Fumonisin B1 Accumulates in Chicken Tissues over Time and This Accumulation Was Reduced by Feeding Algo-Clay
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental Diets and Study Design
2.2. Performances and Organ Weight
2.3. Plasma Biochemistry and Sphingoid Bases
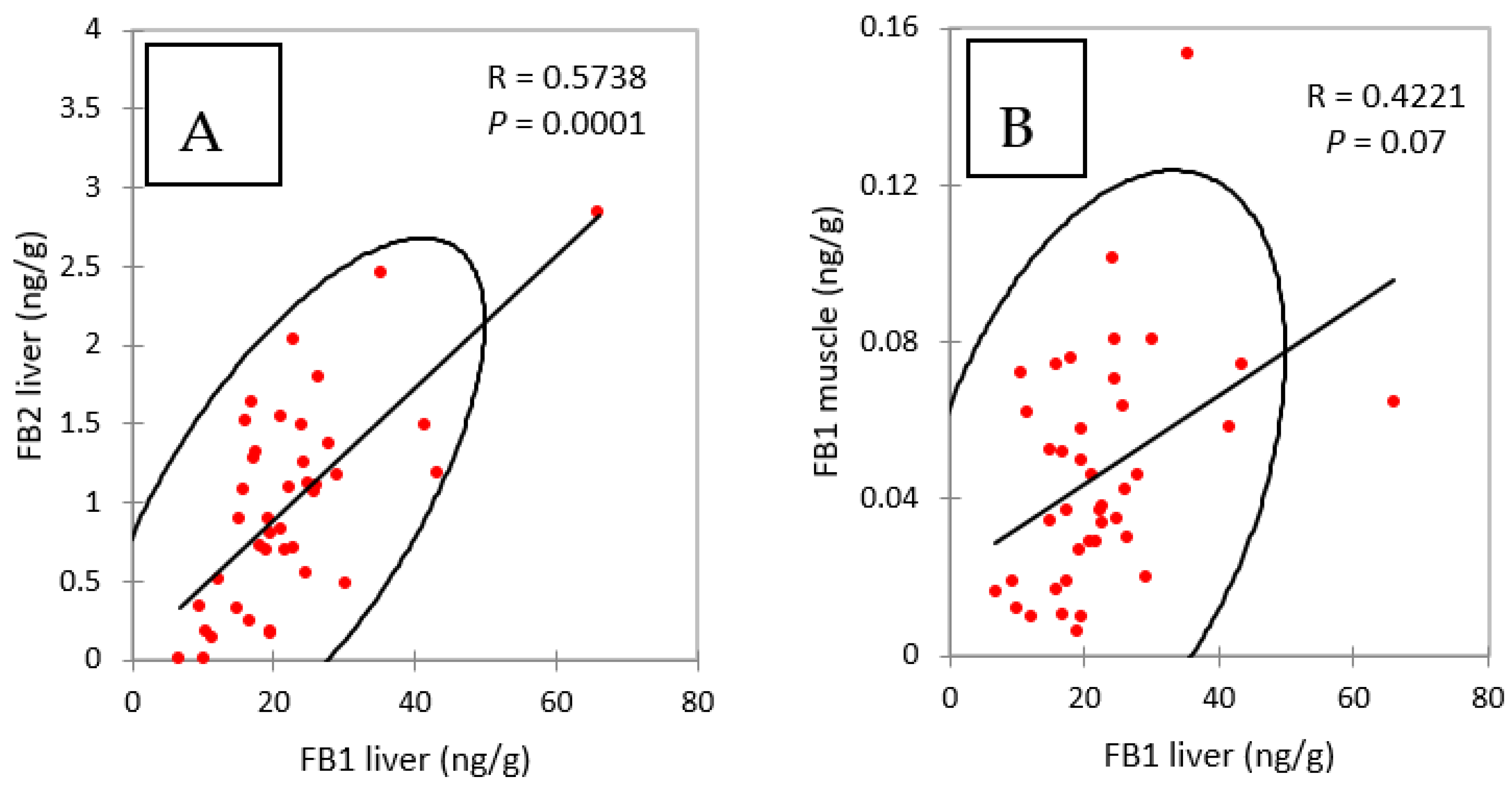
2.4. FB in Liver of Chickens Fed FB Alone
2.5. FB in Muscle of Chickens Fed FB Alone
2.6. FB in Tissues of Chickens Fed FB Plus Algo-Clay
3. Material and Methods
3.1. Chemicals and Reagents
3.2. Experimental Diets and Analysis of Mycotoxins in Feed
3.3. Animal Husbandry and Sample Collection
3.4. Experimental Blinding
3.5. Biochemistry and Sphingosine and Sphinganine in Liver
3.6. Fumonisins in Tissues
3.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FB | Fumonisins B |
| FB1 | Fumonisin B1 |
| FB2 | Fumonisin B2 |
| FB3 | Fumonisin B3 |
| AC | AlgoClay |
| BW | Body Weight |
| FI | Feed Intake |
| FCR | Feed Conversion Ratio |
| Sa | Sphinganine |
| So | Sphingosine |
| OPA | orthophtaldialdehyde |
| IS | Internal Standard |
| IA | Immunoanity |
| RSD | Relative Standard Deviation |
References
- Scott, P.M. Recent Research on Fumonisins: A Review. Food Addit. Contam. Part A 2012, 29, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Guerre, P. Worldwide Mycotoxins Exposure in Pig and Poultry Feed Formulations. Toxins 2016, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; EFSA Panel on Contaminants in the Food Chain (CONTAM); et al. Risks for Animal Health Related to the Presence of Fumonisins, Their Modified Forms and Hidden Forms in Feed. EFSA J. 2018, 16, e05242. [Google Scholar] [CrossRef]
- Voss, K.A.; Smith, G.W.; Haschek, W.M. Fumonisins: Toxicokinetics, Mechanism of Action and Toxicity. Anim. Feed Sci. Technol. 2007, 137, 299–325. [Google Scholar] [CrossRef]
- Diaz, G.J.; Boermans, H.J. Fumonisin Toxicosis in Domestic Animals: A Review. Vet. Hum. Toxicol. 1994, 36, 548–555. [Google Scholar] [PubMed]
- Riley, R.T.; Merrill, A.H. Ceramide Synthase Inhibition by Fumonisins: A Perfect Storm of Perturbed Sphingolipid Metabolism, Signaling, and Disease. J. Lipid Res. 2019, 60, 1183–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, P.C.; Eppley, R.M.; Stack, M.E.; Warbritton, A.; Voss, K.A.; Lorentzen, R.J.; Kovach, R.M.; Bucci, T.J. Fumonisin B1 Carcinogenicity in a Two-Year Feeding Study Using F344 Rats and B6C3F1 Mice. Environ. Health Perspect. 2001, 109 (Suppl. S2), 277–282. [Google Scholar] [CrossRef] [Green Version]
- International Agency for Research on Cancer–IARC. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; IARC: Lyon, France, 2002; 590p. [Google Scholar]
