Penicillium expansum Impact and Patulin Accumulation on Conventional and Traditional Apple Cultivars
Abstract
:1. Introduction
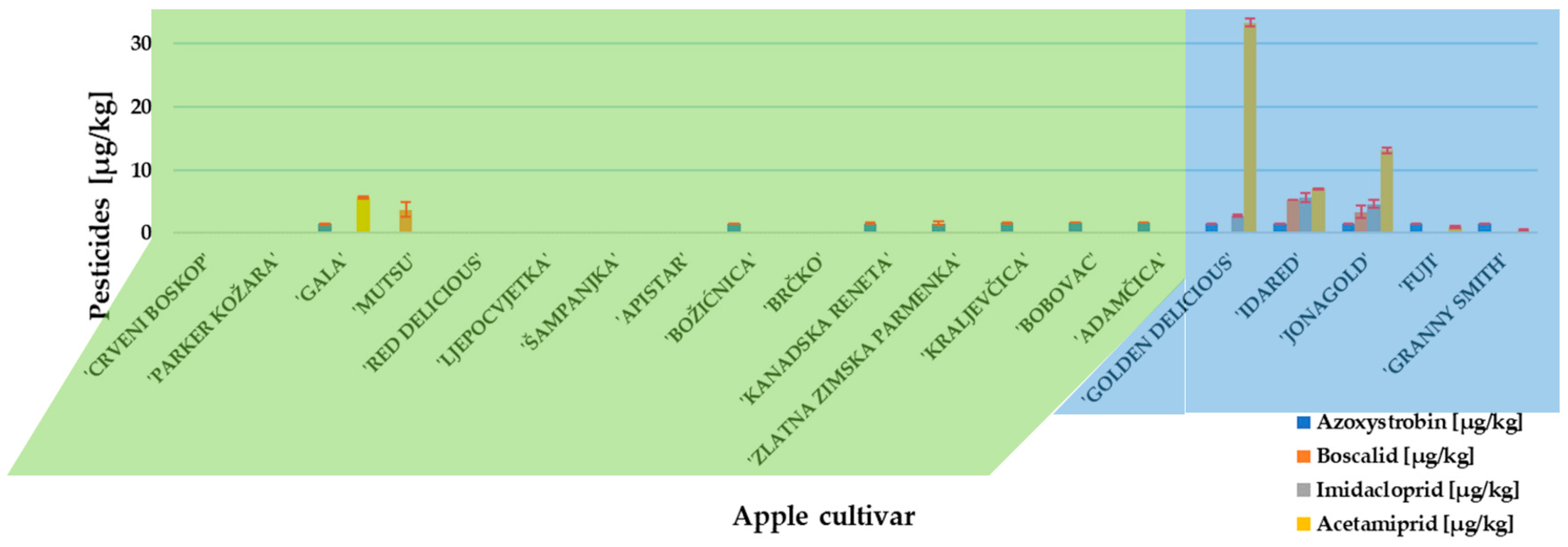
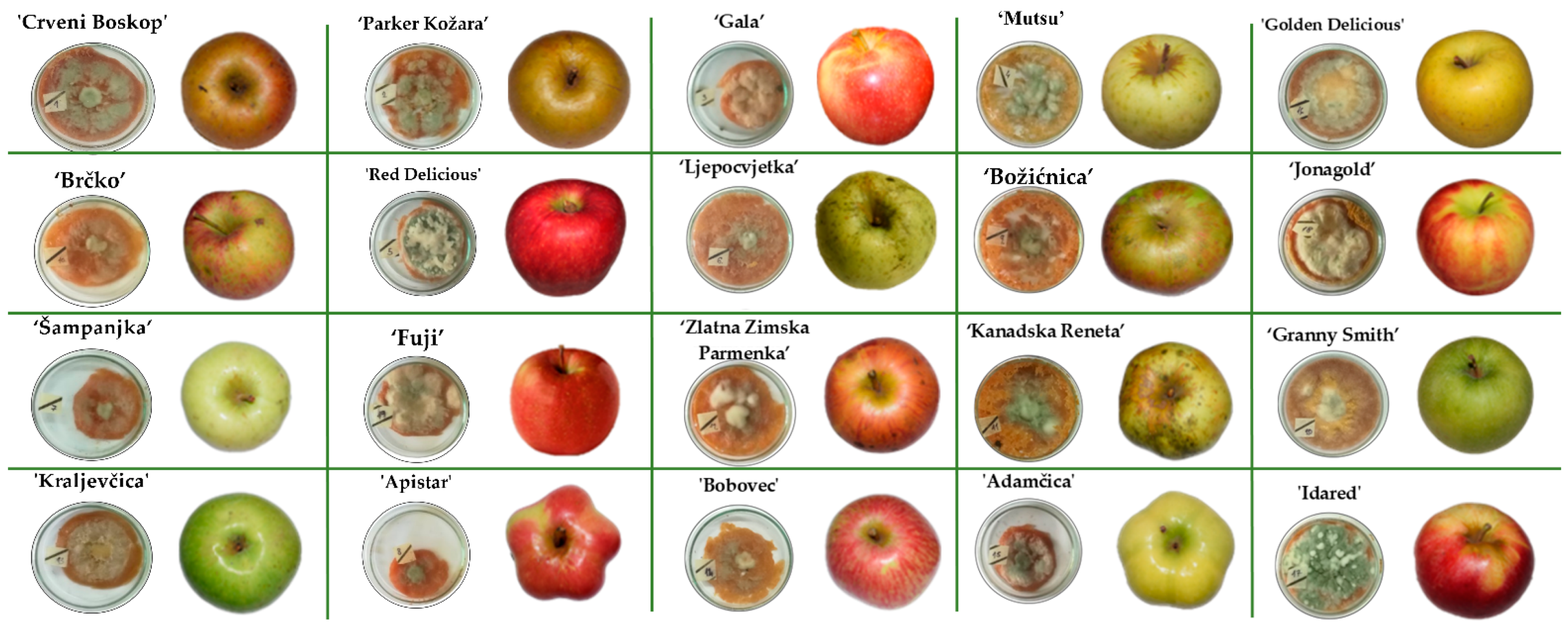
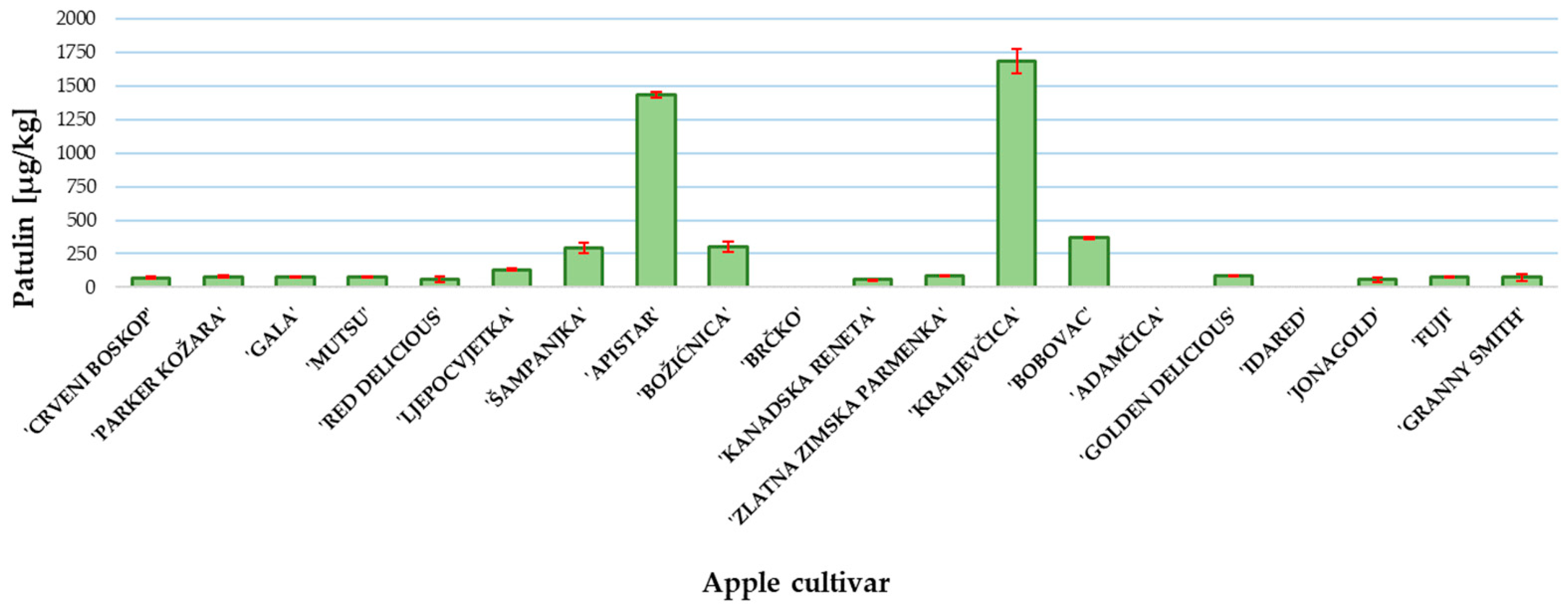
2. Results and Discussion
