Advances in Colorimetric Strategies for Mycotoxins Detection: Toward Rapid Industrial Monitoring
Abstract
:1. Introduction
2. Common Colorimetric Probes
2.1. Enzymes-Based Probes
2.2. Nanomaterial-Based Probes
3. Colorimetric Strategies for Mycotoxins Detection
3.1. Solution-Based Assays
3.2. Enzyme-Linked Immunosorbent Assay (ELISA)
3.3. Lateral Flow Assays
3.4. Microfluidics-Based Assays
4. Recent Advances toward Practical Applications
4.1. Mycotoxin Extraction Methods
4.2. Inventory of Commercially Available Kits for Mycotoxins Detection
4.3. Interesting Examples from Literature with Great Potential for Industrial Applications
5. Conclusions and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuiper-Goodman, T. Risk assessment and risk management of mycotoxins in food. In Mycotoxins in Food; Magan, N., Olsen, E.M., Eds.; Woodhead Publishing: Cambridge, UK, 2004; pp. 3–31. ISBN 978-1-85573-733-4. [Google Scholar]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—the IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Edite Bezerra da Rocha, M.; Freire, F.D.C.O.; Erlan Feitosa Maia, F.; Izabel Florindo Guedes, M.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food: Perspectives in a global and European context. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovina, K.; Nurul Shaeera, S.; Merrylin Vonnie, J.; Xin Yi, S. Recent Biosensors Technologies for Detection of Mycotoxin in Food Products. In Mycotoxins and Food Safety; IntechOpen: London, UK, 2020. [Google Scholar]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Nolan, P.; Auer, S.; Spehar, A.; Elliott, C.T.; Campbell, K. Current trends in rapid tests for mycotoxins. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2019, 36, 800–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Zheng, H.; Sun, M.; Guo, Y.; Luo, F.; Guo, L.; Qiu, B.; Lin, Z.; Chen, G. Highly sensitive colorimetric aptasensor for ochratoxin A detection based on enzyme-encapsulated liposome. Anal. Chim. Acta 2018, 1002, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, R.I.; Osalaye, D.S.; Ralebitso-Senior, T.K.; Boddis, A.; Reid, A.J.; Fatokun, A.A.; Powell, A.K.; Malomo, S.O.; Olorunniji, F.J. Catalytic activities of multimeric G-quadruplex dnazymes. Catalysts 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, Y.; Liu, Z.; Lin, B.; Yi, H.; Xu, F.; Nie, Z.; Yao, S. Insight into G-quadruplex-hemin DNAzyme/RNAzyme: Adjacent adenine as the intramolecular species for remarkable enhancement of enzymatic activity. Nucleic Acids Res. 2016, 44, 7373–7384. [Google Scholar] [CrossRef] [PubMed]
- Kosman, J.; Zukowski, K.; Juskowiak, B. Comparison of Characteristics and DNAzyme Activity of G4–Hemin Conjugates Obtained via Two Hemin Attachment Methods. Molecules 2018, 23, 1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zeng, L.; Li, N.; Liu, C.; Chen, J. A wash-free and label-free colorimetric biosensor for naked-eye detection of aflatoxin B1 using G-quadruplex as the signal reporter. Food Chem. 2019, 298, 125034. [Google Scholar] [CrossRef] [PubMed]
- Santovito, E.; Greco, D.; D’Ascanio, V.; Sanzani, S.M.; Avantaggiato, G. Development of a DNA-based biosensor for the fast and sensitive detection of ochratoxin A in urine. Anal. Chim. Acta 2020, 1133, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Setlem, K.; Mondal, B.; Shylaja, R.; Parida, M. Dual Aptamer-DNAzyme based colorimetric assay for the detection of AFB1 from food and environmental samples. Anal. Biochem. 2020, 608, 113874. [Google Scholar] [CrossRef]
- Chrouda, A.; Zinoubi, K.; Soltane, R.; Alzahrani, N.; Osman, G.; Al-Ghamdi, Y.O.; Qari, S.; Al mahri, A.; Algethami, F.K.; Majdoub, H.; et al. An acetylcholinesterase inhibition-based biosensor for aflatoxin B1 detection using sodium alginate as an immobilization matrix. Toxins 2020, 12, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirón-Mérida, V.A.; Wu, M.; Gong, Y.Y.; Guo, Y.; Holmes, M.; Ettelaie, R.; Goycoolea, F.M. Genipin cross-linked chitosan for signal enhancement in the colorimetric detection of aflatoxin B1 on 3MM chromatography paper. Sens. Bio-Sens. Res. 2020, 29, 100339. [Google Scholar] [CrossRef]
- Bazin, I.; Tria, S.A.; Hayat, A.; Marty, J.L. New biorecognition molecules in biosensors for the detection of toxins. Biosens. Bioelectron. 2017, 87, 285–298. [Google Scholar] [CrossRef]
- Seok, Y.; Byun, J.Y.; Shim, W.B.; Kim, M.G. A structure-switchable aptasensor for aflatoxin B1 detection based on assembly of an aptamer/split DNAzyme. Anal. Chim. Acta 2015, 886, 182–187. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Sun, L.; Liu, L.; Xu, C.; Kuang, H. Nanoparticle-based sensors for food contaminants. TrAC Trends Anal. Chem. 2019, 113, 74–83. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Liu, B.; Cui, X.; Li, Y.; Tang, J.; Wang, H.; Zhang, D.; Li, Z. Recent advances in aptasensors for mycotoxin detection: On the surface and in the colloid. Talanta 2021, 223, 121729. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G.; Hianik, T. Aptamer-based biosensors for mycotoxin detection. In Nanomycotoxicology: Treating Mycotoxins in the Nano Way; Rai, M., Abd-Elsalam, K.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 35–70. ISBN 9780128179987. [Google Scholar]
- Luan, Y.; Chen, Z.; Xie, G.; Chen, J.; Lu, A.; Li, C.; Fu, H.; Ma, Z.; Wang, J. Rapid Visual Detection of Aflatoxin B1 by Label-Free Aptasensor Using Unmodified Gold Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Phanchai, W.; Srikulwong, U.; Chompoosor, A.; Sakonsinsiri, C.; Puangmali, T. Insight into the Molecular Mechanisms of AuNP-Based Aptasensor for Colorimetric Detection: A Molecular Dynamics Approach. Langmuir 2018, 34, 6161–6169. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Upadhyay, N.; Nara, S. Recent advancements in lateral flow immunoassays: A journey for toxin detection in food. Crit. Rev. Food Sci. Nutr. 2018, 58, 1715–1734. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, Q.; Wang, J.; Liang, Z.; Li, J.; Wu, J.; Shen, X.; Lei, H.; Li, X. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens. Bioelectron. 2020, 158, 112178. [Google Scholar] [CrossRef]
- Yu, L.; Li, P.; Ding, X.; Zhang, Q. Graphene oxide and carboxylated graphene oxide: Viable two-dimensional nanolabels for lateral flow immunoassays. Talanta 2017, 165, 167–175. [Google Scholar] [CrossRef]
- Tian, M.; Xie, W.; Zhang, T.; Liu, Y.; Lu, Z.; Li, C.M.; Liu, Y. A sensitive lateral flow immunochromatographic strip with prussian blue nanoparticles mediated signal generation and cascade amplification. Sens. Actuators B Chem. 2020, 309, 127728. [Google Scholar] [CrossRef]
- Kong, D.; Liu, L.; Song, S.; Suryoprabowo, S.; Li, A.; Kuang, H.; Wang, L.; Xu, C. A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nanoscale 2016, 8, 5245–5253. [Google Scholar] [CrossRef]
