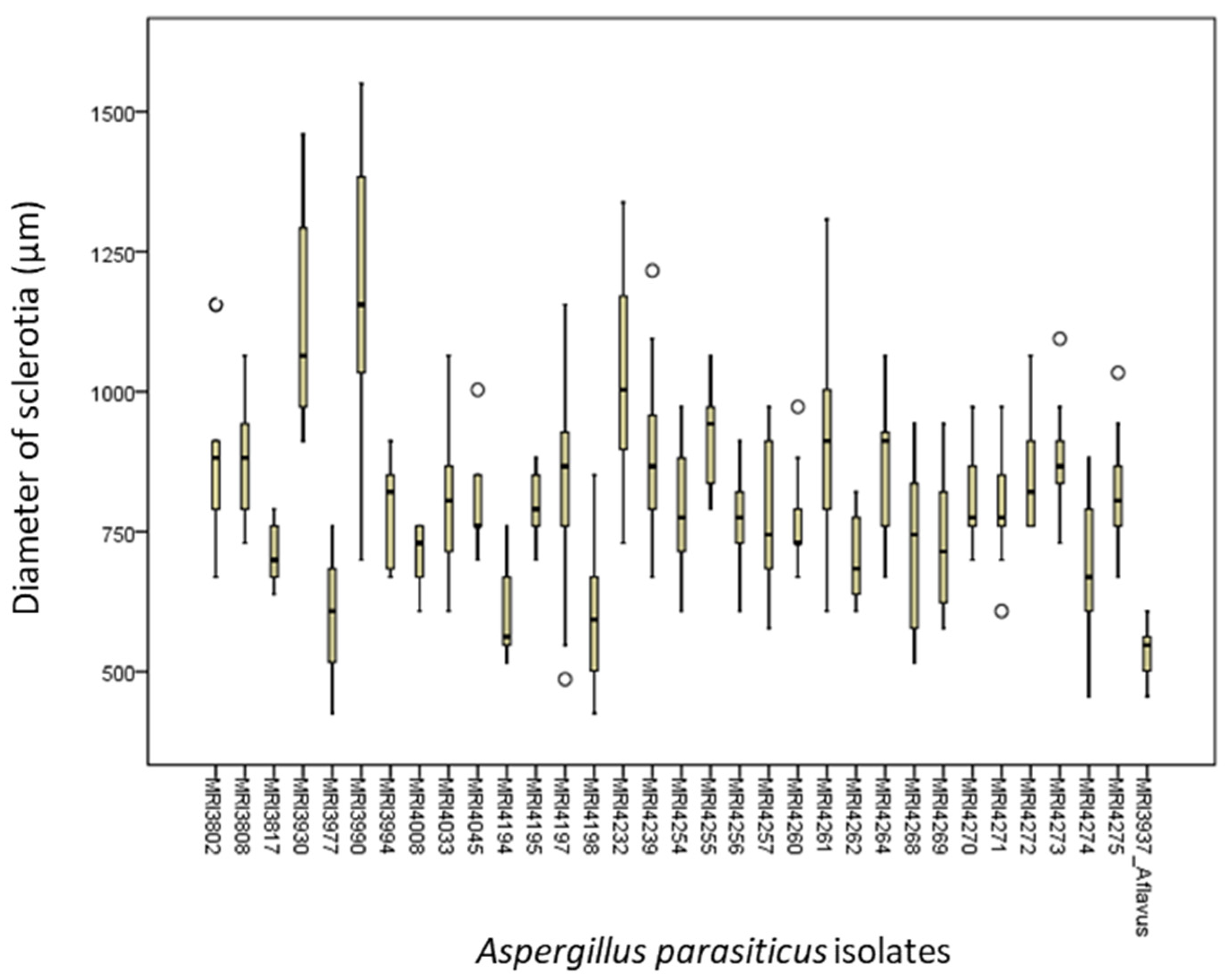
2.2. Morphology of Sclerotia
2.3. Molecular Identification of Aspergillus parasiticus Isolates
2.3.1. RFLP Analysis of ITS rDNA Region
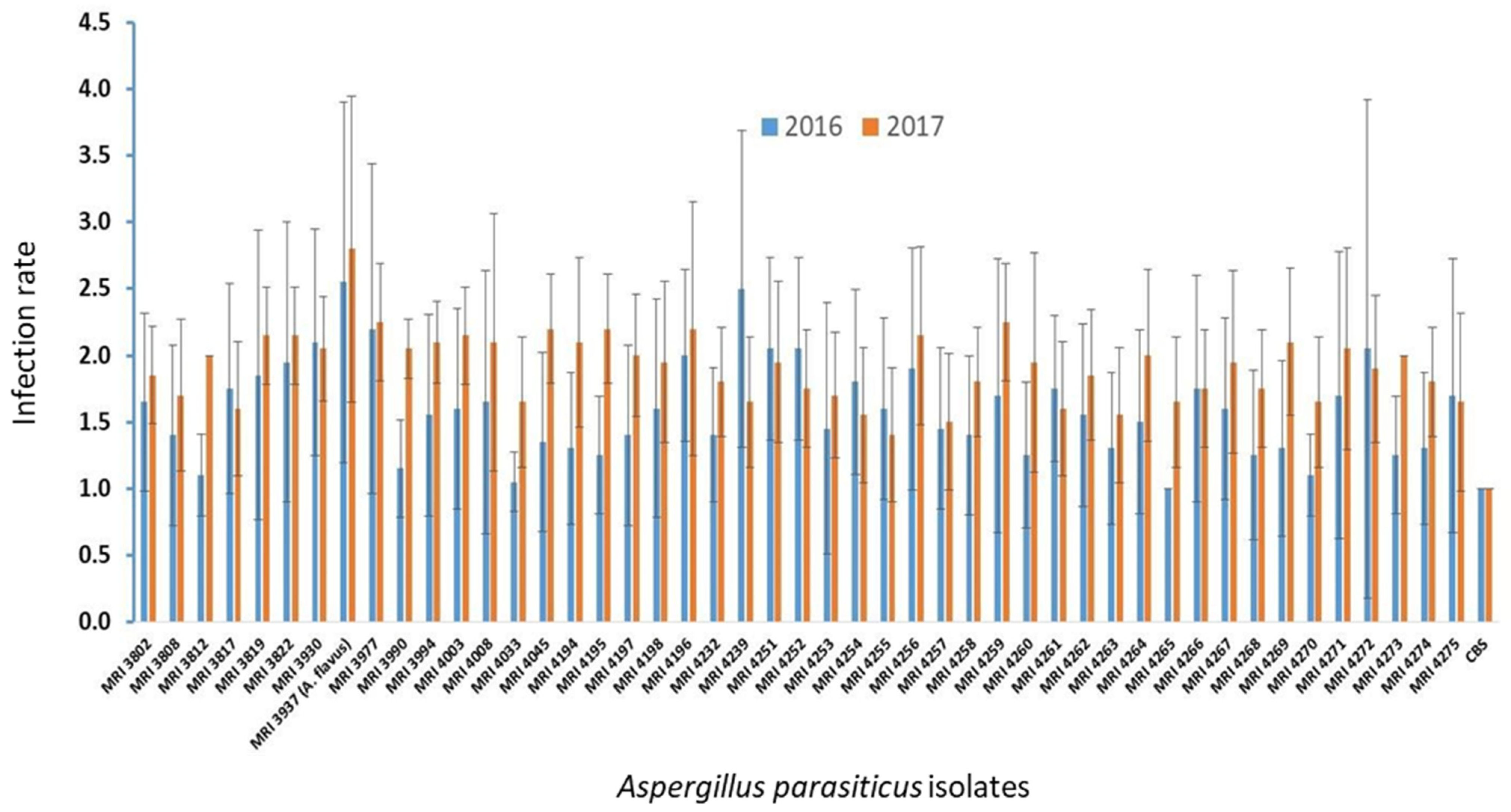
2.3.2. Toxicological Profile of Aspergillus parasiticus Isolates
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sample Collection and Fungal Isolation
5.2. Pathogenicity Test
5.3. Morphological Characterization
5.4. Molecular Analysis
5.4.1. DNA Extraction
5.4.2. PCR Amplification
5.4.3. Restriction Site Analysis of PCR Products
5.5. Aflatoxins Production
5.5.1. Sample Preparation for the Mycotoxin Assessment
5.5.2. HPLC, Extraction, and Quantitation of Aflatoxins
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, B.W. Ecology and population biology of aflatoxigenic fungi in soil. In Aflatoxin and Food Safety; Abbas, H.K., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 95–116. [Google Scholar]
- Nesci, A.; Etcheverry, M. Aspergillus section Flavi populations from field maize in Argentina. Lett. Appl. Microbiol. 2002, 34, 343–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitt, J.I.; Hocking, A.D.; Miscamble, B.F.; Dharmaputra, O.S.; Kuswanto, K.R.; Rahayu, E.S. Sardjono the mycoflora of food commodities from Indonesia. J. Food Mycol. 1998, 1, 41–60. [Google Scholar]
- Horn, B.W. Ecology and Population Biology of Aflatoxigenic Fungi in Soil. J. Toxicol. Toxin Rev. 2003, 22, 351–379. [Google Scholar] [CrossRef]
- Klich, M.; Pitt, J. Differentiation of Aspergillus flavus from A. parasiticus and other closely related species. Trans. Br. Mycol. Soc. 1988, 91, 99–108. [Google Scholar] [CrossRef]
- Vaamonde, G.; Patriarca, A.; Pinto, V.F.; Comerio, R.; Degrossi, C. Variability of aflatoxin and cyclopiazonic acid production by Aspergillus section flavi from different substrates in Argentina. Int. J. Food Microbiol. 2003, 88, 79–84. [Google Scholar] [CrossRef]
- Mphande, F.A.; Siame, B.A.; Taylor, J.E. Fungi, Aflatoxins, and Cyclopiazonic Acid Associated with Peanut Retailing in Botswana. J. Food Prot. 2004, 67, 96–102. [Google Scholar] [CrossRef]
