Overall Exposure of European Adult Population to Mycotoxins by Statistically Modelled Biomonitoring Data
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dataset
2.2. PDI Calculation
2.2.1. European Scenario
2.2.2. North/South Europe Scenarios
2.3. Moving Forward
3. Conclusions
4. Materials and Methods
4.1. Dataset
4.2. Data Clustering
4.3. Statistical Analysis
4.4. PDI Calculation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raspor, P. Total food chain safety: How good practices can contribute? Trends Food Sci. Technol. 2008, 19, 405–412. [Google Scholar] [CrossRef]
- EFSA, Scientific Committee. Statement on the applicability of the Margin of Exposure approach for the safety assessment of impurities which are both genotoxic and carcinogenic in substances added to food/feed. EFSA J. 2012, 10, 2578. [Google Scholar] [CrossRef]
- More, S.J.; Bampidis, V.; Benford, D.; Bennekou, S.H.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Koutsoumanis, K.P.; Naegeli, H.; EFSA, Scientific Committee; et al. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J. 2019, 17, e05634. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, D.; Royce, S.G.; Alexander, J.A.; Buckley, B.; Isukapalli, S.S.; Bandera, E.; Zarbl, H.; Georgopoulos, P.G. Physiologically-Based Toxicokinetic Modeling of Zearalenone and Its Metabolites: Application to the Jersey Girl Study. PLoS ONE 2014, 9, e113632. [Google Scholar] [CrossRef] [Green Version]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2019, 60, 2773–2789. [Google Scholar] [CrossRef]
- Leggieri, M.C.; Lanubile, A.; Dall’Asta, C.; Pietri, A.; Battilani, P. The impact of seasonal weather variation on mycotoxins: Maize crop in 2014 in northern Italy as a case study. World Mycotoxin J. 2020, 13, 25–36. [Google Scholar] [CrossRef]
- Turner, P.; Snyder, J. Development and Limitations of Exposure Biomarkers to Dietary Contaminants Mycotoxins. Toxins 2021, 13, 314. [Google Scholar] [CrossRef]
- Arce-López, B.; Lizarraga, E.; Vettorazzi, A.; González-Peñas, E. Human Biomonitoring of Mycotoxins in Blood, Plasma and Serum in Recent Years: A Review. Toxins 2020, 12, 147. [Google Scholar] [CrossRef] [Green Version]
- Battilani, P.; Palumbo, R.; Giorni, P.; Dall’Asta, C.; Dellafiora, L.; Gkrillas, A.; Toscano, P.; Crisci, A.; Brera, C.; De Santis, B.; et al. Mycotoxin mixtures in food and feed: Holistic, innovative, flexible risk assessment modelling approach. EFSA Support. Publ. 2020, 17, 1757E. [Google Scholar] [CrossRef] [Green Version]
- Brera, C.; De Santis, B.; Debegnach, F.; Miano, B.; Moretti, G.; Lanzone, A.; Del Sordo, G.; Buonsenso, D.; Chiaretti, A.; Hardie, L.; et al. Experimental study of deoxynivalenol biomarkers in urine. EFSA Support. Publ. 2015, 12, 818E. [Google Scholar] [CrossRef] [Green Version]
- Birolini, A. Reliability Engineering: Theory and Practice; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Menčík, J. Weibull Distribution, Concise Reliability for Engineers; Intech Open: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority. Available online: https://www.efsa.europa.eu/en/microstrategy/foodex2-level-7 (accessed on 21 April 2021).