- Tian, F.; Zhou, J.; Jiao, B.; He, Y. A nanozyme-based cascade colorimetric aptasensor for amplified detection of ochratoxin A. Nanoscale 2019, 11, 9547–9555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, D.; Zhou, X.; Yu, Y.; Liu, J.; Hu, N.; Wang, H.; Li, G.; Wu, Y. Recent progress in the construction of nanozyme-based biosensors and their applications to food safety assay. TrAC Trends Anal. Chem. 2019, 121, 115668. [Google Scholar] [CrossRef]
- Wang, C.; Qian, J.; Wang, K.; Yang, X.; Liu, Q.; Hao, N.; Wang, C.; Dong, X.; Huang, X. Colorimetric aptasensing of ochratoxin A using Au@Fe3O4 nanoparticles as signal indicator and magnetic separator. Biosens. Bioelectron. 2016, 77, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tian, F.; Zhou, J.; Zhao, Q.; Fu, R.; Jiao, B. Colorimetric aptasensor for ochratoxin A detection based on enzyme-induced gold nanoparticle aggregation. J. Hazard. Mater. 2020, 388, 121758. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Chen, J.; Li, C.; Xie, G.; Fu, H.; Ma, Z.; Lu, A. Highly sensitive colorimetric detection of ochratoxin a by a label-free aptamer and gold nanoparticles. Toxins 2015, 7, 5377–5385. [Google Scholar] [CrossRef] [Green Version]
- Xiao, R.; Wang, D.; Lin, Z.; Qiu, B.; Liu, M.; Guo, L.; Chen, G. Disassembly of gold nanoparticle dimers for colorimetric detection of ochratoxin A. Anal. Methods 2015, 7, 842–845. [Google Scholar] [CrossRef]
- Liu, B.; Huang, R.; Yu, Y.; Su, R.; Qi, W.; He, Z. Gold Nanoparticle-Aptamer-Based LSPR Sensing of Ochratoxin A at a Widened Detection Range by Double Calibration Curve Method. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhao, R.; Feng, S.; Xie, Y. Colorimetric zearalenone assay based on the use of an aptamer and of gold nanoparticles with peroxidase-like activity. Microchim. Acta 2018, 185, 535. [Google Scholar] [CrossRef]
- Chotchuang, T.; Cheewasedtham, W.; Jayeoye, T.J.; Rujiralai, T. Colorimetric determination of fumonisin B1 based on the aggregation of cysteamine-functionalized gold nanoparticles induced by a product of its hydrolysis. Microchim. Acta 2019, 186, 655. [Google Scholar] [CrossRef]
- Althagafi, I.I.; Ahmed, S.A.; El-Said, W.A. Colorimetric aflatoxins immunoassay by using silica nanoparticles decorated with gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 118999. [Google Scholar] [CrossRef]
- Khansili, N.; Rattu, G.; Kumar, A.; Krishna, P.M. Development of colorimetric sensor with zinc oxide nanoparticles for rapid detection of aflatoxin B1 in rice. Mater. Today Proc. 2020, 21, 1846–1855. [Google Scholar] [CrossRef]
- Zhu, W.; Li, L.; Zhou, Z.; Yang, X.; Hao, N.; Guo, Y.; Wang, K. A colorimetric biosensor for simultaneous ochratoxin A and aflatoxins B1 detection in agricultural products. Food Chem. 2020, 319, 126544. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhang, Z.; Xiong, Y. Water dispersed two-dimensional ultrathin Fe(III)-modified covalent triazine framework nanosheets: Peroxidase like activity and colorimetric biosensing applications. Nanoscale 2018, 10, 20120–20125. [Google Scholar] [CrossRef] [PubMed]
- Sheini, A. Colorimetric aggregation assay based on array of gold and silver nanoparticles for simultaneous analysis of aflatoxins, ochratoxin and zearalenone by using chemometric analysis and paper based analytical devices. Microchim. Acta 2020, 187, 167. [Google Scholar] [CrossRef] [PubMed]
- Demers, L.M.; Östblom, M.; Zhang, H.; Jang, N.-H.; Liedberg, B.; Mirkin, C.A. Thermal Desorption Behavior and Binding Properties of DNA Bases and Nucleosides on Gold. J. Am. Chem. Soc. 2002, 124, 11248–11249. [Google Scholar] [CrossRef]
- Rhouati, A.; Bulbul, G.; Latif, U.; Hayat, A.; Li, Z.-H.; Marty, J.; Rhouati, A.; Bulbul, G.; Latif, U.; Hayat, A.; et al. Nano-Aptasensing in Mycotoxin Analysis: Recent Updates and Progress. Toxins 2017, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- YIN, X.; WANG, S.; LIU, X.; HE, C.; TANG, Y.; LI, Q.; LIU, J.; SU, H.; TAN, T.; DONG, Y. Aptamer-based Colorimetric Biosensing of Ochratoxin A in Fortified White Grape Wine Sample Using Unmodified Gold Nanoparticles. Anal. Sci. 2017, 33, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M. Aptamer-based Colorimetric and Chemiluminescence Detection of Aflatoxin B1 in Foods Samples. Acta Chim. Slov. 2015, 62, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lu, T.; Wang, Y.; Diao, C.; Zhou, Y.; Zhao, L.; Chen, H. Selection of a DNA Aptamer against Zearalenone and Docking Analysis for Highly Sensitive Rapid Visual Detection with Label-Free Aptasensor. J. Agric. Food Chem. 2018, 66, 12102–12110. [Google Scholar] [CrossRef]
- Tian, F.; Zhou, J.; Fu, R.; Cui, Y.; Zhao, Q.; Jiao, B.; He, Y. Multicolor colorimetric detection of ochratoxin A via structure-switching aptamer and enzyme-induced metallization of gold nanorods. Food Chem. 2020, 320, 126607. [Google Scholar] [CrossRef]
- Hao, N.; Lu, J.; Zhou, Z.; Hua, R.; Wang, K. A pH-Resolved Colorimetric Biosensor for Simultaneous Multiple Target Detection. ACS Sens. 2018, 3, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lin, Y.; Wang, X.; Xu, L.; Wang, Z.; Fu, F.F. Exonuclease-assisted multicolor aptasensor for visual detection of ochratoxin A based on G-quadruplex-hemin DNAzyme-mediated etching of gold nanorod. Microchim. Acta 2018, 185. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.S.; Haddad, M.A.; Parisi, S. Validation of HPLC and Enzyme-Linked Immunosorbent Assay (ELISA) techniques for detection and quantification of aflatoxins in different food samples. Foods 2020, 9, 661. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Mehta, A. Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: A review. Food Sci. Nutr. 2020, 8, 2183–2204. [Google Scholar] [CrossRef]
- Xiong, Y.; Pei, K.; Wu, Y.; Xiong, Y. Colorimetric ELISA based on glucose oxidase-regulated the color of acid–base indicator for sensitive detection of aflatoxin B1 in corn samples. Food Control 2017, 78, 317–323. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications Toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Xiong, Y.; Xu, B.; Wu, K.; Li, X.; Jiang, H.; Xiong, Y. Colorimetric ELISA for ochratoxin A detection based on the urease-induced metallization of gold nanoflowers. Sens. Actuators B Chem. 2018, 262, 102–109. [Google Scholar] [CrossRef]
- Zhan, S.; Hu, J.; Li, Y.; Huang, X.; Xiong, Y. Direct competitive ELISA enhanced by dynamic light scattering for the ultrasensitive detection of aflatoxin B1 in corn samples. Food Chem. 2020, 128327. [Google Scholar] [CrossRef]
- Mukherjee, M.; Nandhini, C.; Bhatt, P. Colorimetric and chemiluminescence based enzyme linked apta-sorbent assay (ELASA) for ochratoxin A detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 244, 118875. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Long, L.L.; Chen, Y.Q.; Chen, M.L.; Cheng, Y.H. A nanozyme-linked immunosorbent assay based on metal–organic frameworks (MOFs) for sensitive detection of aflatoxin B1. Food Chem. 2021, 338, 128039. [Google Scholar] [CrossRef]
- Urusov, A.; Zherdev, A.; Petrakova, A.; Sadykhov, E.; Koroleva, O.; Dzantiev, B. Rapid Multiple Immunoenzyme Assay of Mycotoxins. Toxins 2015, 7, 238–254. [Google Scholar] [CrossRef] [PubMed]
