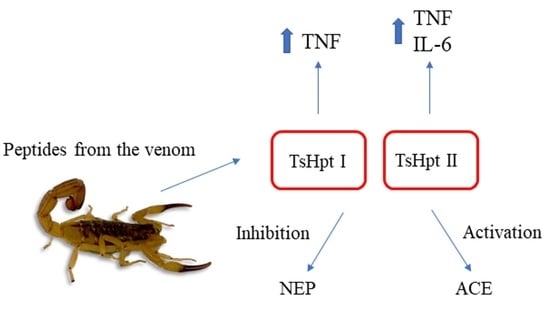
New Insights into the Hypotensins from Tityus serrulatus Venom: Pro-Inflammatory and Vasopeptidases Modulation Activities
Abstract
:1. Introduction
2. Results
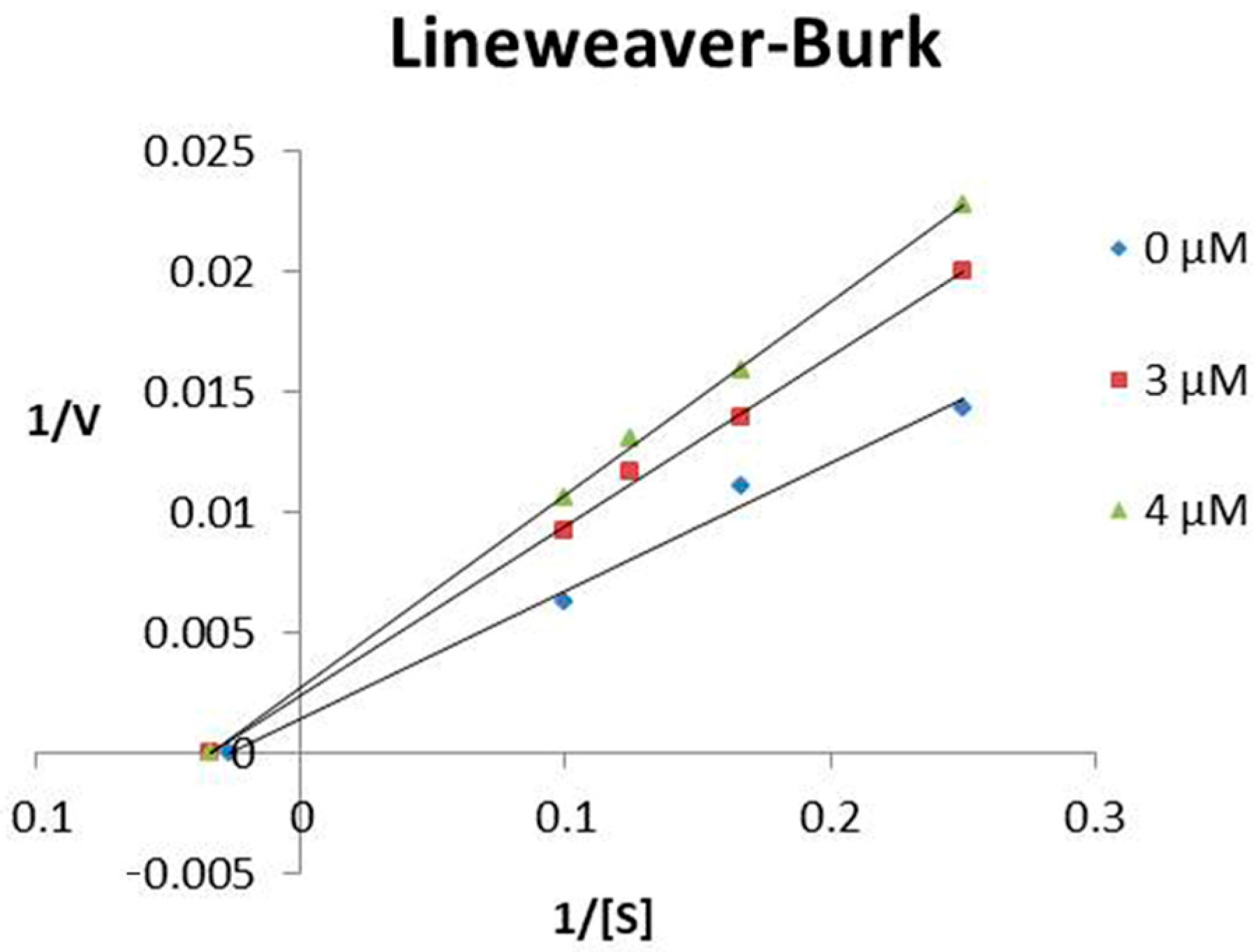
2.1. Modulation of ACE and NEP Activities by Hypotensins
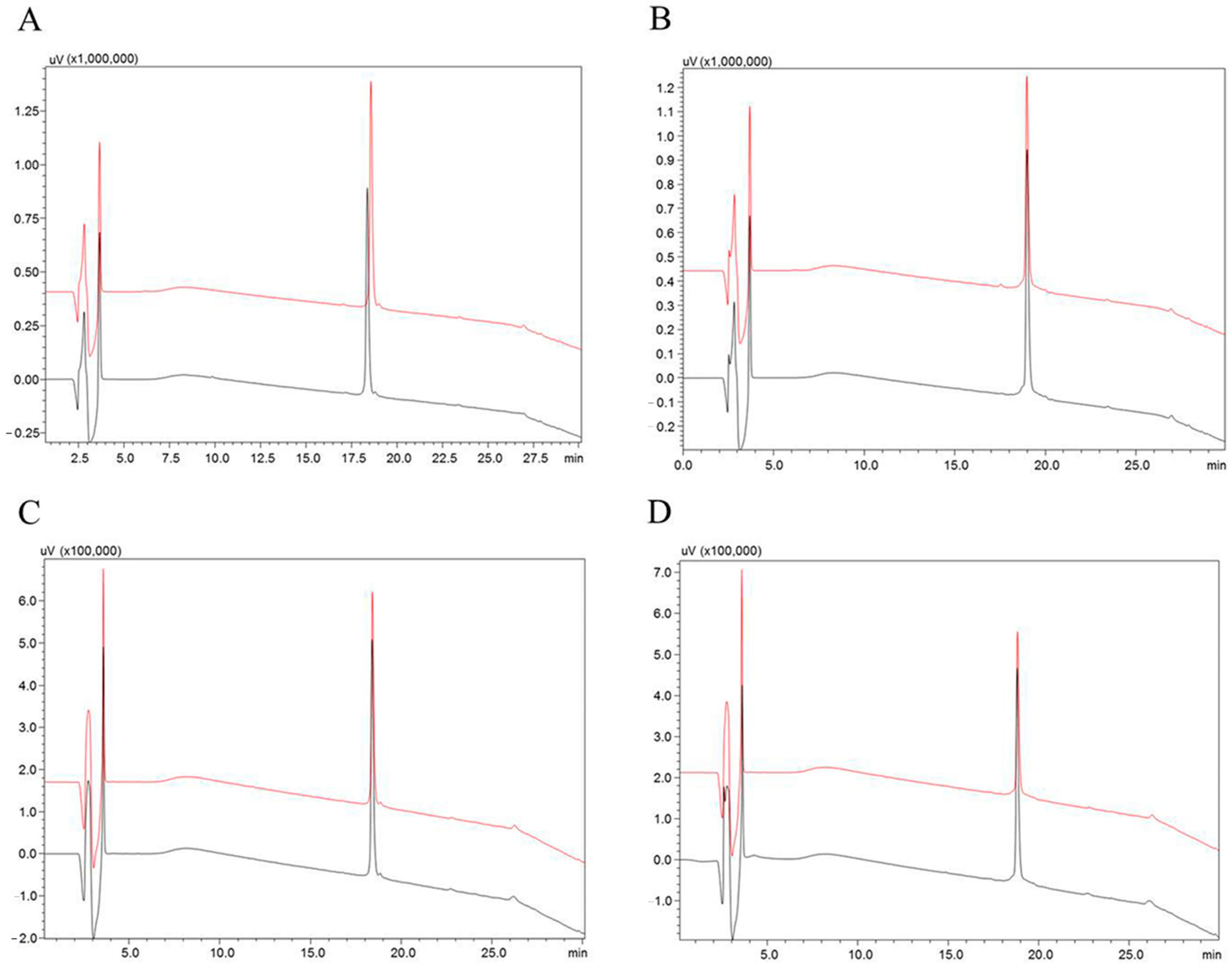
2.2. Stability of Hypotensins against Metallopeptidases ACE and NEP
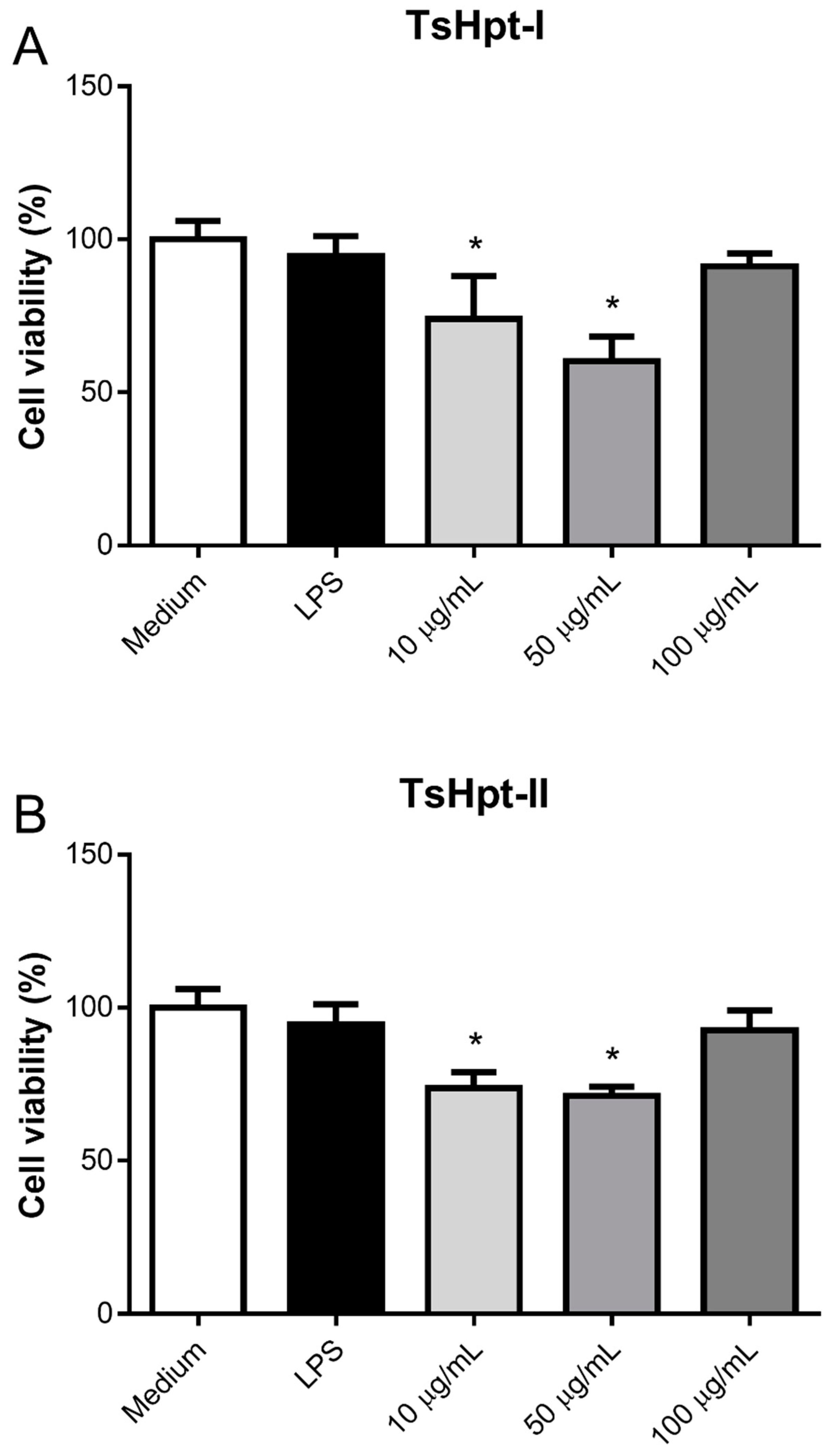
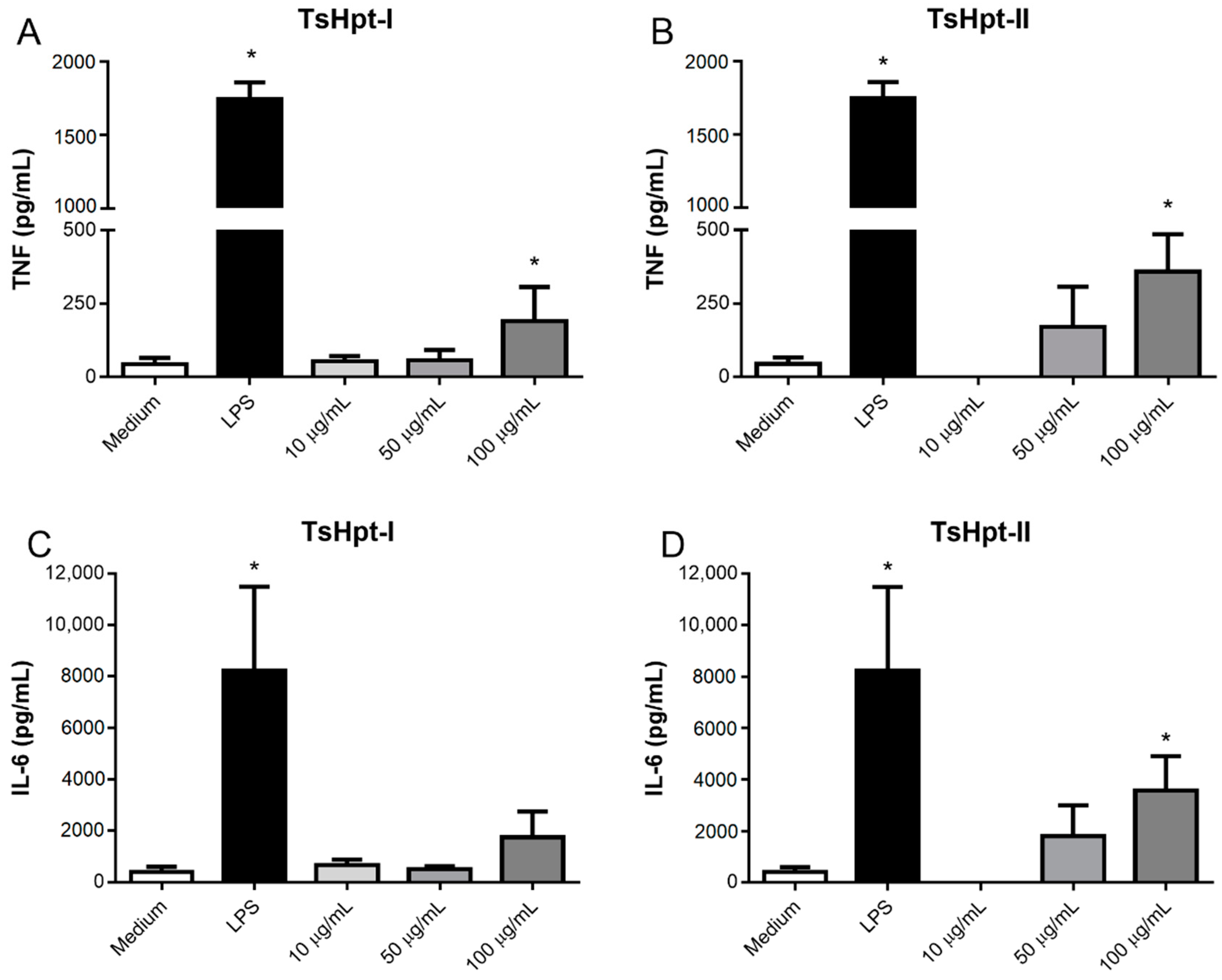
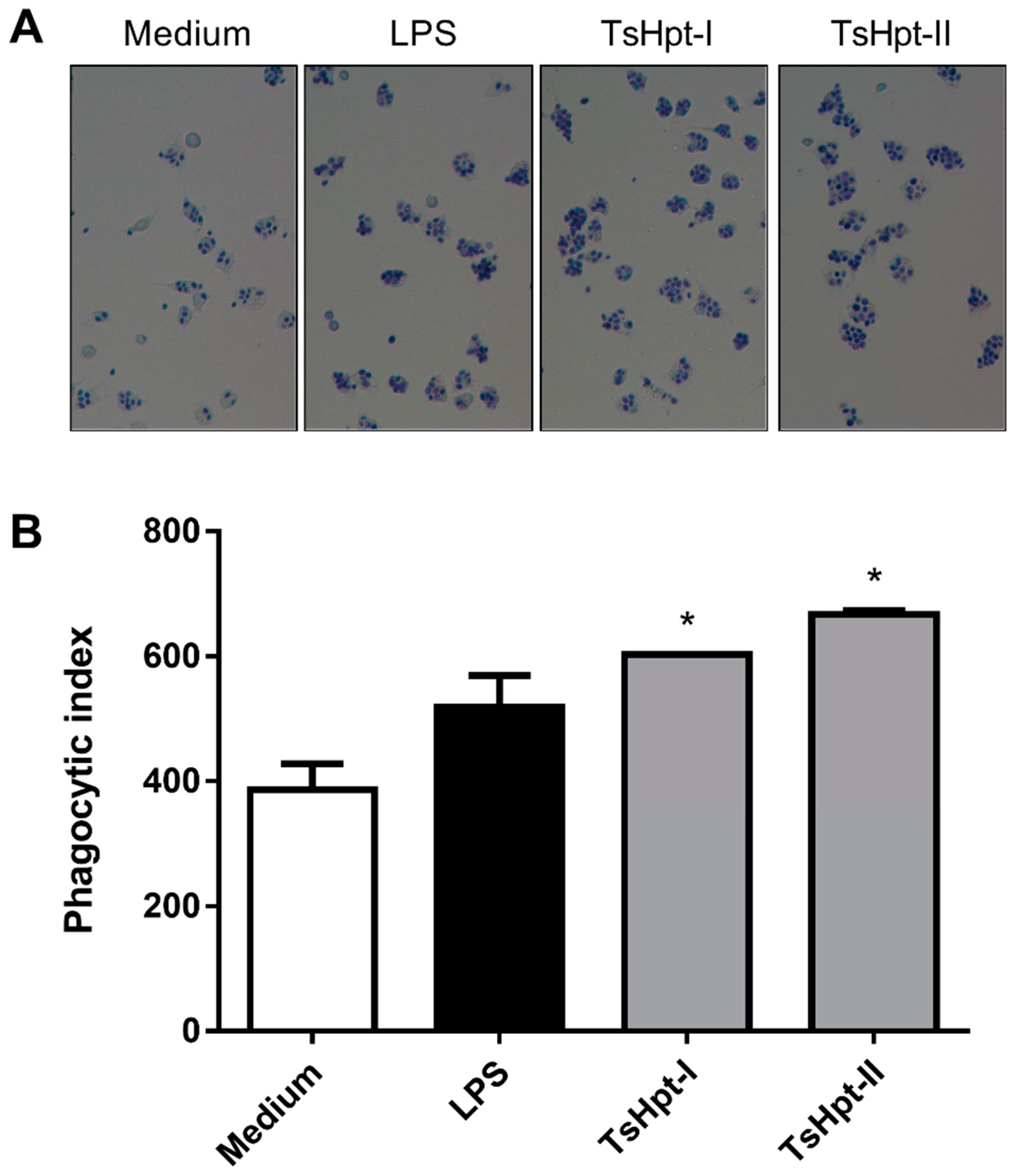
2.3. In Vitro Pro-Inflammatory Effects of TsHpt I and -II on Mouse Macrophages
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Reagents
5.2. Interactions of Hypotensins with Vasopeptidases ACE and NEP
5.3. Stability Test of the Synthetic Peptides
5.4. Characterization of TsHpt-I as a NEP Inhibitor
5.5. Cell Assays
5.5.1. Murine Peritoneal Macrophages Obtainment
5.5.2. Effect of Hypotensins on the Cell Viability and Production of Inflammatory Mediators by Murine Peritoneal Macrophages Stimulated In Vitro with Hypotensins
5.5.3. Effect of TsHpt-I and -II on Phagocytic Function of Murine Peritoneal Macrophages