- Alkadri, D.; Rubert, J.; Prodi, A.; Pisi, A.; Mañes, J.; Soler, C. Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chem. 2014, 157, 111–118. [Google Scholar] [CrossRef]
- Nikolic, M.; Nikolic, A.; Jaukovic, M.; Savic, I.; Petrovic, T.; Bagi, F.; Stankovic, S. Differentiation between Aspergillus flavus and Aspergillus parasiticus isolates originated from wheat. Genetika 2018, 50, 143–152. [Google Scholar] [CrossRef]
- Nikolić, M.; Savić, I.; Obradović, A.; Srdić, J.; Stanković, G.; Stevanović, M. First Report of Aspergillus parasiticus on Barley Grain in Serbia. Plant Dis. 2020, 104, 987. [Google Scholar] [CrossRef]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int. J. Food Microbiol. 2007, 113, 330–338. [Google Scholar] [CrossRef]
- Klich, M.A. Biogeography of Aspergillus Species in Soil and Litter. Mycol. 2002, 94, 21–27. [Google Scholar] [CrossRef]
- Stanković, S.; Lević, J.; Nikolić, M.; Krnjaja, V.; Jauković, M. Prvi Nalaz Aspergillus parasiticus u Proizvodnji Kukuruza u Srbiji. In Proceedings of the XIII Savetovanje o Zaštiti Bilja, Zlatibor, Serbia, 23–27 October 2015; pp. 32–33. [Google Scholar]
- Yu, J.; Chang, P.-K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Clustered Pathway Genes in Aflatoxin Biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253–1262. [Google Scholar] [CrossRef] [Green Version]
- CAST (Council for Agricultural Science and Technology). Mycotoxins: Risks in Plant, Animal, and Human Systems; Task Force Report No. 139; CAST: Ames, IA, USA, 2003. [Google Scholar]
- Sinovec, Z.; Resanović, R.; Sinovec, S. Mycotoxins-Occurrence, Effects and Prevention; Faculty of Veterinary Medicine; Univerziteta u Beogradu: Belgrade, Serbia, 2006. [Google Scholar]
- Logrieco, A.F.; Moretti, A. Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; Bandyopadhyay, R., Leslie, J.F., Visconti, A., Eds.; Cromwell Press: Trowbridge, Wiltshire, UK, 2008; pp. 139–153. [Google Scholar]
- Levic, J.; Gosic-Dondo, S.; Ivanovic, D.; Stankovic, S.; Krnjaja, V.; Bocarov-Stancic, A.; Stepanic, A. An outbreak of Aspergillus species in response to environmental conditions in Serbia. Pestic. Fitomed. 2013, 28, 167–179. [Google Scholar] [CrossRef]