- McNamee, S.E.; Bravin, F.; Rosar, G.; Elliott, C.T.; Campbell, K. Development of a nanoarray capable of the rapid and simultaneous detection of zearalenone, T2-toxin and fumonisin. Talanta 2017, 164, 368–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Pei, K.; Wu, Y.; Duan, H.; Lai, W.; Xiong, Y. Plasmonic ELISA based on enzyme-assisted etching of Au nanorods for the highly sensitive detection of aflatoxin B1 in corn samples. Sens. Actuators B Chem. 2018, 267, 320–327. [Google Scholar] [CrossRef]
- Majdinasab, M.; Zareian, M.; Zhang, Q.; Li, P. Development of a new format of competitive immunochromatographic assay using secondary antibody–europium nanoparticle conjugates for ultrasensitive and quantitative determination of ochratoxin A. Food Chem. 2019, 275, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. TrAC Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Nara, S. Evaluation of gold nanoparticle based lateral flow assays for diagnosis of enterobacteriaceae members in food and water. Food Chem. 2015, 170, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef] [Green Version]
- Di Nardo, F.; Alladio, E.; Baggiani, C.; Cavalera, S.; Giovannoli, C.; Spano, G.; Anfossi, L. Colour-encoded lateral flow immunoassay for the simultaneous detection of aflatoxin B1 and type-B fumonisins in a single Test line. Talanta 2019, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Chen, J.; Chen, X.; Ma, T.; Cai, X.; Duan, H.; Leng, Y.; Huang, X.; Xiong, Y. A novel magneto-gold nanohybrid-enhanced lateral flow immunoassay for ultrasensitive and rapid detection of ochratoxin A in grape juice. Food Chem. 2021, 336, 127710. [Google Scholar] [CrossRef]
- Shao, Y.; Duan, H.; Zhou, S.; Ma, T.; Guo, L.; Huang, X.; Xiong, Y. Biotin–Streptavidin System-Mediated Ratiometric Multiplex Immunochromatographic Assay for Simultaneous and Accurate Quantification of Three Mycotoxins. J. Agric. Food Chem. 2019, 67, 9022–9031. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Yi, C.; Jiang, L.; Wu, J.; Chen, X.; Shen, X.; Sun, Y.; Lei, H. A smartphone-based quantitative detection device integrated with latex microsphere immunochromatography for on-site detection of zearalenone in cereals and feed. Sens. Actuators B Chem. 2019, 290, 170–179. [Google Scholar] [CrossRef]
- Wu, S.; Liu, L.; Duan, N.; Li, Q.; Zhou, Y.; Wang, Z. Aptamer-Based Lateral Flow Test Strip for Rapid Detection of Zearalenone in Corn Samples. J. Agric. Food Chem. 2018, 66, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Kong, W.; Dou, X.; Zhao, M.; Ouyang, Z.; Yang, M. An aptamer based lateral flow strip for on-site rapid detection of ochratoxin A in Astragalus membranaceus. J. Chromatogr. B 2016, 1022, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Dong, X.; Xu, T.; Yuan, J.; Yan, W.; Sui, X.; Zhao, X. Analysis of multiple mycotoxins-contaminated wheat by a smart analysis platform. Anal. Biochem. 2020, 610, 113928. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ren, M.; Li, Y.; Huang, Z.; Shu, M.; Yang, H.; Xiong, Y.; Xu, Y. Detection of aflatoxin B1 with immunochromatographic test strips: Enhanced signal sensitivity using gold nanoflowers. Talanta 2015, 142, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Q.; Han, M.; Zhou, J.; Gong, L.; Niu, Y.; Zhang, Y.; He, L.; Zhang, L. Development and optimization of a multiplex lateral flow immunoassay for the simultaneous determination of three mycotoxins in corn, rice and peanut. Food Chem. 2016, 213, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, H.; Li, C.; Zhang, S.; Shen, J.; Wang, Z. Gold nanoparticles-based lateral flow immunoassay with silver staining for simultaneous detection of fumonisin B1 and deoxynivalenol. Food Control 2015, 54, 347–352. [Google Scholar] [CrossRef]
- Zhang, T.; Lei, L.; Tian, M.; Ren, J.; Lu, Z.; Liu, Y.; Liu, Y. Multifunctional Fe3O4@Au supraparticle as a promising thermal contrast for an ultrasensitive lateral flow immunoassay. Talanta 2021, 222, 121478. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Y.; Zhang, B. Application of Microfluidics in Biosensors. In Advances in Microfluidic Technologies for Energy and Environmental Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Machado, J.M.D.; Soares, R.R.G.; Chu, V.; Conde, J.P. Multiplexed capillary microfluidic immunoassay with smartphone data acquisition for parallel mycotoxin detection. Biosens. Bioelectron. 2018, 99, 40–46. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, Y. Development and application of analytical detection techniques for droplet-based microfluidics-A review. Anal. Chim. Acta 2020, 1113, 66–84. [Google Scholar] [CrossRef]
- Alsaeed, B.; Mansour, F.R. Distance-based paper microfluidics; principle, technical aspects and applications. Microchem. J. 2020, 155, 104664. [Google Scholar] [CrossRef]
- LIANG, S.-J.; MAO, J.-K.; GONG, C.; YU, D.-D.; ZHOU, J.-G. Research and Application Progress of Paper-based Microfluidic Sample Preconcentration. Chinese J. Anal. Chem. 2019, 47, 1878–1886. [Google Scholar] [CrossRef]
- Man, Y.; Li, A.; Li, B.; Liu, J.; Pan, L. A microfluidic colorimetric immunoassay for sensitive detection of altenariol monomethyl ether by UV spectroscopy and smart phone imaging. Anal. Chim. Acta 2019, 1092, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kasoju, A.; Shrikrishna, N.S.; Shahdeo, D.; Khan, A.A.; Alanazi, A.M.; Gandhi, S. Microfluidic paper device for rapid detection of aflatoxin B1 using an aptamer based colorimetric assay. RSC Adv. 2020, 10, 11843–11850. [Google Scholar] [CrossRef]
- Jayawardane, B.M.; Wei, S.; McKelvie, I.D.; Kolev, S.D. Microfluidic Paper-Based Analytical Device for the Determination of Nitrite and Nitrate. Anal. Chem. 2014, 86, 7274–7279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wu, J.; Yao, K.; Yin, Y.; Gong, M.M.; Yang, C.; Lin, F. Paper-Based Microfluidic Device (DON-Chip) for Rapid and Low-Cost Deoxynivalenol Quantification in Food, Feed, and Feed Ingredients. ACS Sens. 2019, 4, 3072–3079. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Wu, D.; Li, X.; Yu, Y.; Luo, P.; Chen, J.; Dai, C.; Wu, Y. Recent advances in emerging nanomaterials based food sample pretreatment methods for food safety screening. TrAC Trends Anal. Chem. 2019, 121, 115669. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Yang, Q.; Hu, N.; Zhang, W.; Zhu, W.; Wang, R.; Suo, Y.; Wang, J. Patulin removal from apple juice using a novel cysteine-functionalized metal-organic framework adsorbent. Food Chem. 2019, 270, 1–9. [Google Scholar] [CrossRef]
- Rahmani, A.; Jinap, S.; Soleimany, F. Qualitative and Quantitative Analysis of Mycotoxins. Compr. Rev. Food Sci. Food Saf. 2009, 8, 202–251. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Analysis and Detection of Major Mycotoxins in Foods. Foods 2020, 9, 518. [Google Scholar] [CrossRef] [Green Version]
- TECNICA ASTORI Aflatoxins/Mycotoxins ELISA Kits. Available online: https://www.astorilab.com/index.php/en/products-catalogue/11-aflatoxins-mycotoxins-elisa-kits (accessed on 15 November 2020).