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bortoluzzi, L.R.; Querol, M.V.M.; Querol, E. Notas sobre a ocorrência de Tityus serrulatus Lutz & Mello, 1922 (Scorpiones, Buthidae) no oeste do Rio Grande do Sul, Brasil. Biota Neotrop. 2007, 7, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, J.; França, F.S.; Wen, F.H.; Málaque, C.M.S.; Haddad, V., Jr. Animais Peçonhentos no Brasil. Biologia, Clínica e Terapêutica dos Acidentes, 2nd ed.; Sarvier: São Paulo, Brazil, 2009. [Google Scholar]
- Lourenço, W.R.; Von Eickstedt, V.R.D.; Cardoso, J.L.C.; França, F.O.S.; Wen, F.H.; Málaque, C.M.S. Escorpiões de importância médica. In Animais Peçonhentos no Brasil. Biologia, Clínica e Terapêutica dos Acidentes; Sarvier: São Paulo, Brazil, 2003; ISBN 8573781335. [Google Scholar]
- Marcussi, S.; Arantes, E.C.; Soares, A.M.; Giglio, J.R.; Mazzi, M.V. Escorpiões: Biologia, Envenenamento e Mecanismos de Ação de suas Toxinas, 1st ed.; FUNPEC: Ribeirão Preto, Brazil, 2011. [Google Scholar]
- FUNASA. Manual de Diagnóstico e Tratamento de Acidentes por Animais Peçonhentos, 2nd ed.; Ministério da Saúde. Fundação Nacional de Saúde: Brasília, Brazil, 2001; ISBN 85-7346-014-8. [Google Scholar]
- Matthiesen, F.A. The breeding of Tityus serrulatus Lutz & Mello, 1927, in captivity (Scorpions, Buthidae). Rev. Bras. Pesqui. Med. Biol. 1971, 4, 299–300. [Google Scholar]