- World Health Organization. Safety Evaluation of Certain Food Additives and Contaminants. WHO Food Additives Series: 63. FAO JECFA MONOGRAPHS 8; WHO Press: Geneva, Switzerland, 2011. [Google Scholar]
- Tardieu, D.; Tran, S.T.; Auvergne, A.; Babilé, R.; Benard, G.; Bailly, J.D.; Guerre, P. Effects of Fumonisins on Liver and Kidney Sphinganine and the Sphinganine to Sphingosine Ratio during Chronic Exposure in Ducks. Chem. Biol. Interact. 2006, 160, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Benlasher, E.; Geng, X.; Nguyen, N.T.X.; Tardieu, D.; Bailly, J.-D.; Auvergne, A.; Guerre, P. Comparative Effects of Fumonisins on Sphingolipid Metabolism and Toxicity in Ducks and Turkeys. Avian Dis. 2012, 56, 120–127. [Google Scholar] [CrossRef]
- Guerre, P. Fusariotoxins in Avian Species: Toxicokinetics, Metabolism and Persistence in Tissues. Toxins 2015, 7, 2289–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardieu, D.; Travel, A.; le Bourhis, C.; Metayer, J.-P.; Mika, A.; Cleva, D.; Boissieu, C.; Guerre, P. Fumonisins and Zearalenone Fed at Low Levels Can Persist Several Days in the Liver of Turkeys and Broiler Chickens after Exposure to the Contaminated Diet Was Stopped. Food Chem. Toxicol. 2021, 148, 111968. [Google Scholar] [CrossRef]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [Green Version]
- Boudergue, C.; Burel, C.; Dragacci, S.; Favrot, M.-C.; Fremy, J.-M.; Massimi, C.; Prigent, P.; Debongnie, P.; Pussemier, L.; Boudra, H. Review of Mycotoxin-Detoxifying Agents Used as Feed Additives: Mode of Action, Efficacy and Feed/Food Safety. EFSA Support. Publ. 2009, 6, 22E. [Google Scholar] [CrossRef]
- Robinson, A.; Johnson, N.M.; Strey, A.; Taylor, J.F.; Marroquin-Cardona, A.; Mitchell, N.J.; Afriyie-Gyawu, E.; Ankrah, N.A.; Williams, J.H.; Wang, J.S.; et al. Calcium Montmorillonite Clay Reduces Urinary Biomarkers of Fumonisin B₁ Exposure in Rats and Humans. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 809–818. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, N.J.; Xue, K.S.; Lin, S.; Marroquin-Cardona, A.; Brown, K.A.; Elmore, S.E.; Tang, L.; Romoser, A.; Gelderblom, W.C.A.; Wang, J.-S.; et al. Calcium Montmorillonite Clay Reduces AFB1 and FB1 Biomarkers in Rats Exposed to Single and Co-Exposures of Aflatoxin and Fumonisin. J. Appl. Toxicol. JAT 2014, 34, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Baglieri, A.; Reyneri, A.; Gennari, M.; Nègre, M. Organically Modified Clays as Binders of Fumonisins in Feedstocks. J. Environ. Sci. Health B 2013, 48, 776–783. [Google Scholar] [CrossRef]