- Charm Sciences INC Mycotoxin Tests. Available online: https://www.charm.com/products/test-and-kits/mycotoxin-tests/ (accessed on 15 November 2020).
- Cusabio Mycotoxin Rapid Test. Available online: https://www.cusabio.com/mycotoxin.html (accessed on 15 November 2020).
- Elabscience Mycotoxin Testing. Available online: https://www.elabscience.com/Products-mycotoxins-215.html (accessed on 15 November 2020).
- EnviroLogix Mycotoxin Test Kits. Available online: https://www.envirologix.com/mycotoxin-testing/mycotoxin-test-kits/ (accessed on 15 November 2020).
- Eurofins Mycotoxins. Available online: https://tecna.eurofins-technologies.com/home/products/mycotoxins/ (accessed on 15 November 2020).
- HELICA ELISA Kits, Research Products. Available online: https://gentaur.com/search/Supplier/Helica (accessed on 15 November 2020).
- NEOGEN Mycotoxin Testing. Available online: https://www.neogen.com/en-gb/categories/mycotoxins/?q=12&s=MostPopular (accessed on 15 November 2020).
- R-Biopharm Tests for the detection of mycotoxins in food. Available online: https://food.r-biopharm.com/analytes/mycotoxins/ (accessed on 16 November 2020).
- Romer Labs Mycotoxin Test Kits|Fast & Reliable Mycotoxin Detection. Available online: https://www.romerlabs.com/en/products/test-kits/mycotoxin-test-kits/ (accessed on 16 November 2020).
- Vicam Mycotoxin Testing Solutions. Available online: http://vicam.com/products (accessed on 16 November 2020).
- Beacon Anaytical Systems Mycotoxins Plate Kits. Available online: https://www.beaconkits.com/mycotoxins (accessed on 16 November 2020).
- Creative Diagnostics Mycotoxins Analysis. Available online: https://www.creative-diagnostics.com/food-analysis/tag-mycotoxins-2.htm (accessed on 16 November 2020).
- PerkinElmer Mycotoxins Analysis in Food. Available online: https://www.perkinelmer.com/uk/category/mycotoxins-in-food (accessed on 16 November 2020).
- Unisensor Mycotoxins Sensor Kits. Available online: https://unisensor.be/en (accessed on 16 November 2020).
- Pribolab—Solutions in Food Safety and Testing-mycotoxin Testing. Available online: http://www.pribolab.com/ (accessed on 16 November 2020).
- Randox Feed & Cereals—Randox Food. Available online: https://www.randoxfood.com/feed-and-cereals-analysis/#1521620960005-d87fa0aa-85bd (accessed on 16 November 2020).
- Novakits Mycotoxines. Available online: http://www.novakits.com/20-mycotoxines (accessed on 16 November 2020).
- Sigma-Aldrich Mycotoxin Testing. Available online: https://www.sigmaaldrich.com/life-science/cell-biology/antibodies/antibody-products.html?TablePage=114571894 (accessed on 16 November 2020).
- Bio-Check Mycotoxin Testing Kits. Mycotoxin Lab and on-site Detection Tests. Available online: https://www.biocheck.uk/mycotoxins (accessed on 16 November 2020).
- Li, W.; Powers, S.; Dai, S.Y. Using commercial immunoassay kits for mycotoxins: ‘joys and sorrows’? World Mycotoxin J. 2014, 7, 417–430. [Google Scholar] [CrossRef]


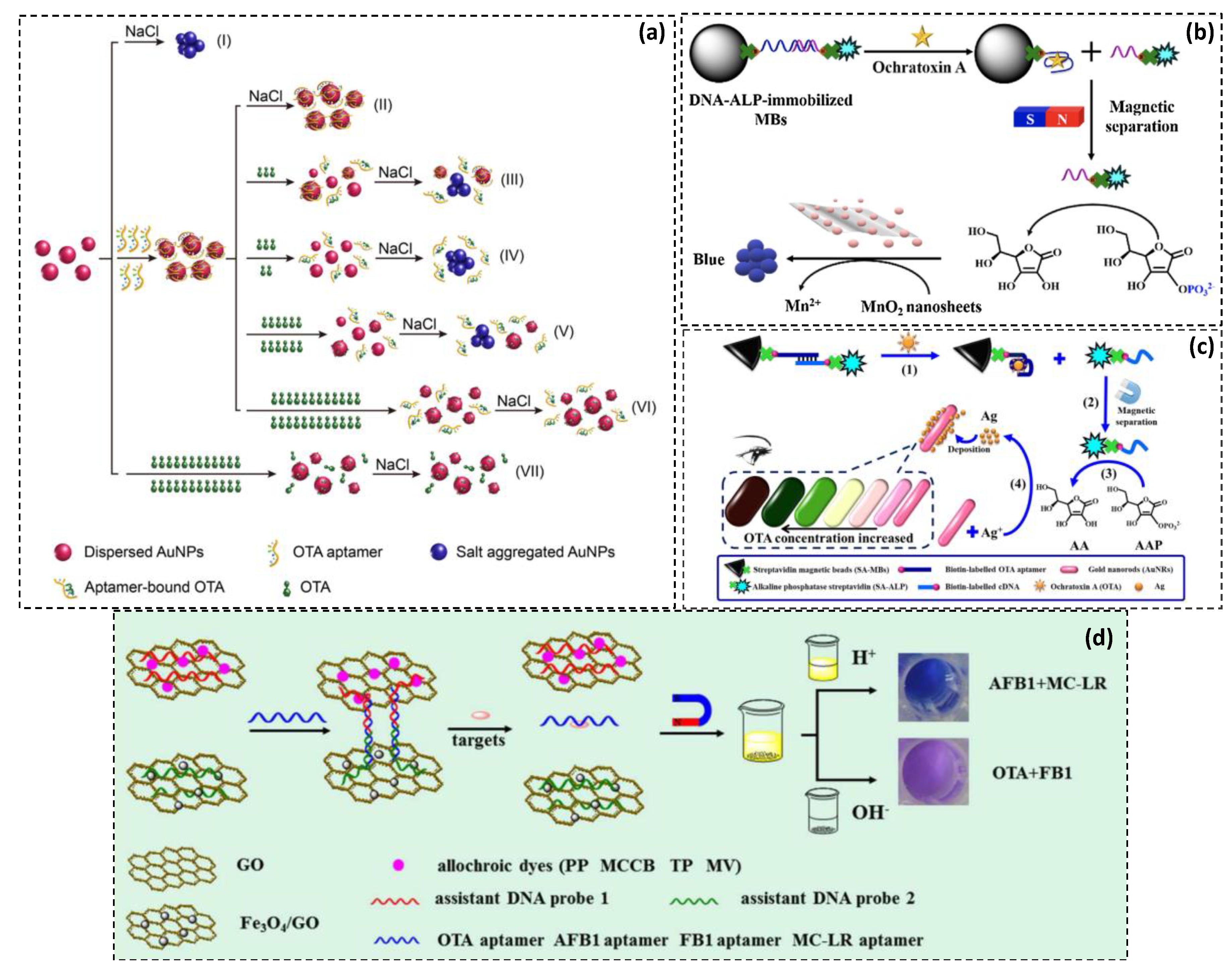

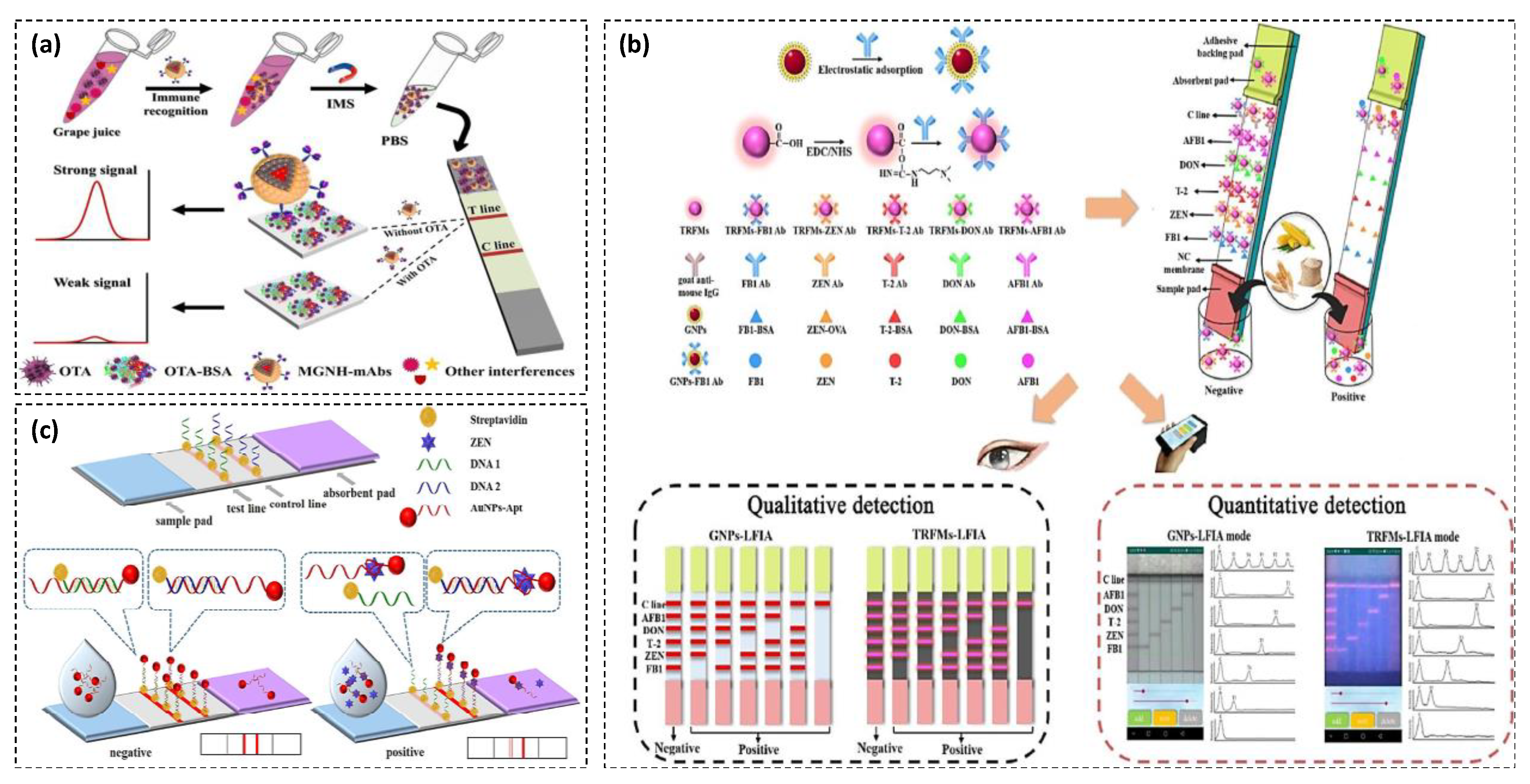
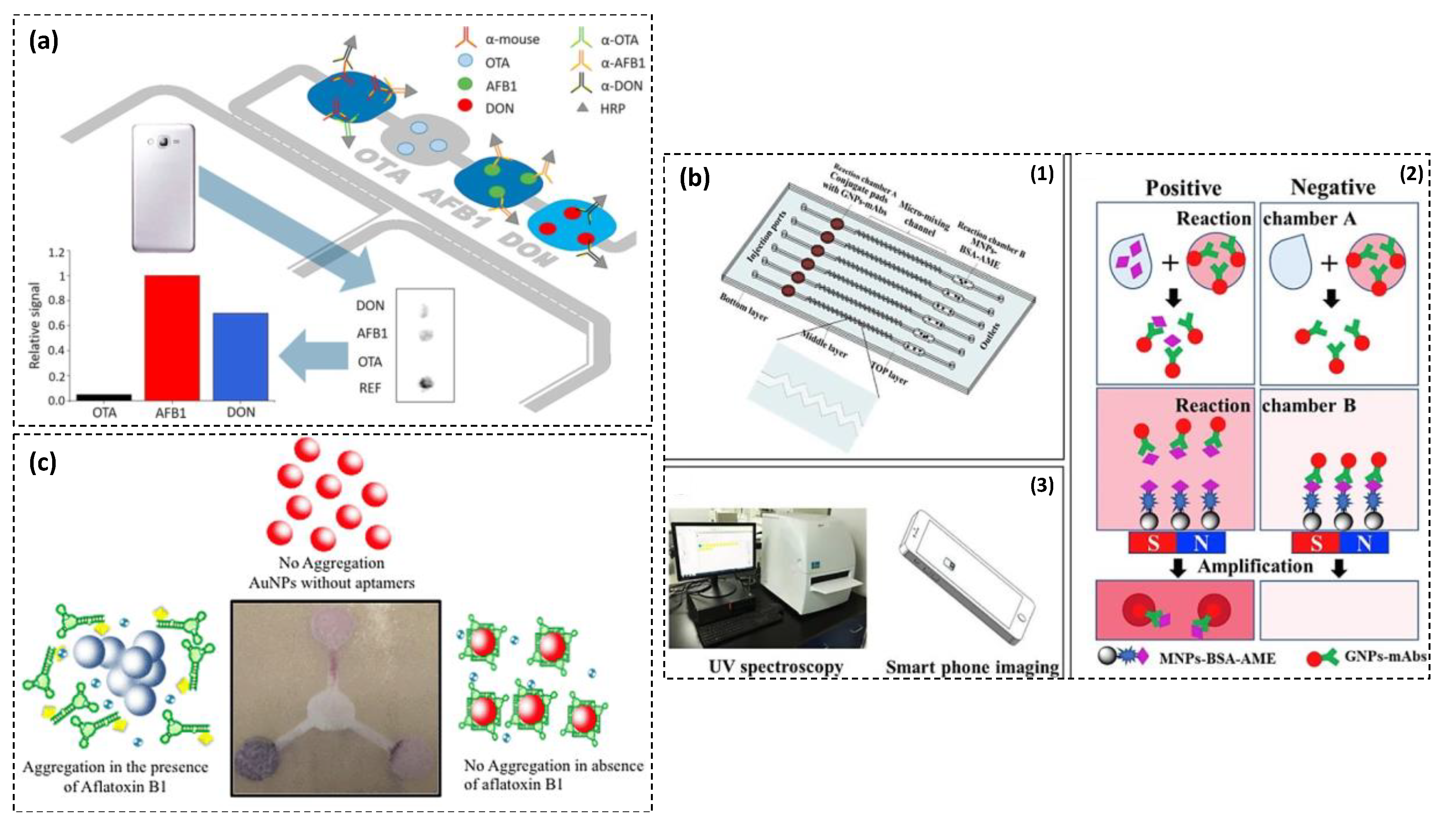
| Strategy | Detection Probe | Target | LOD | Linear Range | Specificity | Sample | Advantages/Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|---|
| Colorimetric aptasensor based on HRP-encapsulated liposome which catalyzed TMB oxidation | HRP | OTA | 0.023 ng·mL−1 | 0.05–2.0 ng·mL−1 | High | Corn | Simple, low-cost, highly selective, sensitive and reliable/long detection time (60 min) | [11] |
| Combination of an ingenious hairpin DNA probe with exonuclease III (Exo III)-assisted signal amplification and TMB oxidation | DNAzyme | AFB1 | 1 pM | 1 pM–100 nM | High | Peanut | High sensitivity, good selectivity, simple operation, wash-free, label-free format, low-cost, applicability to samples with complex matrices/long incubation time (40 min) | [15] |
| Dual aptamer-DNAzyme in combination with apta-magnetic separation | DNAzyme | AFB1 | 22.6 ppb | 0–200 ppb | Good | Corn, rice, groundnut, black pepper, chili | High sensitivity, good selectivity, low-cost, reliable/long incubation time of DNAzyme (30 min) | [17] |
| Detection aptamer containing a DNAzyme sequence and an RCA priming sequence for the isothermal DNA amplification | DNAzyme | OTA | 1.09 × 10−12 ng·mL−1 | 10−12–10 ng·mL−1 | High | Urine | High sensitivity and selectivity, applicability to biological samples with complex matrices/long incubation time and complicated operation with multiple steps of washing and separation | [16] |