3. Conclusion
4. Materials and Methods
4.1. Chemicals
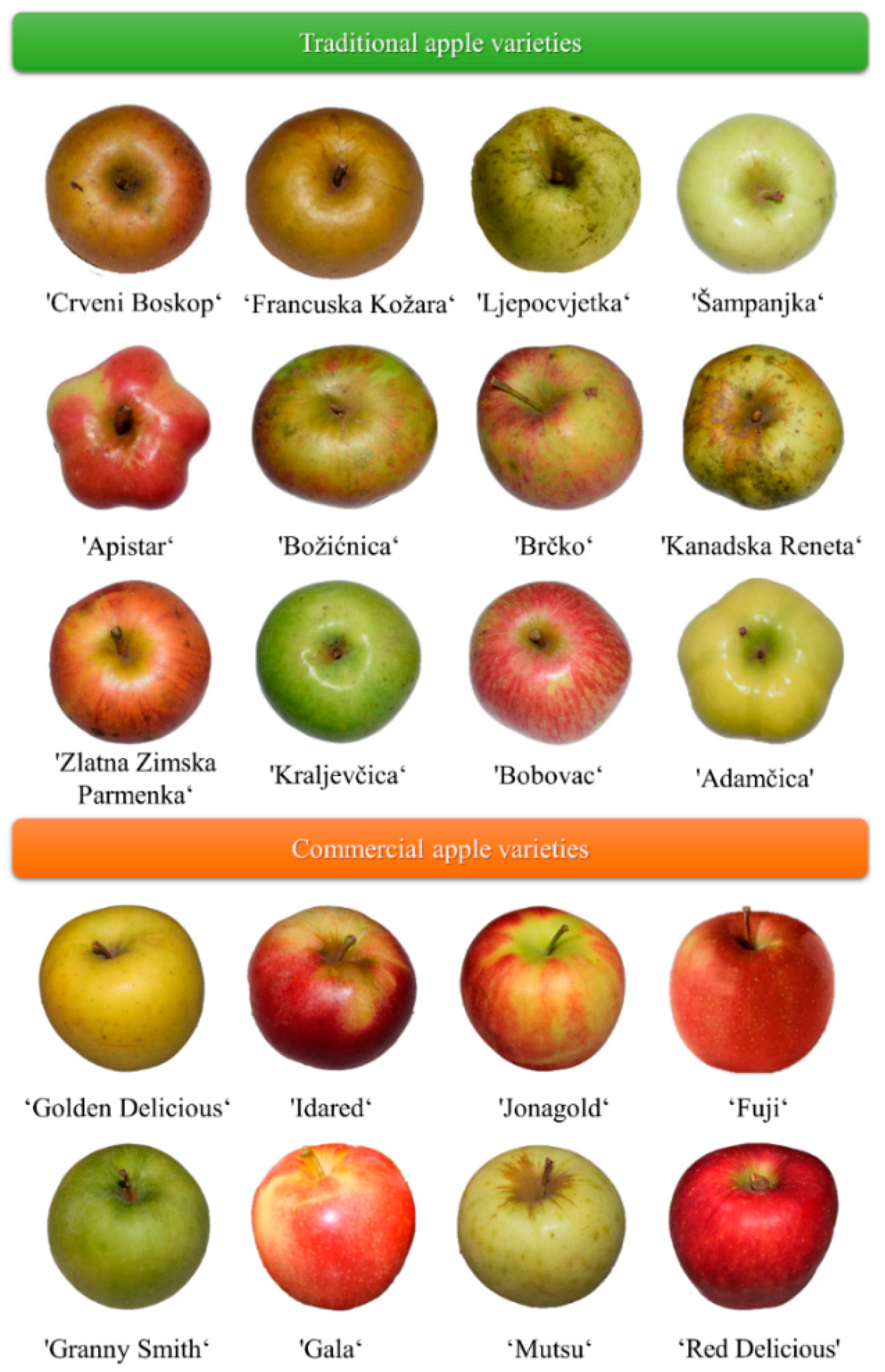
4.2. Plant Material
4.3. Identification of Polyphenols
4.4. Determination of Antiradical Activity
4.5. Determination of the Resistance of the Selected Apple Cultivars to P. expansum
4.6. Sample Preparation Procedure for Patulin and Pesticide Determination
4.7. Patulin and Pesticide Determination
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. FAOSTAT: Statistical Database. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 3 October 2021).