- Liao, Y.-J.; Yang, J.-R.; Chen, S.-E.; Wu, S.-J.; Huang, S.-Y.; Lin, J.-J.; Chen, L.-R.; Tang, P.-C. Inhibition of Fumonisin B1 Cytotoxicity by Nanosilicate Platelets during Mouse Embryo Development. PLoS ONE 2014, 9, e112290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Nekeety, A.A.; El-Kady, A.A.; Abdel-Wahhab, K.G.; Hassan, N.S.; Abdel-Wahhab, M.A. Reduction of Individual or Combined Toxicity of Fumonisin B1 and Zearalenone via Dietary Inclusion of Organo-Modified Nano-Montmorillonite in Rats. Environ. Sci. Pollut. Res. Int. 2017, 24, 20770–20783. [Google Scholar] [CrossRef]
- Yuan, C.-W.; Huang, J.-T.; Chen, C.-C.; Tang, P.-C.; Huang, J.-W.; Lin, J.-J.; Huang, S.-Y.; Chen, S.-E. Evaluation of Efficacy and Toxicity of Exfoliated Silicate Nanoclays as a Feed Additive for Fumonisin Detoxification. J. Agric. Food Chem. 2017, 65, 6564–6571. [Google Scholar] [CrossRef] [PubMed]
- Elliott, C.T.; Connolly, L.; Kolawole, O. Potential Adverse Effects on Animal Health and Performance Caused by the Addition of Mineral Adsorbents to Feeds to Reduce Mycotoxin Exposure. Mycotoxin Res. 2020, 36, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadziakiewicza, M.; Kehoe, S.; Micek, P. Physico-Chemical Properties of Clay Minerals and Their Use as a Health Promoting Feed Additive. Animals 2019, 9, 714. [Google Scholar] [CrossRef] [Green Version]
- Lahaye, M.; Robic, A. Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Holanda, D.M.; Kim, S.W. Mycotoxin Occurrence, Toxicity, and Detoxifying Agents in Pig Production with an Emphasis on Deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef]
- Döll, S.; Dänicke, S. On the Efficacy of Detoxifying Agents in the Prevention of Fusariotoxicoses—A Critical Evaluation of the Situation. Mycotoxin Res. 2003, 19, 185–189. [Google Scholar] [CrossRef]
- Hort, V.; Nicolas, M.; Travel, A.; Jondreville, C.; Maleix, C.; Baéza, E.; Engel, E.; Guérin, T. Carry-over Assessment of Fumonisins and Zearalenone to Poultry Tissues after Exposure of Chickens to a Contaminated Diet—A Study Implementing Stable-Isotope Dilution Assay and UHPLC-MS/MS. Food Control 2020, 107, 106789. [Google Scholar] [CrossRef]
- Murugesan, G.R.; Ledoux, D.R.; Naehrer, K.; Berthiller, F.; Applegate, T.J.; Grenier, B.; Phillips, T.D.; Schatzmayr, G. Prevalence and Effects of Mycotoxins on Poultry Health and Performance, and Recent Development in Mycotoxin Counteracting Strategies. Poult. Sci. 2015, 94, 1298–1315. [Google Scholar] [CrossRef] [PubMed]
- Metayer, J.-P.; Travel, A.; Mika, A.; Bailly, J.-D.; Cleva, D.; Boissieu, C.; Guennec, J.L.; Froment, P.; Albaric, O.; Labrut, S.; et al. Lack of Toxic Interaction Between Fusariotoxins in Broiler Chickens Fed throughout Their Life at the Highest Level Tolerated in the European Union. Toxins 2019, 11, 455. [Google Scholar] [CrossRef] [Green Version]