- EL Khoury, A.; Atoui, A.; Rizk, T.; Lteif, R.; Kallassy, M.; Lebrihi, A. Differentiation between Aspergillus flavus and Aspergillus parasiticus from Pure Culture and Aflatoxin-Contaminated Grapes Using PCR-RFLP Analysis of aflR-aflJ Intergenic Spacer. J. Food Sci. 2011, 76, M247–M253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, P.; Santos, C.; Venâncio, A.; Lima, N. Species identification of Aspergillus section Flavi isolates from Portuguese almonds using phenotypic, including MALDI-TOF ICMS, and molecular approaches. J. Appl. Microbiol. 2011, 111, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.; Hubka, V.; Ezekiel, C.; Hong, S.-B.; Novakova, A.; Chen, A.; Arzanlou, M.; Larsen, T.O.; Sklenář, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef]
- Dovičičová, M.; Tančinová, D.; Labuda, R.; Sulyok, M. Aspergillus parasiticus from wheat grain of Slovak origin and its toxigenic potency. Czech J. Food Sci. 2012, 30, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, K.C.; Montalbano, B.G.; Cotty, P.J. Analysis of single nucleotide polymorphisms in three genes shows evidence for genetic isolation of certain Aspergillus flavus vegetative compatibility groups. FEMS Microbiol. Lett. 2007, 268, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.I.; Hocking, A.D. Aspergillus and Related Teleomorphs. In Fungi and Food Spoilage; Springer Science+Business Media: New York, NY, USA, 2009; pp. 275–337. [Google Scholar]
- Kozakiewicz, Z. Fungal Identification Techniques. In Paper Presented in Proceedings of the Workshop in Barcelona, Barcelona, Spain, 5–8 April 1995; p. 183. [Google Scholar]
- Baquião, A.C.; de Oliveira, M.M.M.; Reis, T.A.; Zorzete, P.; Atayde, D.D.; Corrêa, B. Monitoring and Determination of Fungi and Mycotoxins in Stored Brazil Nuts. J. Food Prot. 2013, 76, 1414–1420. [Google Scholar] [CrossRef]
- Nyongesa, B.W.; Okoth, S.; Ayugi, V. Identification Key for Aspergillus Species Isolated from Maize and Soil of Nandi County, Kenya. Adv. Microbiol. 2015, 5, 205–229. [Google Scholar] [CrossRef] [Green Version]