- Le Gall, S.; Even, S.; Lahaye, M. Fast Estimation of Dietary Fiber Content in Apple. J. Agric. Food Chem. 2016, 64, 1401–1405. [Google Scholar] [CrossRef]
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Barad, S.; Sionov, E.; Keller, N.P.; Prusky, D.B. Does the host contribute to modulation of mycotoxin production by fruit pathogens? Toxins 2017, 9, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Q.; Gong, X.; Wang, C.; Zhao, J.; Zhang, H.; Tian, F.; Chen, W. Food-borne patulin toxicity is related to gut barrier disruption and can be prevented by docosahexaenoic acid and probiotic supplementation. Food Funct. 2019, 10, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Singh, N.; Ansari, K.M. Toxicological effects of patulin mycotoxin on the mammalian system: An overview. Toxicol. Res. 2017, 6, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Kovač, T.; Šarkanj, B.; Borišev, I.; Djordjevic, A.; Jović, D.; Lončarić, A.; Babić, J.; Jozinović, A.; Krska, T.; Gangl, J.; et al. Fullerol C60(OH)24 Nanoparticles Affect Secondary Metabolite Profile of Important Foodborne Mycotoxigenic Fungi In Vitro. Toxins 2020, 12, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovač, M.; Šubarić, D.; Bulaić, M.; Kovač, T.; Šarkanj, B. Yesterday masked, today modified; what do mycotoxins bring next? Arch. Ind. Hyg. Toxicol. 2018, 69, 196–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The European Parliament and the Council of the European Union Commission Regulation (EC). No 1881/2006, Maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Szymczyk, K.; Szteke, B.; Goszcz, H. Patulin content in Polish apple juices [Wystepowanie patuliny w krajowych sokach jabukowych.]. Rocz. Panstw. Zakl. Hig. 2004, 55, 255–260. [Google Scholar]
- Spadaro, D.; Ciavorella, A.; Frati, S.; Garibaldi, A.; Gullino, M.L. Incidence and level of patulin contamination in pure and mixed apple juices marketed in Italy. Food Control 2007, 18, 1098–1102. [Google Scholar] [CrossRef]
- HAH. Croatian Food Agency—Scientific Report About Patulin in Apple Juice; Croatian Food Agency: Osijek, Croatia, 2017; pp. 1–28. [Google Scholar]
- McCallum, J.L.; Tsao, R.; Zhou, T. Factors affecting patulin production by Penicillium expansum. J. Food Prot. 2002, 65, 1937–1942. [Google Scholar] [CrossRef]
- Jackson, L.S.; Beacham-Bowden, T.; Keller, S.E.; Adhikari, C.; Taylor, K.T.; Chirtel, S.J.; Merker, R.I. Apple quality, storage, and washing treatments affect patulin levels in apple cider. J. Food Prot. 2003, 66, 618–624. [Google Scholar] [CrossRef]
- Zhong, L.; Carere, J.; Lu, Z.; Lu, F.; Zhou, T. Patulin in apples and apple-based food products: The burdens and the mitigation strategies. Toxins 2018, 10, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chávez, R.A.S.; Peniche, R.Á.M.; Medrano, S.A.; Muñoz, L.S.; Ortíz, M.d.S.C.; Espasa, N.T.; Sanchis, R.T. Effect of maturity stage, ripening time, harvest year and fruit characteristics on the susceptibility to Penicillium expansum link of apple genotypes from Queretaro, Mexico. Sci. Hortic. 2014, 180, 86–93. [Google Scholar] [CrossRef]
- Jurick, W.M.; Janisiewicz, W.J.; Saftner, R.A.; Vico, I.; Gaskins, V.L.; Park, E.; Forsline, P.L.; Fazio, G.; Conway, W.S. Identification of wild apple germplasm (Malus spp.) accessions with resistance to the postharvest decay pathogens Penicillium expansum and Colletotrichum acutatum. Plant Breed. 2011, 130, 481–486. [Google Scholar] [CrossRef]
- Nybom, H.; Ahmadi-Afzadi, M.; Rumpunen, K.; Tahir, I. Review of the impact of apple fruit ripening, texture and chemical contents on genetically determined susceptibility to storage rots. Plants 2020, 9, 831. [Google Scholar] [CrossRef]