- Frobose, H.L.; Erceg, J.A.; Fowler, S.Q.; Tokach, M.D.; deRouchey, J.M.; Woodworth, J.C.; Dritz, S.S.; Goodband, R.D. The Progression of Deoxynivalenol-Induced Growth Suppression in Nursery Pigs and the Potential of an Algae-Modified Montmorillonite Clay to Mitigate These Effects. J. Anim. Sci. 2016, 94, 3746–3759. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority. Opinion of the Scientific Panel on Contaminants in Food Chain on a Request from the Commission Related to Fumonisins as Undesirable Substances in Animal Feed. EFSA J. 2005, 235, 1–32. [Google Scholar]
- Travel, A.; Metayer, J.-P.; Mika, A.; Bailly, J.-D.; Cleva, D.; Boissieu, C.; Guennec, J.L.; Albaric, O.; Labrut, S.; Lepivert, G.; et al. Toxicity of Fumonisins, Deoxynivalenol, and Zearalenone Alone and in Combination in Turkeys Fed with the Maximum European Union–Tolerated Level. Avian Dis. 2019, 63, 703–712. [Google Scholar] [CrossRef]
- Tardieu, D.; Travel, A.; Metayer, J.-P.; le Bourhis, C.; Guerre, P. Fumonisin B1, B2 and B3 in Muscle and Liver of Broiler Chickens and Turkey Poults Fed with Diets Containing Fusariotoxins at the EU Maximum Tolerable Level. Toxins 2019, 11, 590. [Google Scholar] [CrossRef] [Green Version]
- Tardieu, D.; Bailly, J.-D.; Skiba, F.; Grosjean, F.; Guerre, P. Toxicokinetics of Fumonisin B1 in Turkey Poults and Tissue Persistence after Exposure to a Diet Containing the Maximum European Tolerance for Fumonisins in Avian Feeds. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 3213–3218. [Google Scholar] [CrossRef] [Green Version]
- De Baere, S.; Croubels, S.; Novak, B.; Bichl, G.; Antonissen, G. Development and Validation of a UPLC-MS/MS and UPLC-HR-MS Method for the Determination of Fumonisin B1 and Its Hydrolysed Metabolites and Fumonisin B2 in Broiler Chicken Plasma. Toxins 2018, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Fodor, J.; Balogh, K.; Weber, M.; Miklós, M.; Kametler, L.; Pósa, R.; Mamet, R.; Bauer, J.; Horn, P.; Kovács, F.; et al. Absorption, Distribution and Elimination of Fumonisin B (1) Metabolites in Weaned Piglets. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Marasas, W.F.; Kellerman, T.S.; Gelderblom, W.C.; Coetzer, J.A.; Thiel, P.G.; van der Lugt, J.J. Leukoencephalomalacia in a Horse Induced by Fumonisin B1 Isolated from Fusarium Moniliforme. Onderstepoort. J. Vet. Res. 1988, 55, 197–203. [Google Scholar]
- Kellerman, T.S.; Marasas, W.F.; Thiel, P.G.; Gelderblom, W.C.; Cawood, M.; Coetzer, J.A. Leukoencephalomalacia in Two Horses Induced by Oral Dosing of Fumonisin B1. Onderstepoort. J. Vet. Res. 1990, 57, 269–275. [Google Scholar]
- ANSES_Guide Validation. Available online: https://www.anses.fr/fr/system/files/ANSES_GuideValidation.pdf (accessed on 19 December 2018).