- Giorni, P. Impact on Environmental and Plant Actors on Aspergillus Section Flavi Isolated from Maize in Italy. Ph.D. Thesis, Faculty of Medicine and Biosciences, Cranfield Univesity, Cranfield, UK, 2007. [Google Scholar]
- Okoth, S.; Nyongesa, B.; Ayugi, V.; Kang’ethe, E.; Korhonen, H.; Joutsjoki, V. Toxigenic potential of Aspergillus species oc-curring on maize kernels from two agro-ecological zones in Kenya. Toxins 2012, 4, 991–1007. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Ahmad, M.; Ali, A.; Hamid, R.; Javed, S.; Abdin, M.Z. Detection of Aspergillus flavus and Aspergillus parasiticus from aflatoxin-contaminated peanuts and their differentiation using PCR-RFLP. Ann. Microbiol. 2014, 64, 1597–1605. [Google Scholar] [CrossRef]
- Donner, M.; Atehnkeng, J.; Sikora, R.A.; Bandyopadhyay, R.; Cotty, P.J. Distribution of Aspergillus section Flavi in soils of maize fields in three agroecological zones of Nigeria. Soil Biol. Biochem. 2009, 41, 37–44. [Google Scholar] [CrossRef]
- Rodrigues, P. Mycobiota and Aflatoxigenic Profile of Portuguese Almonds and Chestnuts from Production to Commercialisation. Ph.D. Thesis, Universidade do Minho, Braga, Portugal, 2011. [Google Scholar]
- Nikolić, M.; Stanković, S.; Savić, I. Comparison of methods for determination of the toxigenic potential of Aspergillus parasiticus Speare and Aspergillus flavus Link isolated from maize. J. Nat. Sci. 2017, 133, 95–104. [Google Scholar]
- Baranyi, N.; Despot, D.J.; Palágyi, A.; Kiss, N.; Kocsubé, S.; Szekeres, A.; Kecskeméti, A.; Bencsik, O.; Vágvölgyi, C.; Klarić, M.Š. Identification ofAspergillusspecies in Central Europe able to produce G-type aflatoxins. Acta Biol. Hung. 2015, 66, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C. Introduction to Food- and Airborne Fungi, 7th ed.; Centraalbureau voor Schim-Melcultures: Utrecht, The Netherlands, 2004; p. 389. [Google Scholar]
- Carbone, I.; Jakobek, J.L.; Ramirez-Prado, J.H.; Horn, B.W. Recombination, balancing selection and adaptive evolution in the aflatoxin gene cluster of Aspergillus parasiticus. Mol. Ecol. 2007, 16, 4401–4417. [Google Scholar] [CrossRef] [PubMed]