- Chippaux, J.-P. Epidemiology of envenomations by terrestrial venomous animals in Brazil based on case reporting: From obvious facts to contingencies. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucca, M.B.; Cerni, F.A.; Pinheiro Junior, E.L.; Bordon, K.D.C.F.; Amorim, F.G.; Cordeiro, F.A.; Longhim, H.T.; Cremonez, C.M.; Oliveira, G.H.; Arantes, E.C. Tityus serrulatus venom—A lethal cocktail. Toxicon 2015, 108, 272–284. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, U.C.; Nishiyama, M.Y.; Santos, M.B.V.; Chalkidis, D.M.; Santos-da-Silva, A.D.P.; Souza-Imberg, A.; Candido, D.M.; Yamanouye, N.; Junqueira-de-Azevedo, I.L.M. Proteomic endorsed transcriptomic profiles of venom glands from Tityus obscurus and T. serrulatus scorpions. PLoS ONE 2018, 13, e0193739. [Google Scholar] [CrossRef] [PubMed]
- Rates, B.; Ferraz, K.K.F.; Borges, M.H.; Richardson, M.; De Lima, M.E.; Pimenta, A.M.C. Tityus serrulatus venom peptidomics: Assessing venom peptide diversity. Toxicon 2008, 52, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Verano-Braga, T.; Dutra, A.A.A.; León, I.R.; Melo-Braga, M.N.; Roepstorff, P.; Pimenta, A.M.C.; Kjeldsen, F. Moving pieces in a venomic puzzle: Unveiling post-translationally modified toxins from Tityus serrulatus. J. Proteome Res. 2013, 12, 3460–3470. [Google Scholar] [CrossRef]