- Riley, R.T.; Wang, E.; Merrill, A.H.J. Liquid Chromatographic Determination of Sphinganine and Sphingosine: Use of the Free Sphinganine-to-Sphingosine Ratio as a Biomarker for Consumption of Fumonisins. J. AOAC Int. USA 1994, 77, 533–540. [Google Scholar] [CrossRef]
- Tran, S.T.; Bailly, J.D.; Tardieu, D.; Durand, S.; Benard, G.; Guerre, P. Sphinganine to Sphingosine Ratio and Predictive Biochemical Markers of Fumonisin B1 Exposure in Ducks. Chem. Biol. Interact. 2003, 146, 61–72. [Google Scholar] [CrossRef]


| Mycotoxin | Control | AC | FB | FB + AC |
|---|---|---|---|---|
| Fumonisin B1 | 0.04 ± 0.01 | 0.03 ± 0.01 | 15.2 ± 4.3 | 14.0 ± 3.3 |
| Fumonisin B2 | 0.02 ± 0.01 | <0.01 | 5.59 ± 0.56 | 5.57 ± 0.48 |
| Fumonisin B3 | <0.01 | <0.01 | 0.89 ± 0.15 | 0.89 ± 0.14 |
| Variable | Control | AC 4d | AC 9d | FB 4d | FB + AC 4d | FB 9d | FB + AC 9d |
|---|---|---|---|---|---|---|---|
| BW D10 | 274 ± 26 | 278 ± 40 | 280 ± 28 | 272 ± 35 | 276 ± 38 | 274 ± 31 | 278 ± 27 |
| BW D17 | 661 ± 59 | 703 ± 109 | 696 ± 67 | 738 ± 98 | 648 ± 115 | 673 ± 90 | 688 ± 81 |
| BW D21 | 994 ± 117 | 985 ± 141 | 1050 ± 115 | 1091 ± 132 | 943 ± 180 | 1031 ± 140 | 1016 ± 114 |
| FI D12 | 2407 ± 8 | 2562 ± 107 | 2509 ± 56 | 2611 ± 138 | 2556 ± 60 | 2582 ± 26 | 2627 ± 111 |
| FI D13-D16 | 1615 ± 29 | 1744 ± 57 | 1772 ± 4 | 1794 ± 33 | 1717 ± 158 | 1702 ± 108 | 1798 ± 46 |
| FI D17-D21 | 2435 ± 470 | 2000 ± 16 | 2574 ± 409 | 2472 ± 195 | 2219 ± 269 | 2563 ± 652 | 2536 ± 670 |
| FCR | 1.3 | 1.28 | 1.31 | 1.26 | 1.38 | 1.33 | 1.37 |
| Liver (%) | 2.09 ± 0.169 | 2.059 ± 0.203 | 2.045 ± 0.186 | 2.148 ± 0.236 | 2.22 ± 0.254 | 2.108 ± 0.325 | 2.321 ± 0.323 |
| Gizzard (%) | 2.384 ± 0.459 | 2.383 ± 0.203 | 2.328 ± 0.491 | 2.178 ± 0.384 | 2.57 ± 0.552 | 2.392 ± 0.446 | 2.631 ± 0.642 |
| Heart (%) | 0.569 ± 0.054 | 0.573 ± 0.059 | 0.577 ± 0.069 | 0.57 ± 0.061 | 0.564 ± 0.068 | 0.595 ± 0.119 | 0.608 ± 0.064 |
| Variable 1 | Control | AC 4d | AC 9d | FB 4d | FB + AC 4d | FB 9d | FB + AC 9d |
|---|---|---|---|---|---|---|---|
| Uric Acid 2 | 628 ± 263 | 590 ± 189 | 600 ± 173 | 393 ± 167 | 515 ± 267 | 551 ± 207 | 433 ± 149 |
| Cholesterol 3 | 3.65 ± 0.62 | 3.5 ± 0.53 | 3.9 ± 0.58 | 3.85 ± 0.54 | 3.95 ± 1.79 | 3.81 ± 0.72 | 3.86 ± 0.34 |
