Abstract
This study was performed to assess the effects of dietary aflatoxin B1 (AFB1) on the growth, antioxidant and immune response, digestive enzyme activities, and intestinal morphology of Lateolabrax maculatus during a 56-day feeding trial. Four diets were formulated including 0, 0.1, 0.5, and 1.0 mg/kg of AFB1. Each diet was randomly assigned to 3 fish tanks with 40 fish per tank. Results indicated that the fish’s final body weight, weight gain rate, specific growth rate, feed intake, condition factor, viscerosomatic index, hepatosomatic index, and intestinesomatic index decreased (p < 0.01) as dietary AFB1 increased. AFB1 levels in diets increased (p < 0.05) serum total antioxidant capacity (TAOC), superoxide (SOD), catalase, malondialdehyde (MDA), alkaline phosphatase (AKP), and lysozyme (LZM), and increased (p < 0.05) the TAOC, SOD, MDA, AKP, LZM, and immunoglobulin M in the livers of the fish. Dietary AFB1 decreased (p < 0.05) intestinal trypsin activity and induced intestinal injury. In summary, dietary AFB1 up to 1.0 mg/kg was toxic to L. maculatus as judged by reduced growth, enhanced antioxidant and immune response, decreased intestinal trypsin activity, and impaired intestinal morphology.
Key Contribution:
The present study reports the comprehensive response of Lateolabrax maculatus to dietary AFB1. These results provide a reference for further studies on AFB1 in aquaculture.
1. Introduction
Aflatoxin B1 (AFB1) is mainly produced by Aspergillus flavus, which exists in some raw feed materials for animal use, such as maize, peanut, and wheat flour [1]. AFB1 is a major challenge to aquaculture due to its high toxicity to aquatic animals and great threat to food safety. The biotransformation of AFB1 mainly occurs in the liver and intestine [2]. Intake may induce an inflammatory response, interrupt intestinal integrity, and eventually inhibit the growth of fish [3,4]. At present, research in this field has focused mainly on the effects of dietary AFB1 on growth, bioaccumulation, muscle quality, immune response, hematology, and hepatic function indices of aquatic animals [5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Little information is available that evaluates the effect of AFB1 on the health of the gut, the largest digestive and immune organ in the animal body. Strengthening research in this area can provide a better understanding of the mechanism for AFB1-induced changes in the growth and intestinal health of aquatic animals.
The biological effects AFB1 has on aquatic animals are directly associated with the animal species and the dietary concentrations of AFB1 [5]. The effects of AFB1 on the growth and health of tilapia (Oreochromis niloticus × O. aureus) [6], grass carp (Ctenopharyngodon idella) [7], common carp (Cyprinus carpio) [8], channel catfish [9], Clarias batrachus [10], rainbow trout (Oncorhynchus mykiss) [4,11], Sciaenos ocedatas [12], Litopanaeus vannamei [13,14,15], turbot (Scophthalmus maximus) [16], gibel carp [17], and Indian carp (Labeo rohita) [18] have been assessed. However, information is rare regarding Lateolabrax maculatus, a popular carnivorous fish in the southern region of China in recent years, known for its rapid growth and superb taste [19]. In 2020, the nationwide fish yield exceeded 160,000 tons, according to the 2020 China Fishery Statistical Yearbook. Previous studies documented that Litopanaeus vannamei and gibel carp (Carassius auratus gibelio) could tolerate dietary AFB1 up to 2 mg/kg [13] and 0.5 mg/kg [17], respectively. Other species tolerated dietary AFB1 ranging from 0.1 to 2.5 mg/kg [6,7,9]. This had not yet been evaluated for L. maculatus, however. The present study was conducted to assess the effects of dietary AFB1 on growth, antioxidant and immune response, digestive enzyme activities, and intestinal morphology of L. maculatus.
2. Results
2.1. Growth Performance
Increasing dietary AFB1 from 0 to 1.0 mg/kg linearly and quadratically decreased (p < 0.01) the final body weight (FBW), weight gain rate (WGR), specific growth rate (SGR), and feed intake (FI), but did not alter the feed coefficient (FC) or survival rate (SR) of the fish (p > 0.05) (Table 1). The condition factor (CF), viscerosomatic index (VSI), and intestinesomatic index (ISI) linearly decreased (p < 0.05), and the hepatosomatic index (HIS) linearly and quadratically decreased (p < 0.05) as dietary AFB1 increased.