| Combination of DNA aptamer and two split hemin-binding DNAzyme halves and G-quadruplex formation of a split DNAzyme-hemin/aptamer complex with peroxidase mimicking activity in the absence of AFB1 | DNAzyme | AFB1 | 0.1 ng·mL−1 | 0.1–104 ng·mL−1 | High | Corn | High sensitivity and selectivity simple and low-cost/long incubation time (70 min) | [21] |
| Inhibition of chitosan-immobilized AchE activity by AFB1and Ellman’s method | AchE | AFB1 | Not reported | Not reported | Good | Corn | Simple, rapid (detection time ≈ 8 min), low-cost, portable/failure to report LOD and linear range | [19] |
| Strategy | Detection Probe | Target | LOD | Linear Range | Specificity | Sample | Advantages/Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|---|
| Aptamer assay based on AuNPs aggregation by poly diallyldimethyl ammonium chloride polymer (PDDA). | AuNPs | OTA | 0.009 ng·mL−1 | 0.05–50 ng·mL−1 | High | Chinese liquor sample | Cost-effectiveness, few steps, rapid detection (15 min), good sensitivity/Possible cross-reactivity at high target concentrations. | [38] |
| AuNP dimer disassembly by the target-induced release of complementary DNA probes. | AuNPs | OTA | 0.05 nM | 0.2–250 nM | High | Red wine | Improved sensitivity, low-cost, short detection time (15 min)/Difficult applicability to colored complex samples | [39] |
| Double calibration curve of label free aptasensing assay based on salt-induced aggregation of AuNPs. | AuNPs | OTA | 0.03 ng·mL−1 | 0.03–316 ng·mL−1 | Good | Corn | Widened detection range, enhanced sensitivity, reliability, rapid detection/low selectivity | [40] |
| Peroxidase-like activity of AuNPs in the presence of H2O2 and TMS substrate. | AuNPs | ZEN | 10 ng·mL−1 | 10–250 ng·mL−1 | High | Corn and corn oil | Simple one-step assay, short detection time/Relatively high detection limit | [41] |
| Chemical nano-sensor based on cysteamine-modified AuNPs aggregation via electrostatic interaction with hydrolyzed target. | AuNPs | FB1 | 0.90 μg·kg−1 | 2–8 μg·kg−1 | Low | Corn | Simple one-step assay, Rapid homogenous test (3 min)/Real sample interferences, low sensitivity and selectivity. | [42] |
| ALP- induced gold nanoparticle aggregation mediated by MnO2 nanosheets reduction in the presence of generated ascorbic acid. | AuNPs, MnO2 nanosheets | OTA | 5.0 nM | 6.25–750 nM | High | Grape juice & red wine | Enzymatic amplification, high selectivity/multi reaction steps, possible cross reactivity in real samples | [37] |
| Colorimetric aflatoxins immunoassay by using mesoporous silica nanoparticles decorated with gold nanoparticles. | AuNPs@m-SiNPs nanocomposite | AFs (AFB1, AFB2, and AFG2) | 0.16 ng·mL−1 | 1–75 ng·mL−1 for AFB1 | High | Nuts, cornflakes, cornmeal, peanuts, peanut butter and pecan nuts | High sensitivity, versatile real matrix applicability, 30 min incubation time/long synthesis and modification of transducer | [43] |
| AFB1 hydrolyzed to phenolate anions react with curcumin enol form-Zn red complex to give curcumin enol form-ZnO-Phenol yellow complex. | ZnO NPs | AFB1 | 11 µg·kg−1 | 0–36 µg·kg−1 | Good | Rice | Simple and rapid detection, bioreceptor-free sensor, HPLC validation/Chemical modification of target | [44] |
| Cascade aptasensor by double catalytic amplifications using ALP activity combined to the inhibition of the MnO2 oxidase-mimicking activity. | MnO2 nanosheets | OTA | 0.07 nM | 1.25–250 nM | Excellent | Grape juice | Amplified colorimetric signal, high sensitivity and selectivity/Many washing and addition steps | [34] |
| Simultaneous dual target detection via the combination of two the catalysis of TMB under acidic conditions and the release of TP under alkaline conditions. | Fe3O4-GO nanocomposite and AuNPs | AFB1 | 1.5 ng·mL−1 | 5–250 ng·mL−1 | High | Peanuts | Multiplexed detection, high sensitivity and selectivity/Tedious probes synthesis, specific pH and temperature conditions, long incubation time (90 min), multiple steps of washing and separation | [45] |
| OTA | 0.15 ng·mL−1 | 0.5–80 ng·mL−1 | ||||||
| Salt-induced coagulation of iron-modified 2D covalent triazine framework nanosheets (2D Fe-CTFs) that showed strong peroxidase-like activity | 2D Fe-CTFs | OTA | NR | 0.2–0.8 μM | NR | NR | Promising proof of concept/limited detection range, analytical performances not reported | [46] |