| Proteins 4 | 24.6 ± 1.9 | 25.2 ± 2.3 | 26.4 ± 2.1 | 26.7 ± 2.1 | 23.3 ± 6 | 27.8 ± 2.8 | 26.6 ± 2.2 |
| Albumin 4 | 12.1 ± 0.8 | 12.6 ± 2.5 | 12.6 ± 0.8 | 12.6 ± 0.6 | 11.6 ± 2.1 | 13.3 ± 1.1 | 12.9 ± 0.9 |
| Globulins 4 | 12.7 ± 1.3 | 12.6 ± 1.7 | 14.3 ± 2.7 | 14.2 ± 1.7 | 13 ± 1.2 | 14.4 ± 1.8 | 13.6 ± 1.8 |
| AST 5 | 207 ± 26 | 252 ± 89 | 207 ± 32 | 226 ± 60 | 203 ± 77 | 225 ± 31 | 195 ± 32 |
| ALT 5 | 8.2 ± 2.2 | 8.7 ± 1.95 | 9.38 ± 2.33 | 7.4 ± 1.58 | 10.7 ± 8.91 | 7.89 ± 2.15 | 7.3 ± 2.36 |
| LDH 5 | 1512 ± 487 | 1865 ± 720 | 1448 ± 389 | 1609 ± 532 | 1453 ± 729 | 1388 ± 326 | 1317 ± 355 |
| CPK 5 | 6169 ± 2629 | 5424 ± 2829 | 6363 ± 311 | 7554 ± 2424 | 3967 ± 3171 | 5866 ± 2728 | 6061 ± 2669 |
| PAL 5 | 4527 ± 2285 | 5839 ± 1512 | 5128 ± 1809 | 5498 ± 2271 | 3586 ± 2031 | 4240 ± 1635 | 5778 ± 2979 |
| Sa 6 | 0.66 ± 0.3 DE | 0.37 ± 0.18 E | 0.5 ± 0.18 DE | 0.93 ± 0.23 CD | 1.15 ± 0.45 C | 2.48 ± 0.96 A | 1.88 ± 0.71 B |
| So 6 | 7.21 ± 3.08 BC | 3.74 ± 1.16 D | 8.11 ± 2.4 B | 6.63 ± 2.19 BC | 5.55 ± 1.51 CD | 11.86 ± 4.1 A | 8.14 ± 2.04 B |
| Sa/So | 0.1 ± 0.03 BC | 0.11 ± 0.06 BC | 0.07 ± 0.03 C | 0.15 ± 0.04 B | 0.21 ± 0.06 A | 0.24 ± 0.09 A | 0.23 ± 0.09 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurain, J.; Tardieu, D.; Matard-Mann, M.; Rodriguez, M.A.; Guerre, P. Fumonisin B1 Accumulates in Chicken Tissues over Time and This Accumulation Was Reduced by Feeding Algo-Clay. Toxins 2021, 13, 701. https://doi.org/10.3390/toxins13100701
Laurain J, Tardieu D, Matard-Mann M, Rodriguez MA, Guerre P. Fumonisin B1 Accumulates in Chicken Tissues over Time and This Accumulation Was Reduced by Feeding Algo-Clay. Toxins. 2021; 13(10):701. https://doi.org/10.3390/toxins13100701
Chicago/Turabian StyleLaurain, Julia, Didier Tardieu, Maria Matard-Mann, Maria Angeles Rodriguez, and Philippe Guerre. 2021. "Fumonisin B1 Accumulates in Chicken Tissues over Time and This Accumulation Was Reduced by Feeding Algo-Clay" Toxins 13, no. 10: 701. https://doi.org/10.3390/toxins13100701
APA StyleLaurain, J., Tardieu, D., Matard-Mann, M., Rodriguez, M. A., & Guerre, P. (2021). Fumonisin B1 Accumulates in Chicken Tissues over Time and This Accumulation Was Reduced by Feeding Algo-Clay. Toxins, 13(10), 701. https://doi.org/10.3390/toxins13100701