Table 1.
Effects of AFB1 on survival and growth of L. maculatus.
2.2. Antioxidant and Immune Response
The serum total antioxidant capacity (TAOC), superoxide dismutase (SOD), and catalase (CAT) linearly increased (p < 0.05), and malondialdehyde (MDA) linearly and quadratically increased (p < 0.01) as dietary AFB1 increased and reached significance at the level of 1.0 mg/kg (Table 2).

Table 2.
Effects of AFB1 on the antioxidant and immune response of L. maculatus.
Increased dietary AFB1 linearly and quadratically increased (p < 0.05) serum alkaline phosphatase (AKP) and linearly increased (p < 0.05) lysozyme (LZM). The serum glutathione peroxidase (GPx) was similar among groups (p > 0.05).
The liver TAOC and MDA were linearly increased (p < 0.05), and SOD was linearly and quadratically increased (p < 0.05) as dietary AFB1 increased.
Increased dietary AFB1 linearly increased (p < 0.05) liver AKP, LZM, and immunoglobulin M (IgM). Dietary treatment did not alter the CAT, GPx, or complement C3 (C3) in the liver of fish (p > 0.05).
2.3. Intestinal Digestive Enzyme Activities and Histological Appearance
The intestinal trypsin activity was lower (p < 0.05) in G0.1 and G0.5 than in G0, and was lower (p < 0.05) in G1.0 than in other groups (Figure 1). The activities of lipase and amylase were similar among groups (p > 0.05).
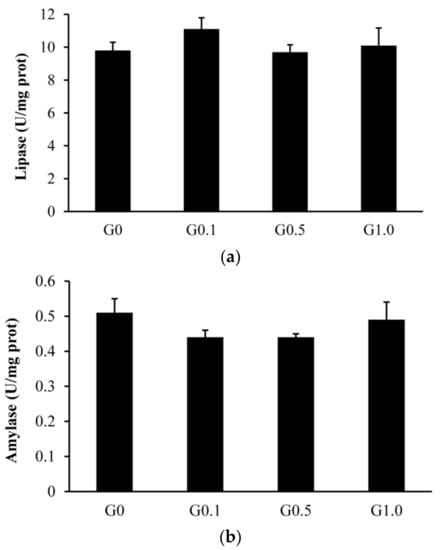
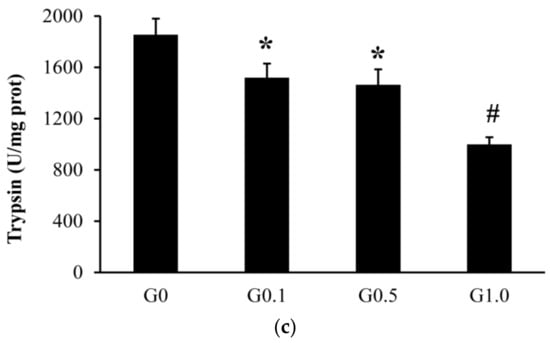
Figure 1.
Effects of AFB1 on the intestinal digestive enzyme activities ((a), lipase; (b), amylase; (c), trypsin) of L. maculatus. G0–G1.0, basal diet added 0, 0.1, 0.5 and1.0 mg/kg of AFB1. * p < 0.05 compared to G0. # p < 0.05 compared to G0.1.
The intestinal villus in G0 was most regular in shape (Figure 2), whereas the villus in G0.1, G0.5, and G1.0 had different degrees of deformation as reflected by the irregular arrangement of the villus.
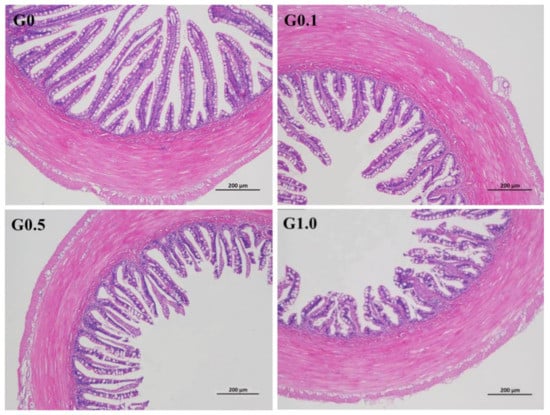
Figure 2.