- EFSA, Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Grasl-Kraupp, B.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar] [CrossRef]
- EFSA, Panel on Contaminants in the Food Chain (CONTAM). Appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14, e04425. [Google Scholar] [CrossRef]
- EFSA, Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J. 2014, 12, 2481. [Google Scholar] [CrossRef] [Green Version]
- EFSA, Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018, 16, e05242. [Google Scholar] [CrossRef]
- EFSA, Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Appropriateness to set a group health based guidance value for T2 and HT2 toxin and its modified forms. EFSA J. 2017, 15, e04655. [Google Scholar] [CrossRef]
- EFSA, Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Appropriateness to set a group health based guidance value for nivalenol and its modified forms. EFSA J. 2017, 15, e04751. [Google Scholar] [CrossRef] [Green Version]
- EFSA, Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; et al. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, e06040. [Google Scholar] [CrossRef]
- EFSA, Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; et al. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, e06113. [Google Scholar] [CrossRef]
- EFSA, Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J. 2012, 10, 2605. [Google Scholar] [CrossRef]
- Turner, P.C.; Hopton, R.P.; Lecluse, Y.; White, K.L.M.; Fisher, J.; LeBailly, P. Determinants of Urinary Deoxynivalenol and De-epoxy Deoxynivalenol in Male Farmers from Normandy, France. J. Agric. Food Chem. 2010, 58, 5206–5212. [Google Scholar] [CrossRef]
- Vidal, A.; Claeys, L.; Mengelers, M.; Vanhoorne, V.; Vervaet, C.; Huybrechts, B.; De Saeger, S.; De Boevre, M. Humans significantly metabolize and excrete the mycotoxin deoxynivalenol and its modified form deoxynivalenol-3-glucoside within 24 hours. Sci. Rep. 2018, 8, 5255. [Google Scholar] [CrossRef] [Green Version]
- Miraglia, M.; Brera, C. Task 3.2.7, Assessment of Dietary Intake of Ochratoxin a by the Population of EU Member State; European Comission: Luxembourg, 2002. [Google Scholar]