| PAD sensor array based on silver and gold nanoparticles aggregation synthesized by three different capping agents. | AgNPs and AuNPs | AFB1 | 2.7 ng·mL−1 | 3.1 ng·mL−1 –7.8 μg·mL−1 | Excellent | Mixtures of pistachio, wheat and coffee, milk | Very fast colorimetric response (5 s), multiplexed detection of five mycotoxins, low cost, device portability/Optical nanoprobes fabrication | [47] |
| AFG1 | 7.3 ng·mL−1 | 8.2 ng·mL−1–8.4 μg.mL−1 | ||||||
| AFM1 | 2.1 ng·mL−1 | 2.5 ng·mL−1–8.2 μg.mL−1 | ||||||
| OTA | 3.3 ng·mL−1 | 4.0 ng·mL−1–3.8 μg.mL−1 | ||||||
| ZEN | 7.0 ng·mL−1 | 8.0 ng·mL−1–7.9 μg.mL−1 |
| Strategy | Target | LOD | Linear Range | Specificity | Sample | Advantages/Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| Colorimetric ELISA based on glucose oxidase-regulated the color of bromocresol purple acid-base indicator | AFB1 | Cutoff limit: 100 pg·mL−1 | 25–200 pg·mL−1 | Excellent | Corn | High sensitivity and selectivity, good repeatability/long incubation time, multi-step washing | [58] |
| Plasmonic ELISA based on the urease-induced metallization of gold nanoflowers | OTA | 8.205 pg·mL−1 | 5.0–640 pg·mL−1 | Excellent | Rice, corn, wheat, white wine | High sensitivity and selectivity, robust, and high-throughput, good repeatability/long incubation time, multi-step washing | [60] |
| DLS-ELISA based on AuNPs aggregation via hydroxyl radicals produced by HRP activity on H2O2 | AFB1 | 0.12 pg·mL−1 | Not reported | Excellent | Corn | High sensitivity and selectivity, reliable, good repeatability, high accuracy/long incubation time, multi-step washing | [61] |
| Apta-ELISA based on target capture by OTA specific aptamer and color development by anti-rabbit secondary antibody labeled with ALP | OTA | 0.84 pg·mL−1 | 1 pg·mL−1–1 µg·mL−1 | Good | Groundnut, coffee bean | High sensitivity, low-cost/long incubation time, multi-step washing, cross-reactivity, matrix interference | [62] |
| A nanozyme-linked immunosorbent assay based on metal–organic frameworks (MOFs) instead of enzyme to catalyze chromogenic TMB | AFB1 | 0.009 ng·mL−1 | 0.01–20 ng·mL−1 | Excellent | Peanut milk, soymilk | High sensitivity and selectivity, high catalytic activity and stability of MOF, good recovery rate and accuracy, avoiding false positive and false negative results/long incubation time, multi-step washing | [63] |
| Multiplexed ELISA based on immobilization of protein-analyte conjugates in separate wells | OTA | 4.0 ng·mL−1 | 4.0–120 ng·mL−1 | Not reported | Poultry, corn | High sensitivity, reduction of simultaneous detection time of three mycotoxins/long incubation time, multi-step washing | [64] |
| AFB1 | 0.1 ng·mL−1 | 0.1–1.0 ng·mL−1 | |||||
| ZEN | 0.3 ng·mL−1 | 0.3–50.0 ng·mL−1 | |||||
| Multiplex nanoarray based on ELISA technique via nano-spotting of mycotoxin-protein conjugates into single wells of a microplate | ZEN T-2 toxin FB1 | IC50: 197.4 0.7 216.7 µg·kg−1 in wheat | Not reported | High | Wheat, corn | High sensitivity and selectivity, reduction of simultaneous detection time of three mycotoxins, throughput, easily adaptable by end users/long incubation time, multi-step washing | [65] |
| Plasmonic ELISA based on the HRO-assisted etching of gold nanorods | AFB1 | Cutoff limit: 12.5 pg·mL−1 | 3.1–150 pg·mL−1 | Excellent | Corn | High sensitivity and selectivity, high accuracy and precision, portable, equipment-free/long incubation time, multi-step washing | [66] |
| Strategy | Target | LOD | Linear Range | Specificity | Sample | Advantages/Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| Dual color AuNPs and a single test line for simultaneous detection of two mycotoxins | AFB1 FBs | 0.5 ng·mL−1 20 ng·mL−1 | Not reported | Not reported | Wheat, pasta | High sensitivity, simple, low-cost, rapid (detection time of 10 min), semi-quantitative, reliable/no evaluation of selectivity and stability | [71] |
| Magneto-gold nanohybrid based LFA for simultaneous separation and target detection | OTA | 0.094 ng·mL−1 | 0.098–12.5 ng·mL−1 | High | Grape juice | High sensitivity and selectivity, simple, low-cost, rapid (detection time of 15 min), semi-quantitative, high precision in complex matrices, high reproducibility/no evaluation of stability | [72] |