Effects of AFB1 on the intestinal histological appearance (×100) of L. maculatus. G0–G1.0, basal diet added 0, 0.1, 0.5 and 1.0 mg/kg of AFB1.
3. Discussion
3.1. Effect of AFB1 on the Growth of L. maculatus
The similar SR of fish among the groups suggested that dietary AFB1 up to 1.0 mg/kg did not alter the survival of L. maculatus. However, the decreased FI, WGR, and SGR as dietary AFB1 increased suggests that AFB1 adversely impacts the palatability of feed and the growth of L. maculatus. To the best of our knowledge, this is the first study assessing the effects of dietary AFB1 on the growth of L. maculatus. Similar results were also reported in tilapia [6], grass carp [7], common carp [8], Pelteobagrus fulvidraco [20], channel catfish [9], Clarias batrachus [10], rainbow trout [11], Litopanaeus vannamei [13], and gibel carp [17]. Growth inhibition is regarded as one of the main toxic effects of AFB1 on aquatic animals [1]. It has been reported that dietary AFB1 inhibits the growth of gibel carp by inducing liver function impairment and metabolic disorders [17]. In this study, the decreased hepatosomatic index, along with the declined intestinesomatic index of L. maculatus as dietary AFB1 increased, suggests that AFB1 may cause dysorganoplasia of the liver and intestine since AFB1 can induce degeneration and hepatocyte necrosis [21] and weaken the intestinal barrier function [22]. Using growth performance as the evaluation index, the recommended inclusion level of AFB1 in the L. maculatus diet is less than 1.0 mg/kg.
3.2. Effects of AFB1 on the Antioxidant and Immune Response of L. maculatus
The assessment of serum antioxidant and immune parameters can provide a better understanding of the mechanism for AFB1-induced damage in the growth and health of L. maculatus. In this study, the increased TAOC in either serum or the liver indicated that dietary AFB1 up to 1.0 mg/kg enhanced the antioxidant response of L. maculatus. Antioxidant enzymes including SOD and CAT have been known to play a key role in alleviating oxidative stress via scavenging reactive oxygen species [23]. The increased SOD and CAT activities, along with increased MDA concentrations in fish fed AFB1-treated diets in this study, suggest that dietary AFB1 causes oxidative stress. It has been reported that SOD could catalyze the dismutation of superoxide anion free radicals and thereby alleviate cellular DNA damage [24]. CAT protects the cell from oxidative injury by catalyzing hydrogen peroxide decomposition [25]. MDA is a product of lipid peroxidation, which can induce oxidative stress [26]. These increased antioxidant parameters in fish fed AFB1-treated diets are most likely in response to the physiological toxicity or oxidative stress stimulated by AFB1 rather than an improved antioxidant capacity of the fish. Similar results were also reported by Wang et al. [14], stating that including 5 mg/kg of AFB1 in Litopenaeus vannamei diets induced dysregulation of the antioxidant system of shrimp by increasing SOD and CAT activities and MDA concentration during the 30 days of the AFB1 challenge.
Similarly, the increased immune parameters as reflected by the increased AKP and LZM in the serum, as well as the increased AKP, LZM, and IgM in the livers of the fish, suggests that dietary AFB1 enhanced the immune response of L. maculatus. AKP, an unconventional immune protein, influences inflammation through the regulation of purinergic signaling [27]. LZM, a critical defense protein in the innate immune system, plays an important role in defending against microbial invasion [28]. IgM is the first antibody to respond to an antigen and is an effective defense factor against adverse stress [29]. Similar results were reported by Li [30], stating that dietary inclusion of 10 mg/kg and 50 mg/kg of AFB1 increased serum LZM and AKP activities and IgM concentration in Cyprinus carp.