- Pasquali, M.; Beyer, M.; Logrieco, A.; Audenaert, K.; Balmas, V.; Basler, R.; Boutigny, A.-L.; Chrpová, J.; Czembor, E.; Gagkaeva, T.; et al. A European Database of Fusarium graminearum and F. culmorum Trichothecene Genotypes. Front. Microbiol. 2016, 7, 406. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Degen, G.H. Urinary biomarkers of exposure to the mycoestrogen zearalenone and its modified forms in German adults. Arch. Toxicol. 2018, 92, 2691–2700. [Google Scholar] [CrossRef]
- HBM4EU. Available online: https://www.hbm4eu.eu/the-project/ (accessed on 15 May 2021).
- Apel, P.; Angerer, J.; Wilhelm, M.; Kolossa-Gehring, M. New HBM values for emerging substances, inventory of reference and HBM values in force, and working principles of the German Human Biomonitoring Commission. Int. J. Hyg. Environ. Health 2017, 220, 152–166. [Google Scholar] [CrossRef] [Green Version]
- Warth, B.; Sulyok, M.; Berthiller, F.; Schuhmacher, R.; Krska, R. New insights into the human metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone. Toxicol. Lett. 2013, 220, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Goodrum, P.E.; Anderson, J.K.; Luz, A.L.; Ansell, G.K. Application of a Framework for Grouping and Mixtures Toxicity Assessment of PFAS: A Closer Examination of Dose-Additivity Approaches. Toxicol. Sci. 2020, 179, 262–278. [Google Scholar] [CrossRef]
- Christensen, K.L.; Makris, S.L.; Lorber, M. Generation of hazard indices for cumulative exposure to phthalates for use in cumulative risk assessment. Regul. Toxicol. Pharmacol. 2014, 69, 380–389. [Google Scholar] [CrossRef]
- Katsikantami, I.; Colosio, C.; Alegakis, A.; Tzatzarakis, M.N.; Vakonaki, E.; Rizos, A.K.; Sarigiannis, D.; Tsatsakis, A.M. Estimation of daily intake and risk assessment of organophosphorus pesticides based on biomonitoring data–The internal exposure approach. Food Chem. Toxicol. 2018, 123, 57–71. [Google Scholar] [CrossRef]