- Marín, S.; Morales, H.; Hasan, H.A.H.; Ramos, A.J.; Sanchis, V. Patulin distribution in Fuji and Golden apples contaminated with Penicillium expansum. Food Addit. Contam. 2006, 23, 1316–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepeljnjak, S.; Šegvić, M.; Ožegović, L. Citrininotoxinogenicity of Penicillium spp. isolated from decaying apples. Braz. J. Microbiol. 2002, 33, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Snini, S.P.; Tannous, J.; Heuillard, P.; Bailly, S.; Lippi, Y.; Zehraoui, E.; Barreau, C.; Oswald, I.P.; Puel, O. Patulin is a cultivar-dependent aggressiveness factor favouring the colonization of apples by Penicillium expansum. Mol. Plant Pathol. 2016, 17, 920–930. [Google Scholar] [CrossRef]
- Norelli, J.L.; Wisniewski, M.; Fazio, G.; Burchard, E.; Gutierrez, B.; Levin, E.; Droby, S. Genotyping-by-sequencing markers facilitate the identification of quantitative trait loci controlling resistance to Penicillium expansum in Malus sieversii. PLoS ONE 2017, 12, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, M.; Fischer, C. Genetic resources as basis for new resistant apple cultivars. J. Fruit Ornam. Plant Res. 2004, 12, 63–76. [Google Scholar]
- Janisiewicz, W.J.; Saftner, R.A.; Conway, W.S.; Forsline, P.L. Preliminary evaluation of apple germplasm from Kazakhstan for resistance to postharvest blue mold in fruit caused by Penicillium expansum. HortScience 2008, 43, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Jakobek, L.; Barron, A.R. Ancient apple varieties from Croatia as a source of bioactive polyphenolic compounds. J. Food Compos. Anal. 2016, 45, 9–15. [Google Scholar] [CrossRef]
- Lončarić, A.; Skendrović Babojelić, M.; Kovač, T.; Šarkanj, B. Pomological Properties and Polyphenol Content of Conventional and Traditional Apple Cultivars from Croatia. Pomol. Prop. Polyphen. Content Conv. Tradit. Apple Cultiv. Croat. 2019, 8, 19–24. [Google Scholar]
- Iacopini, P.; Camangi, F.; Stefani, A.; Sebastiani, L. Antiradical potential of ancient Italian apple varieties of Malus×domestica Borkh. In a peroxynitrite-induced oxidative process. J. Food Compos. Anal. 2010, 23, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi-Afzadi, M.; Nybom, H.; Ekholm, A.; Tahir, I.; Rumpunen, K. Biochemical contents of apple peel and flesh affect level of partial resistance to blue mold. Postharvest Biol. Technol. 2015, 110, 173–182. [Google Scholar] [CrossRef]
- Sun, J.; Janisiewicz, W.J.; Nichols, B.; Jurick, W.M.; Chen, P. Composition of phenolic compounds in wild apple with multiple resistance mechanisms against postharvest blue mold decay. Postharvest Biol. Technol. 2017, 127, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Krivak, P.; Medvidović Kosanović, M.; Šter, A. Dihydrochalcones in old apple varieties from Croatia. In Proceedings of the International Conference 16th Ružička days “TODAY SCIENCE—TOMORROW INDUSTRY”, Vukovar, Croatia, 21–23 September 2016; Jukić, A., Ed.; pp. 146–154. [Google Scholar]
- Guyot, S.; Marnet, N.; Laraba, D.; Sanoner, P.; Drilleau, J.F. Reversed-Phase HPLC following Thiolysis for Quantitative Estimation and Characterization of the Four Main Classes of Phenolic Compounds in Different Tissue Zones of a French Cider Apple Variety (Malus domestica Var. Kermerrien). J. Agric. Food Chem. 1998, 46, 1698–1705. [Google Scholar] [CrossRef]
- Jakobek, L.; Ištuk, J.; Buljeta, I.; Voća, S.; Žlabur, J.Š.; Babojelić, M.S. Traditional, Indigenous Apple Varieties, a Fruit with Potential for Beneficial Effects: Their Quality Traits and Bioactive Polyphenol Contents. Foods 2020, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Lončarić, A.; Matanović, K.; Ferrer, P.; Kovač, T.; Šarkanj, B.; Babojelić, M.S.; Lores, M. Peel of traditional apple varieties as a great source of bioactive compounds: Extraction by micro-matrix solid-phase dispersion. Foods 2020, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Sadeghipour, M.; Terreux, R.; Phipps, J. Flavonoids and tyrosine nitration: Structure–activity relationship correlation with enthalpy of formation. Toxicol. Vitr. 2005, 19, 155–165. [Google Scholar] [CrossRef]
- López, M. Study of phenolic compounds as natural antioxidants by a fluorescence method. Talanta 2003, 60, 609–616. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/products/?event=details&p=23 (accessed on 3 October 2021).