3.3. Effects of AFB1 on Intestinal Digestive Enzyme Activities and the Histological Appearance of L. maculatus
Intestinal digestive enzymes, including trypsin, lipase, and amylase, are often used as indicators to assess the digestive process of fish [31] or as feedback related to changes in feed formula [32]. Although the effects of AFB1 on the intestinal digestion of livestock have been assessed [33,34], information is rarely available for fish. In this study, dietary AFB1 significantly decreased intestinal trypsin activity but did not alter the lipase and amylase activities of L. maculatus. Wang et al. [35] reported that including 55 μg/kg of AFB1 in Cyprinus carpio diets decreased the apparent digestibility of crude protein owing to decreased intestinal trypsin activity. A similar result was observed by Ostrowski–Meissner [36], in that dietary AFB1 of up to 210 μg/kg only decreased the digestibility of crude protein in the intestines of ducklings. However, including 300 μg/kg of AFB1 in piglet diets [33] and 80 μg/kg of AFB1 in broiler diets [34] did not affect their intestinal digestive enzyme activities. Variations among studies seem to be attributed to species differences and may result from different concentrations of AFB1 in diets. In this study, the decreased growth of L. maculatus fed a diet containing 1.0 mg/kg of AFB1 may partly contribute to the decreased digestibility of crude protein, as reflected by the declined intestinal trypsin activity. However, crude protein digestibility was not evaluated in the present study and further research is still needed to confirm this hypothesis.
Changes in histological morphology commonly indicate pathological alteration caused by feed sources [37]. In this study, the atrophic intestinal villus observed in the AFB1-treated groups indicated that dietary AFB1 caused intestinal injury to L. maculatus. This is similar to the report by Wang et al. [38], stating that including 5 mg/kg of AFB1 in Litopenaeus vannamei diets destroyed the histomorphology of the intestine by reducing the height of the intestinal villus and completely detaching epithelial cells from the basement membrane. Others also observed similar results in rainbow trout (Oncorhynchus mykiss) [4] and common carp [8]. Documentation shows that such histological damage interferes with the absorption of nutrients [39,40]. In tilapia, the decreased growth performance of the fish was due to intestinal lesions induced by AFB1 [39]. The intestinal histological damages induced by AFB1 in this study may also account for the decreased growth performance of L. maculatus.
4. Conclusions
Dietary AFB1 up to 1.0 mg/kg enhanced antioxidant and immune response, decreased intestinal trypsin activity, and induced intestinal histological damages in L. maculatus, eventually reducing the growth performance of the fish. The findings of this study provide a better understanding of the mechanism for AFB1-induced damages in the growth and intestinal health of L. maculatus.
5. Materials and Methods
5.1. Experimental Diets
The compositions of the experimental diets are shown in Table 3. Four diets were prepared including 0 (G0), 0.1 (G0.1), 0.5 (G0.5), and 1.0 (G1.0) mg/kg of AFB1 (from Aspergillus flavus, Sigma, Canada). Dietary AFB1 concentrations were determined using liquid chromatography tandem mass spectrometry [41]. The actual AFB1 concentrations in G0, G0.1, G0.5, and G1.0 were 0, 0.09, 0.47, and 1.02 mg/kg, respectively.

Table 3.
Ingredients and proximate composition (g/kg DM) of the basal diet.
5.2. Feeding Trial
In total, 480 juvenile L. maculatus (initial body weight 2.9 ± 0.02 g) were randomly distributed into 12 tanks (40 fish per tank), with 3 tanks per diet. Fish were hand-fed to apparent satiation twice daily at 07:00 and 19:00 for 56 days. During the feeding trial, the water temperature was 25–27 °C, dissolved oxygen was above 6.0 mg/L, pH was 7.4–8.0, and ammonia and nitrite levels were below 0.01 mg/L. The protocol of this study was approved by the Animal Care and Use Committee of Guangdong Academy of Agricultural Sciences (Guangzhou, China).
5.3. Sampling
At the termination of the trial, fish were fasted for 24 h and then anesthetized with 3-aminobenzoic acid ethyl ester methanesulfonate (40 mg/L, Sigma, Oakland, CA, USA) before sampling. Fish per tank were counted and weighed to analyze the SR, FBW, WGR, SGR, and FC. Feed intake (FI) was determined as the gravimetric difference between the feed offered and orts. Six fish in each tank were randomly selected for analysis of CF, VSI, his, and ISI.