| AuNPs and TRFMs-based lateral flow immunoassays for multiplex detection mycotoxins along with a smartphone-based quantitative dual detection mode device | AFB1 ZEN Deoxynivalenol T-2 toxin FB1 | AuNPs-LFA: 0.59 0.24 0.32 0.90 0.27 ng·mL−1 TRFMs-LFA: 0.42 0.10 0.05 0.75 0.04 ng·mL−1 | Not reported | Low | Maize, wheat, bran | High sensitivity, simple, low-cost, rapid, reliable, quantitative, portable/no evaluation of stability, high cross reactivity with other mycotoxin in a single class | [30] |
| Application of GO and carboxylated GO instead of AuNPs as label | AFB1 | 0.3 ng·mL−1 | Not reported | Not reported | Peanut oil, maize, rice | High sensitivity, high stability (4 months), simple, low-cost, rapid, reliable, rapid (detection time of 15 min), acceptable reproducibility/qualitative, no evaluation of specificity | [31] |
| Aptamer-based competitive LFA | ZEN | vLOD: 20 ng·mL−1 qLOD: 5 ng·mL−1 | 5–200 ng·mL−1 | High | Corn | High sensitivity and selectivity, high stability (2 months at room temperature), simple, low-cost, short detection time (5 min), portable/no evaluation of reproducibility | [75] |
| AuNPs-aptamer conjugate as recognition element and label; immobilization of biotinylated DNA probe 1 and probe 2 on test and control lines, respectively | OTA | 1 ng·mL−1 | Not reported | Excellent | Astragalus membranaceus | High sensitivity and selectivity, cost-effective, robust, high stability (6 months at room temperature), simple, short detection time (15 min), portable/no evaluation of reproducibility, qualitative | [76] |
| Design a smart analysis platform for multiplex LFIA based on AuNPs as label and five test lines | AFB1 ZEN Deoxynivalenol T-2 toxin FB1 | 4 40 200 10 20 µg·kg−1 | Not reported | Low | Wheat | High sensitivity, cost-effective, robust, simple, short detection time (15 min), portable, quantitative/no evaluation of reproducibility and stability, high cross reactivity with other mycotoxin in a single class | [77] |
| Enhanced signal sensitivity in LFIA using multi-branched gold nanoflowers | AFB1 | 0.32 pg·mL−1 in rice | 0.5–25 pg·mL−1 | Low | Rice | Excellent sensitivity, cost-effective, simple, short detection time (15 min), portable, quantitative/no evaluation of reproducibility and stability, high cross reactivity with AFG2 | [78] |
| Multiplex LFIA based on AuNPs as label | AFB1 ZEN OTA | 0.1–0.13 0.42–0.46 0.19–0.24 µg·kg−1 | Not reported | Not reported | Corn, rice, peanut | High sensitivity, cost-effective, simple, short detection time (15 min), portable, quantitative/no evaluation of reproducibility, selectivity and stability | [79] |
| AuNPs-based LFIA with silver staining for signal amplification | FB1 Deoxynivalenol | cut-off values: 2.0 40 ng·mL−1 | Not reported | Not reported | Maize | High sensitivity, low-cost, simple, short detection time, portable/no evaluation of reproducibility, selectivity and stability | [80] |
| LFIA based on multifunctional photothermal contrast Fe3O4@Au supraparticle (Fe3O4@Au SP) | OTA | 0.12 pg·mL−1 | 1 pg·mL−1–1 µg·mL−1 | Good | Corn, peanut, soybean | High sensitivity and selectivity, low-cost, simple, reliable/no evaluation of reproducibility and stability | [81] |
| A LFIA using Prussian blue nanoparticle (PBNP) as a peroxidase mimicking label for TMP catalysis | OTA | 10 pg·mL−1 | 10 pg·mL−1–1 µg·mL−1 | High | Human serum | High sensitivity and selectivity, low-cost, simple, reliable/no evaluation of reproducibility and stability | [32] |
| Strategy | Target | LOD | Linear Range | Specificity | Sample | Advantages/Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| PDMS-based microfluidic immunoassay using antibody labeled with HRP | OTA AFB1 ZEN | <40 0.1–0.2 <10 ng·mL−1 | − | High | Corn | Good sensitivity, high selectivity, low-cost, short detection time (10 min), portable/no evaluation of reproducibility and stability, lots of user-intervention steps, semi-quantitative | [83] |
| Microfluidic immunoassay based on combination target binding to AuNPs-mAb and immunogold amplification of AuNPs-mAbs-AME | Altenariol monomethyl ether | 12.5 pg·mL−1 200 pg·mL−1 | 12.5–200 pg·mL−1 200–1000 pg·mL−1 | High | Apple, cherry, orange | High sensitivity and selectivity, low-cost, short detection time (15 min), portable, simultaneous analysis of six samples, quantitative/no evaluation of reproducibility and stability | [87] |
| Development of a µPAD based on salt-induced aggregation of AuNPs in the presence of analyte | AFB1 | 10 nM | 1 pM–1 µM | High | Water | High sensitivity and selectivity, cost-effective, short detection time (> 1 min), portable/no evaluation of reproducibility and stability, no evaluation of food matrix, qualitative | [88] |