- Tannous, J.; Atoui, A.; El Khoury, A.; Francis, Z.; Oswald, I.P.; Puel, O.; Lteif, R. A study on the physicochemical parameters for Penicillium expansum growth and patulin production: Effect of temperature, pH, and water activity. Food Sci. Nutr. 2016, 4, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Qiao, N.; Zhao, J.; Zhang, H.; Tian, F.; Zhai, Q.; Chen, W. Postharvest control of Penicillium expansum in fruits: A review. Food Biosci. 2020, 36, 100633. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Chen, Y.; Zhang, Z.; Qin, G.; Chen, T.; Tian, S. Molecular basis and regulation of pathogenicity and patulin biosynthesis in Penicillium expansum. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3416–3438. [Google Scholar] [CrossRef] [PubMed]
- Finotti, E.; Parroni, A.; Zaccaria, M.; Domin, M.; Momeni, B.; Fanelli, C.; Reverberi, M. Aflatoxins are natural scavengers of reactive oxygen species. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Ballester, A.-R.; Núñez, F.; González-Candelas, L. Evaluation of the activity of the antifungal PgAFP protein and its producer mould against Penicillium spp postharvest pathogens of citrus and pome fruits. Food Microbiol. 2019, 84, 103266. [Google Scholar] [CrossRef]
- Gessler, C.; Patocchi, A.; Sansavini, S.; Tartarini, S.; Gianfranceschi, L. Venturia inaequalis Resistance in Apple. CRC. Crit. Rev. Plant Sci. 2006, 25, 473–503. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Malisardi, G.; Scalambra, E.; Andreotti, E.; Romagnoli, C.; Vicentini, C.; Manfredini, S.; Vertuani, S. Synthesis, Antioxidant and Antimicrobial Activity of a New Phloridzin Derivative for Dermo-Cosmetic Applications. Molecules 2012, 17, 13275–13289. [Google Scholar] [CrossRef] [Green Version]
- Shim, S.-H.; Jo, S.-J.; Kim, J.-C.; Choi, G.-J. Control Efficacy of Phloretin Isolated from Apple Fruits Against Several Plant Diseases. Plant Pathol. J. 2010, 26, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Oleszek, M.; Pecio, Ł.; Kozachok, S.; Lachowska-Filipiuk, Ż.; Oszust, K.; Frąc, M. Phytochemicals of apple pomace as prospect bio-fungicide agents against mycotoxigenic fungal species—in vitro experiments. Toxins 2019, 11, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loncaric, A.; Dugalic, K.; Mihaljevic, I.; Jakobek, L.; Pilizota, V. Effects of sugar addition on total polyphenol content and antioxidant activity of frozen and freeze-dried apple Purée. J. Agric. Food Chem. 2014, 62, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ballester, A.R.; Norelli, J.; Burchard, E.; Abdelfattah, A.; Levin, E.; González-Candelas, L.; Droby, S.; Wisniewski, M. Transcriptomic response of resistant (Pi613981–malus sieversii) and susceptible (“royal gala”) genotypes of apple to blue mold (penicillium expansum) infection. Front. Plant Sci. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, 70, 12–34. [Google Scholar]
- Guidance SANTE. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. European Commission SANTE/12682/2019. 2019. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 3 October 2021).