Blood was collected from the caudal veins of six fish in each tank, kept at 25 °C for 30 min, and centrifuged at 8000× g for 10 min. Serum was stored at −80 °C for subsequent analysis of serum antioxidant and immune indexes.
The livers of three fish per tank were taken to determine antioxidant and immune indexes.
The intestines of three fish per tank were sampled for trypsin analysis (Ultraviolet colorimetry), lipase (colorimetry), and amylase (colorimetry) activities using commercial kits supplied by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The intestines of three other fish per tank were randomly collected for intestinal histological examination [42].
5.4. Sample Analyses
Proximate compositions of diets, including dry matter, crude protein, crude lipid, and ash, were measured following the AOAC method [43].
The TAOC (colorimetry), SOD (hydroxylamine method), CAT (ammonium molybdate spectrophotometric method), GPx (colorimetry), MDA (thiobarbituric acid method), AKP (microplate culture method), and LZM (turbidimetry) in the serum and liver were determined using commercial kits provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Concentrations of liver IgM and complement C3 were determined by ELISA kits using immunoturbidimetry (R&D Systems, Minneapolis, MN, USA).
5.5. Calculations and Statistical Analysis
The WGR, FI, FC, SR, SGR, VSI, his, and ISI were calculated by Peng et al. [44].
All data were analyzed by ANOVA and the SAS Mixed procedure system [45] with a tank as the statistical unit. Polynomial contrasts were used to analyze the linear and/or quadratic responses to dietary AFB1 concentrations. Differences were compared using LSMEANS with the PDIFF option and adjusted with a Tukey test. Significance was regarded as p < 0.05.
Author Contributions
K.P. conceived and designed the experiments; B.C., H.Z. and W.H. performed the experiments; K.P. analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by National Natural Science Foundation of China (31902388), Natural Science Foundation of Guangdong Province of China (2018A0303130301, 2021A1515010850), Science and Technology program of Guangdong Province (2019A050505007), Science and Tech-nology Planning Project of Guangzhou (202002030378), Special Fund for Scientific Innovation Strategy-Construction of High-Level Academy of Agriculture Science (R2018QD-075), Special Funds for 2021 Rural Revitalization Strategy-Agricultural Science and Technology Capacity Im-provement (TS-1-6-17). The authors would also like to thank reviews for their constructive com-ments to improve the quality of this paper.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Animal Care and Use Committee of Guangdong Academy of Agricultural Sciences (protocol code 20201111 and date of approval 2020/04/16).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon request, please contact the contributing authors.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (31902388), Natural Science Foundation of Guangdong Province of China (2018A0303130301, 2021A1515010850), Science and Technology program of Guangdong Province (2019A050505007), Science and Technology Planning Project of Guangzhou (202002030378), Special Fund for Scientific Innovation Strategy-Construction of High-Level Academy of Agriculture Science (R2018QD-075), Special Funds for 2021 Rural Revitalization Strategy-Agricultural Science and Technology Capacity Improvement (TS-1-6-17).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goncalves, R.A.; Schatzmayr, D.; Albalat, A.; Mackenzie, S. Mycotoxins in aquaculture: Feed and food. Rev. Aquacult. 2020, 12, 145–175. [Google Scholar] [CrossRef]