| Colorimetric competitive immunoassay into a paper microfluidic device using AuNPs as signal indicator | Deoxynivalenol | 0.644 ng·mL−1 | 0.01–20 ng·mL−1 | High | Wheat, corn | High sensitivity and selectivity, rapid (detection time of 12 min), low-cost, portable, and reliable/qualitative, no evaluation of reproducibility and stability | [90] |
| Company | Kit | Type of Detection | Mycotoxins | Time (min) | Multiplexing | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AFs | OTA | FUM | ZEN | DON | T2/HT2 | ||||||
| Astori Tecnica | ELISA | QNT, Semi-QNT for OTA | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 20–30 | No | [95] |
| LFIA | QLT, QNT | AFM1 | (−) | (−) | (−) | (−) | (−) | 10 | |||
| Charm Sciences Inc. | LFIA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 3–5 | No | [96] |
| CUSABIO | ELISA | QNT | AFB1 | ✓ | (−) | ✓ | ✓ | T2 | 20 | No | [97] |
| LFIA | QNT | AFB1 | ✓ | (−) | ✓ | ✓ | T2 | 3–5 | |||
| Elabscience | ELISA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 75 | No | [98] |
| LFIA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | |||
| EnviroLogix | LFIA | QLT, QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 2–4 | Yes (AF, ZEN, DON, FUM) | [99] |
| Eurofins | ELISA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 15–75 | No | [100] |
| LFIA | QNT | ✓ | (−) | ✓ | ✓ | ✓ | (−) | 5 | |||
| Helica | ELISA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | (−) | NR | No | [101] |
| Neogen | ELISA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 10–20 | No | [102] |
| LFIA | QLT, QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 3–8 | |||
| R-Biopharm | ELISA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 45–150 | No | [103] |
| LFIA | Semi-QNT, QNT | ✓ | (−) | ✓ | ✓ | ✓ | ✓ | 5 | |||
| Romer Labs | ELISA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 15 | Yes (up to 6 mycotoxins) | [104] |
| LFIA | QLT, QNT | ✓ | ✓ | ✓ | ✓ | ✓ | (−) | 3 | No | ||
| Vicam | LFIA | Semi-QNT, QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | No | [105] |
| Beacon Analytical Systems | ELISA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 15–75 | No | [106] |
| Creative Diagnostics | ELISA | QNT | ✓ | ✓ | ✓ | (−) | ✓ | ✓ | 15–120 | No | [107] |
| LFIA | QLT, Semi-QNT, QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 3–10 | |||
| PerkinElmer | ELISA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 15–60 | No | [108] |
| LFIA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 4–6 | |||
| Unisensor | LFIA | QNT | ✓ | (−) | (−) | (−) | (−) | (−) | 10 | No | [109] |
| Pribolab | ELISA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NR | No | [110] |
| LFIA | QLT, QNT | ✓ | ✓ | ✓ | ✓ | ✓ | (−) | 10–12 | |||
| Randox | ELISA | QNT | ✓ | (−) | (−) | (−) | (−) | (−) | NR | No | [111] |
| Biochip Arrays | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 120 | Yes (up to 10 mycotoxins) | ||
| Novakits | ELISA | Semi-QNT, QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 15–70 | No | [112] |
| LFIA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | Yes (7 mycotoxins) | ||
| Sigma | ELISA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | (−) | NR | No | [113] |
| Bio-Check | ELISA | QNT | ✓ | ✓ | ✓ | ✓ | ✓ | (−) | NR | No | [114] |
| LFIA | QNT | ✓ | ✓ | ✓ | (−) | ✓ | (−) | 3–5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majdinasab, M.; Ben Aissa, S.; Marty, J.L. Advances in Colorimetric Strategies for Mycotoxins Detection: Toward Rapid Industrial Monitoring. Toxins 2021, 13, 13. https://doi.org/10.3390/toxins13010013
Majdinasab M, Ben Aissa S, Marty JL. Advances in Colorimetric Strategies for Mycotoxins Detection: Toward Rapid Industrial Monitoring. Toxins. 2021; 13(1):13. https://doi.org/10.3390/toxins13010013
Chicago/Turabian StyleMajdinasab, Marjan, Sondes Ben Aissa, and Jean Louis Marty. 2021. "Advances in Colorimetric Strategies for Mycotoxins Detection: Toward Rapid Industrial Monitoring" Toxins 13, no. 1: 13. https://doi.org/10.3390/toxins13010013
APA StyleMajdinasab, M., Ben Aissa, S., & Marty, J. L. (2021). Advances in Colorimetric Strategies for Mycotoxins Detection: Toward Rapid Industrial Monitoring. Toxins, 13(1), 13. https://doi.org/10.3390/toxins13010013