- Nascimento, D.G.; Rates, B.; Santos, D.M.; Verano-Braga, T.; Barbosa-Silva, A.; Dutra, A.A.A.; Biondi, I.; Martin-Eauclaire, M.F.; De Lima, M.E.; Pimenta, A.M.C. Moving pieces in a taxonomic puzzle: Venom 2D-LC/MS and data clustering analyses to infer phylogenetic relationships in some scorpions from the Buthidae family (Scorpiones). Toxicon 2006, 47, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Resende, C.; Leão, N.M.; de Lima, M.E.; Santos, R.A.; de Castro Pimenta, A.M.; Verano-Braga, T. Moving pieces in a cryptomic puzzle: Cryptide from Tityus serrulatus Ts3 Nav toxin as potential agonist of muscarinic receptors. Peptides 2017, 98, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Verano-Braga, T.; Rocha-Resende, C.; Silva, D.M.; Ianzer, D.; Martin-Eauclaire, M.F.; Bougis, P.E.; de Lima, M.E.; Santos, R.A.S.; Pimenta, A.M.C. Biochemical and Biophysical Research Communications Tityus serrulatus Hypotensins: A new family of peptides from scorpion venom. Biochem. Biophys. Res. Commun. 2008, 371, 515–520. [Google Scholar] [CrossRef]
- Verano-Braga, T.; Figueiredo-Rezende, F.; Melo, M.N.; Lautner, R.Q.; Gomes, E.R.M.; Mata-Machado, L.T.; Murari, A.; Rocha-Resende, C.; Elena de Lima, M.; Guatimosim, S.; et al. Structure–function