- Mahdavi, R.; Nikniaz, L.; Arefhosseini, S.R.; Jabbari, M.V. Determination of Aflatoxin M1 in Breast Milk Samples in Tabriz–Iran. Matern. Child Health J. 2008, 14, 141–145. [Google Scholar] [CrossRef]
- Gambacorta, L.; Olsen, M.; Solfrizzo, M. Pig Urinary Concentration of Mycotoxins and Metabolites Reflects Regional Differences, Mycotoxin Intake and Feed Contaminations. Toxins 2019, 11, 378. [Google Scholar] [CrossRef] [Green Version]
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Leggieri, M.C.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, R.; Crisci, A.; Venâncio, A.; Abrahantes, J.C.; Dorne, J.-L.; Battilani, P.; Toscano, P. Occurrence and Co-Occurrence of Mycotoxins in Cereal-Based Feed and Food. Microorganisms 2020, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Davillas, A.; Jones, A.M. Parametric models for biomarkers based on flexible size distributions. Health Econ. 2018, 27, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Delignette-Muller, M.L.; Dutang, C. fitdistrplus: An R Package for Fitting Distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Novack-Gottshall, P.; Wang, S.C. Lilliefors-Corrected Kolmogorov-Smirnov Goodness-of-Fit Tests. KS Correct-Package. R Package Version 1.4.0. 2019. Available online: https://CRAN.R-project.org/package=KScorrect (accessed on 21 September 2021).
- EFSA, Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for water. EFSA J. 2010, 8, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.Q.; Zhang, L.S.; Hu, X.; Xiao, Y.; Chen, J.S.; Xu, Y.C.; Fremy, J.; Chu, F.S. Correlation of dietary aflatoxin B1 levels with excretion of aflatoxin M1 in human urine. Cancer Res. 1987, 47, 1848–1852. [Google Scholar]
- Studer-Rohr, I.; Schlatter, J.; Dietrich, D.R. Kinetic parameters and intraindividual fluctuations of ochratoxin A plasma levels in humans. Arch. Toxicol. 2000, 74, 499–510. [Google Scholar] [CrossRef]
- Degen, G.H. Are we ready to estimate daily ochratoxin A intake based on urinary concentrations? Environ. Int. 2016, 97, 254–255. [Google Scholar] [CrossRef]
- Degen, G.H.; Ali, N.; Gundert-Remy, U. Preliminary data on citrinin kinetics in humans and their use to estimate citrinin exposure based on biomarkers. Toxicol. Lett. 2018, 282, 43–48. [Google Scholar] [CrossRef]