- Sergent, T.; Ribonnet, L.; Kolosova, A.; Garsou, S.; Schaut, A.; De Saeger, S.; Schneider, Y.J. Molecular and cellular effects of food contaminants and secondary plant components and their plausible interactions at the intestinal level. Food Chem. Toxicol. 2008, 46, 813–841. [Google Scholar] [CrossRef]
- Souza, C.F.; Baldissera, M.D.; Descovi, S.N.; Zeppenfeld, C.C.; Garzon, L.R.; da Silva, A.S.; Baldisserotto, B. Serum and hepatic oxidative damage induced by a diet contaminated with fungal mycotoxin in freshwater silver catfish Rhamdia quelen: Involvement on disease pathogenesis. Microb. Pathog. 2018, 124, 82–88. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Imani, A.; Niewold, T.A.; Pietsch-Schmied, C.; Moghanolou, K.S. Synergistic toxicity of dietary aflatoxin B1 (AFB1) and zearalenone (ZEN) in rainbow trout (Oncorhynchus mykiss) is attenuated by anabolic effects. Aquaculture 2021, 541, 736793. [Google Scholar] [CrossRef]
- Anater, A.; Manyes, L.; Meca, G.; Ferrer, E.; Luciano, F.B.; Pimpao, C.T.; Font, G. Mycotoxins and their consequences in aquaculture: A review. Aquaculture 2016, 451, 1–10. [Google Scholar] [CrossRef]
- Deng, S.X.; Tian, L.X.; Liu, F.J.; Jin, S.J.; Liang, G.Y.; Yang, H.J.; Du, Z.Y.; Liu, Y.J. Toxic effects and residue of aflatoxin B1 in tilapia (Oreochromis niloticus × O. aureus) during long-term dietary exposure. Aquaculture 2010, 307, 233–240. [Google Scholar] [CrossRef]
- Zeng, Z.; Jiang, W.; Wu, P.; Liu, Y.; Zeng, Y.; Jiang, J.; Kuang, S.; Tang, L.; Zhou, X.; Feng, L. Dietary aflatoxin B1 decreases growth performance and damages the structural integrity of immune organs in juvenile grass carp (Ctenopharyngodon idella). Aquaculture 2019, 500, 1–17. [Google Scholar] [CrossRef]
- Tasa, H.; Imani, A.; Moghanlou, K.S.; Nazdar, N.; Moradi-Ozarlou, M. Aflatoxicosis in fingerling common carp (Cyprinus carpio) and protective effect of rosemary and thyme powder: Growth performance and digestive status. Aquaculture 2020, 527, 735437. [Google Scholar] [CrossRef]
- Jantrarotai, W.; Lovell, R.T. Subchronic toxicity of dietary aflatoxin B1 to channel catfish. J. Aquat. Anim. Health 1990, 2, 248–254. [Google Scholar] [CrossRef]
- Amjad, F.; Durreshahwar, R. Studies on effects of dietary aflatoxin on growth performance and survival rate of fish Clarias batrachus. Int. J. Life Sci. 2016, 4, 121–124. [Google Scholar]
- Arana, S.; Tabata, Y.A.; Sabino, M.; Rigolino, M.G.; Hernandez-Blazquez, F.J. Differential effect of chronic aflatoxin B1 intoxication on the growth performance and incidence of hepatic lesions in triploid and diploid rainbow trout (Oncorhynchus mykiss). Arch. Med. Vet. 2002, 34, 253–263. [Google Scholar] [CrossRef]
- Zeng, Z.L.; Wang, G.J.; Zhu, C.K.; Zhan, H.; Li, Z. Effects of dietary silymarin on growth and toxin accumulation of Sciaenos ocedatas poisoned by aflatoxin B1. Chin. J. Anim. Sci. 2016, 52, 50–55. [Google Scholar]
- Wang, J.; Guo, R.; Su, L.; Xia, H.; Cui, M. Toxic effects of aflatoxin B1 on growth performance, biochemical and hepatopancreas microstructure of Litopanaeus vannamei. J. Fish. China 2012, 36, 952–957. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Liu, M.; Jiang, K.; Wang, M.; Wang, L. Aflatoxin B1 (AFB1) induced dysregulation of intestinal microbiota and damage of antioxidant system in pacific white shrimp (Litopenaeus vannamei). Aquaculture 2018, 495, 940–947. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, L.; Wang, Y.; Deng, Q.; Fang, Z.; Zhao, L.; Zhao, J. Protective mechanism of tea polyphenols against muscle quality deterioration of shrimp (Penaeus vannamei) induced by aflatoxin B1. Aquaculture 2021, 532, 736093. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.; Lin, G.; Li, M.; Zhu, R.; Yiannikouris, A.; Zhang, Y.; Mai, K. The assessment of diet contaminated with aflatoxin B1 in juvenile turbot (Scophthalmus maximus) and the evaluation of the efficacy of mitigation of a yeast cell wall extract. Toxins 2020, 12, 597. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, X.M.; Han, D.; Yang, Y.X.; Xie, S.Q. Growth and aflatoxin B1 accumulation of gibel carp adult fed with diets of different levels of alfatoxin B1. Acta Hydrobiol. Sin. 2012, 36, 818–825. [Google Scholar]