studies of Tityus serrulatus Hypotensin-I (TsHpt-I): A new agonist of B2 kinin receptor. Toxicon 2010, 56, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Duzzi, B.; Cajado-Carvalho, D.; Kuniyoshi, A.K.; Kodama, R.T.; Gozzo, F.C.; Fioramonte, M.; Tambourgi, D.V.; Portaro, F.V.; Rioli, V. [des-Arg 1]-Proctolin: A novel NEP-like enzyme inhibitor identified in Tityus serrulatus venom. Peptides 2016, 80, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Skidgel, R.A.; Erdos, E.G. Novel activity of human angiotensin I converting enzyme: Release of the NH2- and COOH-terminal tripeptides from the luteinizing hormone-releasing hormone. Proc. Natl. Acad. Sci. USA 1985, 82, 1025–1029. [Google Scholar] [CrossRef] [Green Version]
- Roques, B.P.; Noble, F.; Dauge, V.; Fournie-Zaluski, M.C.; Beaumont, A. Neutral endopeptidase 24.11: Structure, inhibition, and experimental and clinical pharmacology. Pharmacol. Rev. 1993, 45, 87–146. [Google Scholar] [PubMed]
- Rawlings, N.D.; Salvesen, G. Handbook of Proteolytic Enzymes, 3rd ed.; Elsevier: Amsterdam, Holland, 2013; ISBN 9780123822192. [Google Scholar]