- Matumba, L.; Monjerezi, M.; Biswick, T.; Mwatseteza, J.; Makumba, W.; Kamangira, D.; Mtukuso, A. A survey of the incidence andlevel of aflatoxin contamination in a range of locally and imported processed foods on Malawian retail market. Food Control 2014, 39, 87–91. [Google Scholar] [CrossRef]
- Barros, G.; Chiotta, M.; Torres, A.; Chulze, S. Genetic diversity in Aspergillus parasiticus population from the peanut agroecosystem in Argentina. Lett. Appl. Microbiol. 2006, 42, 560–566. [Google Scholar] [CrossRef]
- Reid, L.M.; Hamilton, R.I.; Mather, D.E. Screening Maize for Resistance to Gibberella Ear Rot; Technical Bulleten 5E, Departament of Agriculture; Research Branch Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 1996. [Google Scholar]
- Liu, D.; Coloe, S.; Baird, R.; Pedersen, J. Rapid Mini-Preparation of Fungal DNA for PCR. J. Clin. Microbiol. 2000, 38, 471. [Google Scholar] [CrossRef]
- Ehrlich, K.; Montalbano, B.G.; Cotty, P.J. Sequence comparison of aflR from different Aspergillus species provides evidence for variability in regulation of aflatoxin production. Fungal Genet. Biol. 2003, 38, 63–74. [Google Scholar] [CrossRef]
- Abbas, H.K.; Zablotowicz, R.; Weaver, M.A.; Horn, B.W.; Xie, W.; Shier, W.T. Comparison of cultural and analytical methods for determination of aflatoxin production by Mississippi DeltaAspergillusisolates. Can. J. Microbiol. 2004, 50, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwitz, W. Official Methods of Analysis of the Association AOAC International, 17th ed.; Chapter 49; AOAC International: Gaithersburg, MD, USA, 2000; pp. 44–46. [Google Scholar]
| Isolate | AFB1 µg/kg | AFB2 µg/kg | AFG1 µg/kg | AFG2 µg/kg |
|---|---|---|---|---|
| 3802 | 3429.82 | 544.25 | 3461.35 | 260.47 |
| 3808 | 14.24 | × | 3.27 | × |
| 3812 | × | × | × | × |
| 3817 | × | × | × | × |
| 3819 | × | × | × | × |
| 3822 | × | × | × | × |
| 3930 | 3438.01 | 331.92 | 3437.81 | 306.65 |
| 3937 (A. flavus) | 646.05 | 0.27 | 12.04 | × |
| 3977 | 911.03 | 561.35 | 554.22 | 47.69 |
| 3990 | 5698.47 | 303.94 | 6518.88 | 90.52 |
| 3994 | × | × | × | × |
| 4003 | × | × | × | × |
| 4008 | × | × | × | × |
| 4033 | × | × | × | × |
| 4045 | 6716.42 | 1543.80 | 5189.43 | 181.76 |
| 4194 | 7361.03 | 995.41 | 3085.11 | × |