- Kovač, M.; Bulaić, M.; Jakovljević, J.; Nevistić, A.; Rot, T.; Kovač, T.; Dodlek Šarkanj, I.; Šarkanj, B. Mycotoxins, Pesticide Residues, and Heavy Metals Analysis of Croatian Cereals. Microorganisms 2021, 9, 216. [Google Scholar] [CrossRef]
| Apple Cultivar | Gallic Acid | Catechin | Epicatechin | p-Cumaric Acid | Phloridzin | Phloretin | Caffeic Acid | Chlorogenic Acid |
|---|---|---|---|---|---|---|---|---|
| mg/100 g of FW | ||||||||
| ‘Crveni Boskop’ | 2.96 ± 0.09 k* | 56.55 ± 0.62 f | 26.76 ± 0.44 f | 3.84 ± 0.01e f | 1.44 ± 0.05 e | 0.04 ± 0.00 d | 0.64 ± 0.01 d | 1.28 ± 0.02 i |
| ‘Parker Kožara’ | 2.80 ± 0.07 k | 58.85 ± 0.61 e | 30.62 ± 0.33 e | 0.81 ± 0.08 i | 0.45 ± 0.02 j | 0.03 ± 0.01 e | 0.58 ± 0.05 e | 1.89 ± 0.07 g |
| ‘Ljepocvjetka’ | 4.15 ± 0.06 g | 18.42 ± 0.12 i | 11.22 ± 0.11 k | 1.77 ± 0.02 h | 2.39 ± 0.03 a | 0.33 ± 0.00 a | 0.28 ± 0.01 i | 3.78 ± 0.02 b |
| ‘Šampanjka’ | 3.14 ± 0.05 j | 10.92 ± 0.08 m | 7.31 ± 0.05 n | 0.66 ± 0.04 j | 1.10 ± 0.03 g | 0.33 ± 0.01 a | 0.68 ± 0.06 d | 2.56 ± 0.01 e |
| ‘Apistar’ | 3.99 ± 0.12 g | 91.26 ± 0.48 a | 64.26 ± 0.86 b | 3.79 ± 0.12 f | 1.30 ± 0.05 f | <LOD | 0.41 ± 0.05 h | 1.46 ± 0.20 h |
| ‘Božićnica’ | 4.44 ± 0.06 f | 59.85 ± 0.27 d | 58.85 ± 0.21 c | 6.21 ± 0.04 d | 1.76 ± 0.02 d | <LOD | 0.25 ± 0.01 i | 2.11 ± 0.04 f |
| ‘Brčko’ | 5.66 ± 0.10 d | 6.11 ± 0.06 o | 17.78 ± 0.11 h | 0.47 ± 0.01 k | 1.09 ± 0.01 g | <LOD | 0.19 ± 0.01 j | 0.29 ± 0.06 kl |
| ‘Kanadska Reneta’ | 4.42 ± 0.06 f | 59.99 ± 0.13 d | 54.04 ± 0.41 d | 9.10 ± 0.03 a | 1.85 ± 0.01 c | <LOD | 0.47 ± 0.03 g | 2.61 ± 0.01 e |
| ‘Zlatna Zimska Parmenka’ | 5.15 ± 0.03 e | 55.64 ± 0.29 g | 53.97 ± 0.19 d | 6.81 ± 0.05 b | 1.95 ± 0.07 b | <LOD | 0.81 ± 0.01 b | 3.53 ± 0.04 c |
| ‘Kraljevčica’ | 7.40 ± 0.05 b | 80.08 ± 0.28 b | 21.51 ± 0.18 g | 3.07 ± 0.02 g | 0.56 ± 0.05 i | <LOD | 0.20 ± 0.00 j | 0.24 ± 0.03 l |
| ‘Bobovac’ | 8.35 ± 0.04 a | 38.38 ± 0.05 h | 67.00 ± 0.76 a | 6.42 ± 0.16 c | 1.77 ± 0.02 d | 0.06 ± 0.01 c | 0.76 ± 0.03 c | 3.33 ± 0.03 d |
| ‘Adamčica’ | 3.73 ± 0.03 h | 15.73 ± 0.77 k | 7.99 ± 0.36 n | 6.22 ± 0.14 d | 0.72 ± 0.05 h | <LOD | 1.22 ± 0.04 a | 3.95 ± 0.22 a |
| ’Golden Delicious’ | 6.42 ± 0.02 c | 3.12 ± 0.07 p | 10.13 ± 0.08 l | 0.89 ± 0.03 i | 0.40 ± 0.02 j | <LOD | 0.17 ± 0.01 j | 0.38 ± 0.02 jk |
| ‘Idared’ | 5.60 ± 0.30 d | 2.32 ± 0.08 q | 3.13 ± 0.07 q | <LOD | 0.31 ± 0.01 k | <LOD | 0.06 ± 0.00 kl | 0.47 ± 0.01 j |
| ‘Jonagold’ | 2.86 ± 0.02 k | 8.23 ± 0.29 c | 16.34 ± 0.81 i | <LOD | 0.18 ± 0.01 lm | 0.3 ± 0.02 b | 0.10 ± 0.00 k | 0.04 ± 0.00 m |
| ‘Fuji’ | 1.90 ± 0.03 l | 2.88 ± 0.06 p | 8.90 ± 0.44 m | <LOD | 0.18 ± 0.01 lm | <LOD | 0.05 ± 0.00 l | n.d. |
| ‘Granny Smith’ | 4.52 ± 0.25 f | 16.71 ± 0.37 j | 14.98 ± 0.27 j | <LOD | 0.17 ± 0.01 m | <LOD | n.d. | 0.07 ± 0.00 m |
| ‘Gala’ | 2.91 ± 0.05 k | 8.96 ± 0.16 n | 5.91 ± 0.08 p | 0.38 ± 0.01 k | 0.23 ± 0.00 l | 0.02 ± 0.01 f | 0.07 ± 0.00 kl | 0.06 ± 0.00 m |