- Daull, P.; Jeng, A.Y.; Battistini, B. Towards Triple Vasopeptidase Inhibitors for the Treatment of Cardiovascular Diseases. J. Cardiovasc. Pharmacol. 2007, 50, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Cajado-Carvalho, D.; Kuniyoshi, A.K.; Duzzi, B.; Iwai, L.K. Insights into the Hypertensive Effects of Tityus serrulatus Scorpion Venom: Purification of an angiotensin-converting enzyme-like peptidase. Toxins 2016, 8, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sifi, A.; Adi-Bessalem, S.; Laraba-Djebari, F. Involvement of the Endothelin Receptor Type A in the Cardiovascular Inflammatory Response Following Scorpion Envenomation. Toxins 2020, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Cologna, C.; Marcussi, S.; Giglio, J.; Soares, A.; Arantes, E. Tityus serrulatus Scorpion Venom and Toxins: An Overview. Protein Pept. Lett. 2009, 16, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Cerni, F.A.; Pinheiro-Junior, E.L.; Zoccal, K.F.; Bordon, K.D.C.F.; Amorim, F.G.; Peigneur, S.; Vriens, K.; Thevissen, K.; Cammue, B.P.A.; et al. Non-disulfide-bridged peptides from Tityus serrulatus venom: Evidence for proline-free ACE-inhibitors. Peptides 2016, 82, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Cassini-Vieira, P.; Felipetto, M.; Prado, L.B.; Verano-Braga, T.; Andrade, S.P.; Santos, R.A.S.; Teixeira, M.M.; de Lima, M.E.; Pimenta, A.M.C.; Barcelos, L.S. Ts14 from Tityus serrulatus boosts angiogenesis and attenuates inflammation and collagen deposition in sponge-induced granulation tissue in mice. Peptides 2017, 98, 63–69. [Google Scholar] [CrossRef]