| 4195 | 6431.77 | 287.25 | 7191.62 | 110.11 |
| 4197 | 5850.87 | 527.31 | 7122.59 | 395.18 |
| 4198 | 6741.07 | 652.58 | 7421.58 | 374.93 |
| 4196 | 4409.10 | 143.97 | 5357.14 | 68.92 |
| 4232 | 5708.12 | 142.81 | 6495.07 | 86.92 |
| 4239 | × | × | × | × |
| 4251 | 5918.46 | 254.03 | 6606.65 | 98.25 |
| 4252 | 6816.08 | 721.28 | 2166.36 | × |
| 4253 | 15.46 | × | × | × |
| 4254 | × | × | × | × |
| 4255 | 89.67 | × | × | × |
| 4256 | × | × | × | × |
| 4257 | × | × | × | × |
| 4258 | 6884.01 | 1.590,6 | 2946.52 | 10.43 |
| 4259 | × | × | × | × |
| 4260 | × | × | × | × |
| 4261 | 5198.68 | 403.79 | 5.13 | 128.02 |
| 4262 | 5776.73 | 629.68 | 5746.97 | 217.83 |
| 4263 | × | × | × | × |
| 4264 | × | × | × | × |
| 4265 | 5968.77 | 1733.52 | 634.79 | × |
| 4266 | 5168.79 | 351.97 | 5157.56 | 119.18 |
| 4267 | × | × | × | × |
| 4268 | × | × | × | × |
| 4269 | × | × | × | × |
| 4270 | × | × | × | × |
| 4271 | × | × | × | × |
| 4272 | × | × | × | × |
| 4273 | × | × | × | × |
| 4274 | 6029.33 | 500.80 | 6240.54 | 201.47 |
| 4275 | 33.05 | × | 8.03 | × |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, M.; Savić, I.; Nikolić, A.; Jauković, M.; Kandić, V.; Stevanović, M.; Stanković, S. Toxigenic Species Aspergillus parasiticus Originating from Maize Kernels Grown in Serbia. Toxins 2021, 13, 847. https://doi.org/10.3390/toxins13120847
Nikolić M, Savić I, Nikolić A, Jauković M, Kandić V, Stevanović M, Stanković S. Toxigenic Species Aspergillus parasiticus Originating from Maize Kernels Grown in Serbia. Toxins. 2021; 13(12):847. https://doi.org/10.3390/toxins13120847
Chicago/Turabian StyleNikolić, Milica, Iva Savić, Ana Nikolić, Marko Jauković, Vesna Kandić, Milan Stevanović, and Slavica Stanković. 2021. "Toxigenic Species Aspergillus parasiticus Originating from Maize Kernels Grown in Serbia" Toxins 13, no. 12: 847. https://doi.org/10.3390/toxins13120847
APA StyleNikolić, M., Savić, I., Nikolić, A., Jauković, M., Kandić, V., Stevanović, M., & Stanković, S. (2021). Toxigenic Species Aspergillus parasiticus Originating from Maize Kernels Grown in Serbia. Toxins, 13(12), 847. https://doi.org/10.3390/toxins13120847