- van der Westhuizen, L.; Shephard, G.S.; Burger, H.M.; Rheeder, J.P.; Gelderblom, W.C.; Wild, C.P.; Gong, Y.Y. Fumonisin B1 as a Urinary Biomarker of Exposure in a Maize Intervention Study Among South African Subsistence Farmers. Cancer Epidemiol. Biomark. Prev. 2011, 20, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Riley, R.T.; Torres, O.; Showker, J.L.; Zitomer, N.; Matute, J.; Voss, K.A.; Waes, J.G.-V.; Maddox, J.R.; Gregory, S.; Ashley-Koch, A.E. The kinetics of urinary fumonisin B1 excretion in humans consuming maize-based diets. Mol. Nutr. Food Res. 2012, 56, 1445–1455. [Google Scholar] [CrossRef] [Green Version]
- Turner, P.C. Deoxynivalenol and nivalenol occurrence and exposure assessment. World Mycotoxin J. 2010, 3, 315–321. [Google Scholar] [CrossRef]
- Gambacorta, S.; Solfrizzo, H.; Visconti, A.; Powers, S.; Cossalter, A.; Pinton, P.; Oswald, I. Validation study on urinary biomarkers of exposure for aflatoxin B1, ochratoxin A, fumonisin B1, deoxynivalenol and zearalenone in piglets. World Mycotoxin J. 2013, 6, 299–308. [Google Scholar] [CrossRef]

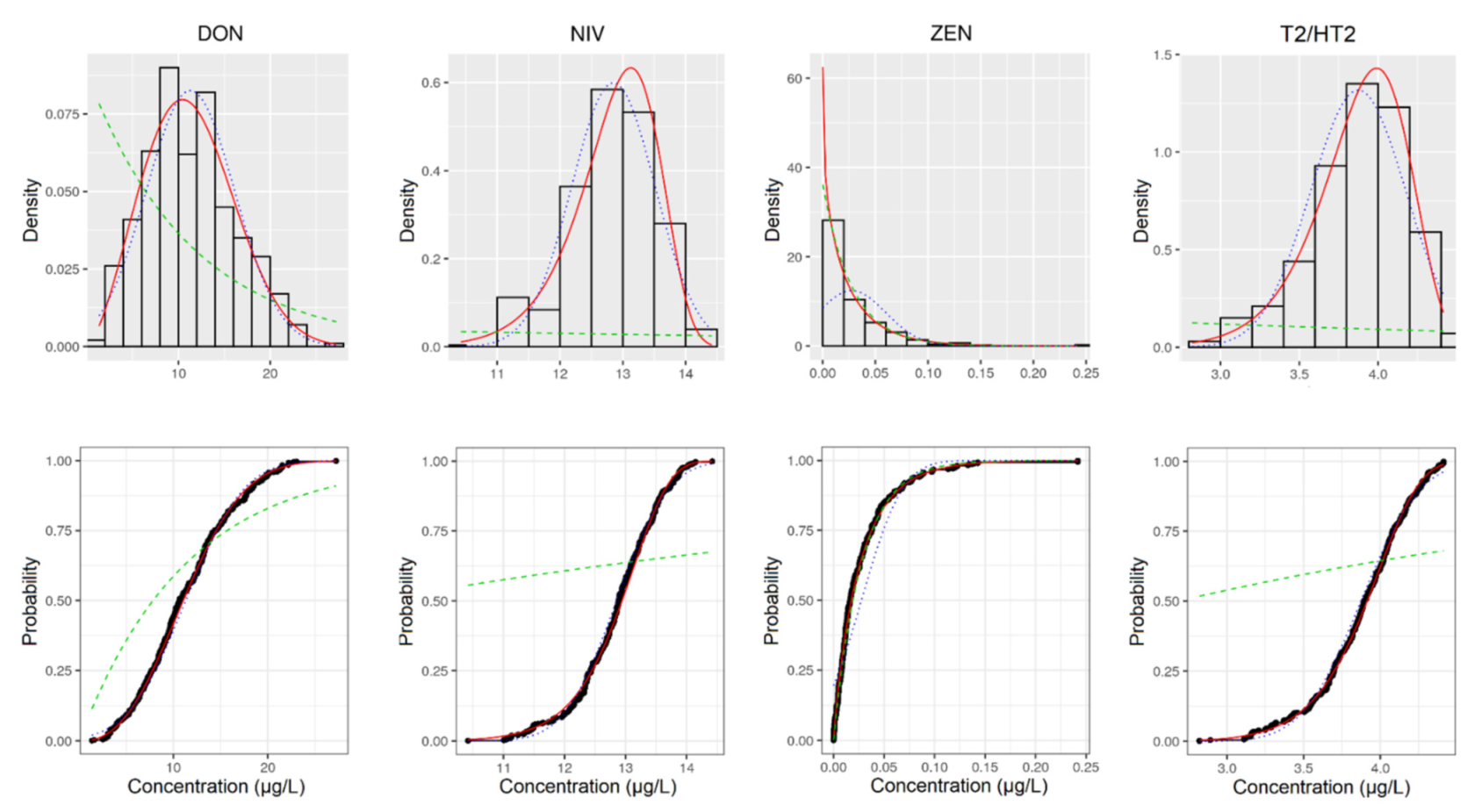
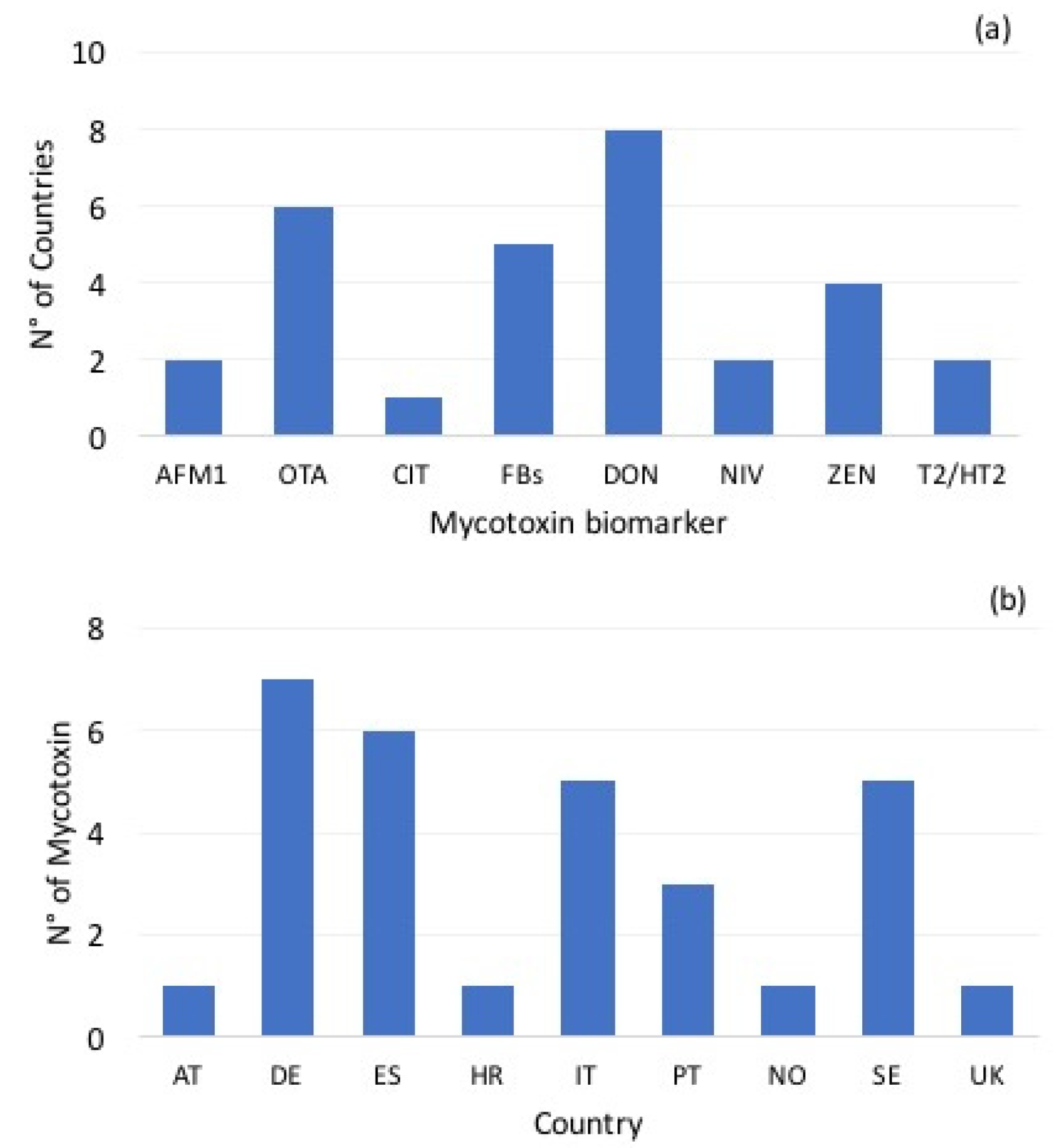
| Toxin | Parameter | AT | DE | ES | HR | IT | NO | PT | SE | UK |
|---|---|---|---|---|---|---|---|---|---|---|
| AFM1 | NR/NS | 1/50 | 4/169 | |||||||
| Mean/SD | 0.01/- | 0.04/0.02 | ||||||||
| OTA | NR/NS | 7/104 | 6/242 | 1/40 | 1/52 | 20/1150 | 1/252 | |||
| Mean/SD | 0.63/1.04 | 0.40/0.28 | 0.93/1.13 | 0.06/0.31 | 0.02/0.004 | 0.46/0.57 | ||||
| CIT | NR/NS | 3/30 | ||||||||
| Mean/SD | 0.172/0.027 | |||||||||
| FBs | NR/NS | 1/50 | 1/27 | 3/157 | 1/68 | 1/252 | ||||
| Mean/SD | 0.005/- | 2.25/0.35 | 0.06/0.03 | 2.5/0.0 | 0.007/0.004 | |||||
| DON | NR/NS | 1/27 | 8/281 | 6/87 | 126/403 | 149/298 | 2/11 | 5/1155 | 129/516 | |
| Mean/SD | 0/2.4 | 3.51/3.48 | 8.45/12.3 | 5.62/5.79 | 6.66/5.04 | 10.8/7.78 | 2.77/1.43 | 21.09/27.47 | ||
| NIV | NR/NS | 5/60 | 1/252 | |||||||
| Mean/SD | 15.44/1.83 | 0.02/0.74 | ||||||||