- Torres-Rêgo, M.; Gláucia-Silva, F.; Rocha Soares, K.S.; de Souza, L.B.F.C.; Damasceno, I.Z.; dos Santos-Silva, E.; Lacerda, A.F.; Chaves, G.M.; da Silva-Júnior, A.A.; de Fernandes-Pedrosa, M.F. Biodegradable cross-linked chitosan nanoparticles improve anti-Candida and anti-biofilm activity of TistH, a peptide identified in the venom gland of the Tityus stigmurus scorpion. Mater. Sci. Eng. C 2019, 103, 109830. [Google Scholar] [CrossRef] [PubMed]
- Barros, N.M.T.; Campos, M.; Bersanetti, P.A.; Oliveira, V.; Juliano, M.A.; Boileau, G.; Juliano, L.; Carmona, A.K. Neprilysin carboxydipeptidase specificity studies and improvement in its detection with fluorescence energy transfer peptides. Biol. Chem. 2007, 388. [Google Scholar] [CrossRef] [PubMed]
- Segel, I.H. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady State Enzyme Systems; Willey: New York, NY, USA, 1975. [Google Scholar]
| Metallopeptidases | ||||
|---|---|---|---|---|
| ACE | NEP | |||
| Activation (%) | Inhibition (%) | Activation (%) | Inhibition (%) | |
| TsHpt-I | 64 | - | - | 75 |