| ‘Mutsu’ | 3.75 ± 0.03 h | 10.66 ± 0.09 m | 6.61 ± 0.02 o | 0.49 ± 0.01 k | 0.32 ± 0.02 k | 0.03 ± 0.02 e | 0.05 ± 0.00 l | 0.06 ± 0.00 m |
| ‘Red Delicious’ | 3.45 ± 0.01 i | 14.67 ± 0.21l | 8.92 ± 0.00 m | 3.95 ± 0.15 e | 0.51 ± 0.05 i | 0.04 ± 0.00 d | 0.52 ± 0.04 f | 0.30 ± 0.02 kl |
| Apple Cultivar | AOA mmol TE/L |
|---|---|
| ‘Crveni Boskop’ | 354.7 ± 9.10 j* |
| ‘Parker Kožara’ | 367.53 ± 2.55 hi |
| ‘Ljepocvjetka’ | 402.37 ± 2.75 bc |
| ‘Šampanjka’ | 376.24 ± 4.67 fg |
| ‘Apistar’ | 409.63 ± 2.93 b |
| ‘Božićnica’ | 407.7 ± 8.08 b |
| ‘Brčko’ | 331.71 ± 1.68 kl |
| ‘Kanadska Reneta’ | 389.79 ± 3.27 e |
| ‘Zlatna Zimska Parmenka’ | 391.97 ± 1.51 de |
| ‘Kraljevčica’ | 416.87 ± 3.02 a |
| ‘Bobovac’ | 368.01 ± 6.17 ghi |
| ‘Adamčica’ | 318.83 ± 2.75 m |
| ‘Red Delicious’ | 370.91 ± 6.26 gh |
| ‘Idared’ | 384.22 ± 1.11 ef |
| ‘Jonagold’ | 398.74 ± 2.75 cd |
| ‘Fuji’ | 375.03 ± 4.42 gh |
| ‘Granny Smith’ | 398.98 ± 3.84 cd |
| ‘Gala’ | 359.54 ± 4.19 ij |
| ‘Mutsu’ | 373.09 ± 0.42 gh |
| ‘Red Delicious’ | 336.07 ± 13.74 k |
| Polyphenols mg/100 g of FW | Patulin µg/kg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | ||||||||||
| Gallic acid | 0.4226 | 0.002 | |||||||||
| Catechin | 0.3717 | 0.008 | |||||||||
| Epicatechin | 0.3305 | 0.019 | |||||||||
| p-cumaric acid | 0.1588 | 0.271 | |||||||||
| Phloridzin | 0.0612 | 0.673 | |||||||||
| Phloretin | −0.1727 | 0.230 | |||||||||
| Caffeic acid | −0.0031 | 0.983 | |||||||||
| Chlorogenic acid | −0.0491 | 0.735 | |||||||||
| r >= | −1 | −0.80 | −0.60 | −0.40 | −0.20 | 0 | 0.20 | 0.40 | 0.60 | 0.80 | 1 |
| p <= | 0.001 | 0.010 | 0.025 | 0.050 | 0.100 | 0.150 | 0.200 | 0.350 | 0.500 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lončarić, A.; Šarkanj, B.; Gotal, A.-M.; Kovač, M.; Nevistić, A.; Fruk, G.; Skendrović Babojelić, M.; Babić, J.; Miličević, B.; Kovač, T. Penicillium expansum Impact and Patulin Accumulation on Conventional and Traditional Apple Cultivars . Toxins 2021, 13, 703. https://doi.org/10.3390/toxins13100703
Lončarić A, Šarkanj B, Gotal A-M, Kovač M, Nevistić A, Fruk G, Skendrović Babojelić M, Babić J, Miličević B, Kovač T. Penicillium expansum Impact and Patulin Accumulation on Conventional and Traditional Apple Cultivars . Toxins. 2021; 13(10):703. https://doi.org/10.3390/toxins13100703
Chicago/Turabian StyleLončarić, Ante, Bojan Šarkanj, Ana-Marija Gotal, Marija Kovač, Ante Nevistić, Goran Fruk, Martina Skendrović Babojelić, Jurislav Babić, Borislav Miličević, and Tihomir Kovač. 2021. "Penicillium expansum Impact and Patulin Accumulation on Conventional and Traditional Apple Cultivars " Toxins 13, no. 10: 703. https://doi.org/10.3390/toxins13100703
APA StyleLončarić, A., Šarkanj, B., Gotal, A.-M., Kovač, M., Nevistić, A., Fruk, G., Skendrović Babojelić, M., Babić, J., Miličević, B., & Kovač, T. (2021). Penicillium expansum Impact and Patulin Accumulation on Conventional and Traditional Apple Cultivars . Toxins, 13(10), 703. https://doi.org/10.3390/toxins13100703