| ZEN | NR/NS | 4/80 | 6/87 | 7/356 | 1/252 | |||||
| Mean/SD | 0.02/0.013 | 1.03/0.44 | 0.05/0.03 | 0.03/0.006 | ||||||
| T2/HT2 | NR/NS | 1/101 | 6/87 | |||||||
| Mean/SD | 0.04/- | 3.95/6.21 |
| Mycotoxin | PDI_Mean (μg/kg bw/Day) | PDI_LB (μg/kg bw/Day) | PDI_UB (μg/kg bw/Day) | TDI (μg/kg bw/Day) |
|---|---|---|---|---|
| OTA 1 | 0.2878 | 0.0988 | 0.3494 | 4.73 3 |
| (0.2772–0.2984) | (0.0964–0.1012) | (0.3436–0.3550) | ||
| FBs 2 | 8.99929 | 0.17597 | 19.65327 | 1 |
| (8.98985–9.00872) | (0.17036–0.18158) | (19.21803–20.08852) | ||
| DON 1 | 0.38846 | 0.23349 | 0.41134 | 1 |
| (0.38507–0.39185) | (0.22973–0.23726) | (0.40335–0.41933) | ||
| ZEN 2 | 0.03483 | 0.00143 | 0.06532 | 0.25 |
| (0.03479–0.03487) | (0.00139–0.00147) | (0.06256–0.06807) | ||
| T2/HT2 1 | 0.16885 | 0.15962 | 0.20799 | 0.02 |
| (0.16859–0.16911) | (0.15401–0.16524) | (0.20258–0.21341) | ||
| AFB1 1 | 0.06385 | 0.4 3 | ||
| (0.06101–0.06668) | ||||
| CIT 1 | 0.009484 | 0.2 4 | ||
| (0.009172–0.009796) | ||||
| NIV 1 | 35.79271 | 1.2 | ||
| (35.75385–35.83157) |
| Mycotoxin | PDI_Mean (μg/kg bw/Day) | PDI_LB (μg/kg bw/Day) | PDI_UB (μg/kg bw/Day) | Area |
|---|---|---|---|---|
| OTA 1,2 | 0.6764 | 0.6764 | 0.6764 | North Europe |
| (0.6662–0.6866) | (0.6662–0.6866) | (0.6662–0.6866) | ||
| DON 1 | 0.48282 | 0.27699 | 0.53178 | North Europe |
| (0.4796–0.48603) | (0.27162–0.28236) | (0.51759–0.54597) | ||
| ZEN 1 | 0.00149 | 0.00145 | 0.00159 | North Europe |
| (0.00146–0.00152) | (0.00143–0.00148) | (0.00156–0.00162) | ||
| OTA 1 | 0.1874 | 0.1746 | 0.2024 | South Europe |
| (0.1844–0.1906) | (0.1688–0.1806) | (0.1954–0.2092) | ||
| DON 1 | 0.24309 | 0.19531 | 0.25879 | South Europe |
| (0.23905–0.24712) | (0.19156–0.19907) | (0.25378–0.26380) | ||
| ZEN 1 | 0.05553 | 0.05552 | 0.10588 | South Europe |
| (0.05551–0.05556) | (0.05550–0.05555) | (0.10380–0.10795) |
| Mycotoxin | Scenario | Concentration (Mean; μg/L) | Lower Confidence Interval (Mean; μg/L) | Upper Confidence Level (Mean; μg/L) | LB Concentration (μg/L) | UB Concentration (μg/L) |
|---|---|---|---|---|---|---|