| TsHpt-II | 44 | - | - | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duzzi, B.; Silva, C.C.F.; Kodama, R.T.; Cajado-Carvalho, D.; Squaiella-Baptistão, C.C.; Portaro, F.C.V. New Insights into the Hypotensins from Tityus serrulatus Venom: Pro-Inflammatory and Vasopeptidases Modulation Activities. Toxins 2021, 13, 846. https://doi.org/10.3390/toxins13120846
Duzzi B, Silva CCF, Kodama RT, Cajado-Carvalho D, Squaiella-Baptistão CC, Portaro FCV. New Insights into the Hypotensins from Tityus serrulatus Venom: Pro-Inflammatory and Vasopeptidases Modulation Activities. Toxins. 2021; 13(12):846. https://doi.org/10.3390/toxins13120846
Chicago/Turabian StyleDuzzi, Bruno, Cristiane Castilho Fernandes Silva, Roberto Tadashi Kodama, Daniela Cajado-Carvalho, Carla Cristina Squaiella-Baptistão, and Fernanda Calheta Vieira Portaro. 2021. "New Insights into the Hypotensins from Tityus serrulatus Venom: Pro-Inflammatory and Vasopeptidases Modulation Activities" Toxins 13, no. 12: 846. https://doi.org/10.3390/toxins13120846
APA StyleDuzzi, B., Silva, C. C. F., Kodama, R. T., Cajado-Carvalho, D., Squaiella-Baptistão, C. C., & Portaro, F. C. V. (2021). New Insights into the Hypotensins from Tityus serrulatus Venom: Pro-Inflammatory and Vasopeptidases Modulation Activities. Toxins, 13(12), 846. https://doi.org/10.3390/toxins13120846