- Sahoo, P.K.; Mukherjee, S.C. Immunosuppressive effects of aflatoxin B1 in Indian major carp (Labeo rohita). Comp. Immunol. Microbiol. Infect. Dis. 2001, 24, 143–149. [Google Scholar] [CrossRef]
- Wang, J.; Tao, Q.; Zhen, W.; Mai, K.; Wei, X.; Zhang, Y.; Ai, Q. Effects of fish meal replacement by soybean meal with supplementation of functional compound additives on intestinal morphology and microbiome of Japanese seabass (Lateolabrax japonicus). Aquacult. Res. 2017, 48, 2186–2197. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Li, Y.; Huang, M.; Gao, Y.; Xue, X.; Zhang, H.; Encarnacao, P.; Santos, G.A.; Goncalves, R.A. Response of yellow catfish (Pelteobagrus fulvidraco) to different dietary concentrations of aflatoxin B1 and evaluation of an aflatoxin binder in offsetting its negative effects. Cienc. Mar. 2016, 42, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.; Chen, B.; Zhao, H.; Huang, W.; Chen, X. Effects of dietary alfatoxin B1 on growth performance, body composition, liver histomorphology and toxin residues of Lateolabrax maculatus. Chin. J. Anim. Nutr. 2021, in press. [Google Scholar]
- Romero, A.; Ares, I.; Ramos, E.; Castellano, V.; Martinez, M.; Martinez-Larranaga, M.; Anadon, A.; Martinez, M. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology 2016, 353, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Lv, X.; Zhao, H.; Chen, B.; Chen, X.; Huang, W. Antioxidant and intestinal recovery function of condensed tannins in Lateolabrax maculatus responded to in vivo and in vitro oxidative stress. Aquaculture 2022, 547, 737399. [Google Scholar] [CrossRef]
- Paulton, M.P.; Thomas, P.C.; Vijayan, K.K. Identification of antioxidant enzyme genes of the Indian edible oyster, Crassostrea madrasensis (Preston) through polymerase chain reaction. Indian J. Fish. 2012, 59, 179–181. [Google Scholar]
- Peng, K.; Shirley, D.C.; Xu, Z.; Huang, Q.; McAllister, T.A.; Chaves, A.V.; Acharya, S.; Liu, C.; Wang, S.; Wang, Y. Effect of purple prairie clover (Dalea purpurea Vent.) hay and its condensed tannins on growth performance, wool growth, nutrient digestibility, blood metabolites and ruminal fermentation in lambs fed total mixed rations. Anim. Feed Sci. Technol. 2016, 222, 100–110. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, Y.; Ye, J.; Du, Z.; Kong, Y. An evaluation of replacing fish meal with fermented soybean meal in the diet of Macrobrachium nipponense: Growth, nonspecific immunity, and resistance to Aeromonas hydrophila. Fish Shellfish Immunol. 2015, 44, 295–301. [Google Scholar] [CrossRef]
- Rader, B.A. Alkaline phosphatase, an unconventional immune protein. Front. Immunol. 2017, 8, 897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defense molecule of fish innate immune system. Aquacult. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Semple, S.L.; Kellendonk, C.J.; Al-Hussinee, L.; MacInnes, J.I.; Lumsden, J.S.; Dixon, B. Serum IgM, MH class IIβ genotype and respiratory burst activity do not differ between rainbow trout families displaying resistance or susceptibility to the coldwater pathogen, Flavobacterium psychrophilum. Aquaculture 2018, 483, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Li, H.B. Study on the Immunotoxicity of AFB1 to Cyprinus Carp and its Intervention Agents. Ph.D. Thesis, Ningbo University, Ningbo, China, 2016. [Google Scholar]