| AFM1 | European | 0.03871 | 0.03692 | 0.04050 | NA | NA |
| OTA | European | 0.26559 | 0.25601 | 0.27517 | 0.08794 | 0.31446 |
| CIT | European | 0.13687 | 0.13237 | 0.13978 | NA | NA |
| FBs | European | 0.96978 | 0.96876 | 0.97080 | 0.01849 | 2.09468 |
| DON | European | 10.07178 | 9.98468 | 10.15888 | 5.94282 | 10.34675 |
| NIV | European | 12.83527 | 12.82103 | 12.84950 | NA | NA |
| ZEN | European | 0.46020 | 0.45964 | 0.46077 | 0.01905 | 0.88318 |
| T2/HT2 | European | 3.63374 | 3.62790 | 3.639577 | 3.56309 | 4.41715 |
| OTA | North Europe | 0.61299 | 0.60379 | 0.62219 | 0.61299 | 0.61299 |
| DON | North Europe | 12.67093 | 12.58648 | 12.75538 | 7.39707 | 13.94056 |
| ZEN | North Europe | 0.01958 | 0.01920 | 0.01996 | 0.01821 | 0.02145 |
| OTA | South Europe | 0.16489 | 0.16204 | 0.16773 | 0.15423 | 0.173492 |
| DON | South Europe | 6.12992 | 6.02877 | 6.23107 | 4.92539 | 6.54026 |
| ZEN | South Europe | 0.71473 | 0.71445 | 0.71502 | 0.01749 | 1.36606 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Santis, B.; Debegnach, F.; Toscano, P.; Crisci, A.; Battilani, P.; Brera, C. Overall Exposure of European Adult Population to Mycotoxins by Statistically Modelled Biomonitoring Data. Toxins 2021, 13, 695. https://doi.org/10.3390/toxins13100695
De Santis B, Debegnach F, Toscano P, Crisci A, Battilani P, Brera C. Overall Exposure of European Adult Population to Mycotoxins by Statistically Modelled Biomonitoring Data. Toxins. 2021; 13(10):695. https://doi.org/10.3390/toxins13100695
Chicago/Turabian StyleDe Santis, Barbara, Francesca Debegnach, Piero Toscano, Alfonso Crisci, Paola Battilani, and Carlo Brera. 2021. "Overall Exposure of European Adult Population to Mycotoxins by Statistically Modelled Biomonitoring Data" Toxins 13, no. 10: 695. https://doi.org/10.3390/toxins13100695
APA StyleDe Santis, B., Debegnach, F., Toscano, P., Crisci, A., Battilani, P., & Brera, C. (2021). Overall Exposure of European Adult Population to Mycotoxins by Statistically Modelled Biomonitoring Data. Toxins, 13(10), 695. https://doi.org/10.3390/toxins13100695