- Abolfathi, M.; Hajimoradloo, A.; Ghorbani, R.; Zamani, A. Effect of starvation and refeeding on digestive enzyme activities in juvenile roach, Rutilus rutilus caspicus. Comp. Biochem. Phys. A Mol. Integr. Physiol. 2012, 161, 166–173. [Google Scholar] [CrossRef]
- Santigosa, E.; Sanchez, J.; Medale, F.; Kaushik, S.; Perez-Sanchez, J.; Gallardo, M.A. Modifications of digestive enzymes in trout (Oncorhynchus mykiss) and sea bream (Sparus aurata) in response to dietary fish meal replacement by plant protein sources. Aquaculture 2008, 282, 68–74. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Yu, B.; He, J.; Mao, X.; Yu, J.; Zheng, P.; Wang, Q.; Wang, H.; Zeng, Z.; et al. Effects of aflatoxin B1 and adsorbent on growth performance and jejunum digestive enzyme activities of weaned piglets. Chin. J. Anim. Sci. 2015, 27, 3501–3508. [Google Scholar]
- Deng, Q.; Liu, N.; Jiang, Q.; Wang, J.; Chen, Y.; Gu, K. Effects of lactic acid bacteria on the growth performance, nutrient apparent digestibility and slaughter performance of broilers diets contaminated with AFB1. China Anim. Husb. Vet. Med. 2016, 43, 1194–1200. [Google Scholar]
- Wang, D.; Liu, L.; Huang, J.; Zhang, Y. Effects of Bacillus subtilis on growth performance, serum biochemical indices, and digestibility and toxin residues of yellow river carp fed AFB1-containing diets. Feed Res. 2015, 12, 42–46. [Google Scholar]
- Ostrowski-Meissner, H.T. Effect of dietary aflatoxins on protein and energy utilization by two Indonesian breeds of ducklings (Alabio and Tegal). Poult. Sci. 1983, 62, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Luo, Z.; Chen, F.; Wei, C.C.; Wu, K.; Zhu, X.M.; Liu, X. Effect of fish meal replacement by chlorella meal with dietary cellulase addition on growth performance, digestive enzymatic activities, histology and myogenic genes’ expression for crucian carp Carassius auratus. Aquacult. Res. 2017, 48, 3244–3256. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Liu, M.; Jiang, K.; Wang, M.; Wang, L. Comparative transcriptome analysis reveals the different roles between hepatopancreas and intestine of Litopenaeus vannamei in immune response to aflatoxin B1 (AFB1) challenge. Comp. Biochem. Phys. Part C 2019, 222, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mehrim, A.I.; Abdelhamid, A.M.; Abou-Shousha, A.; Salem, M.F.I.; El-Sharawy, M.A.M.M. Nutritious attempts to detoxify aflatoxic diets of tilapia fish: 2-clinical, biochemical and histological parameters. J. Arab. Aquacul. Soc. 2006, 1, 69–90. [Google Scholar]
- Liew, W.P.P.; Mohd-Redzwan, S. Mycotoxin: Its impact on gut health and microbiota. Front. Cell. Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Herrman, T.J.; Dai, S.Y. Determination of aflatoxins in animal feeds by liquid chromatography/tandem mass spectrometry with isotope dilution. Rapid Commun. Mass Spectrom. 2011, 25, 1222–1230. [Google Scholar] [CrossRef]
- Peng, K.; Zhao, H.X.; Wang, G.X.; Chen, B.; Mo, W.; Huang, Y. Effects of condensed tannins on growth performance, intestinal immune capacity and bacterial microbiomes of Lateolabrax japonicas. Aquacult. Res. 2021, 52, 5321–5331. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Agricultural Chemists, 16th ed.; 5th rev; AOAC International: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Peng, K.; Wang, G.; Wang, Y.; Chen, B.; Sun, Y.; Mo, W.; Li, G.; Huang, Y. Condensed tannins enhanced antioxidant capacity and hypoxic stress survivability but not growth performance and fatty acid profile of juvenile Japanese seabass (Lateolabrax japonicus). Anim. Feed Sci. Technol. 2020, 269, 114671. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS OnlineDoc® 9.1.3.; SAS Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).