Cell Death Signaling Pathway Induced by Cholix Toxin, a Cytotoxin and eEF2 ADP-Ribosyltransferase Produced by Vibrio cholerae
Abstract
1. Introduction
2. V. cholerae Strains Possessing Cholix Genes
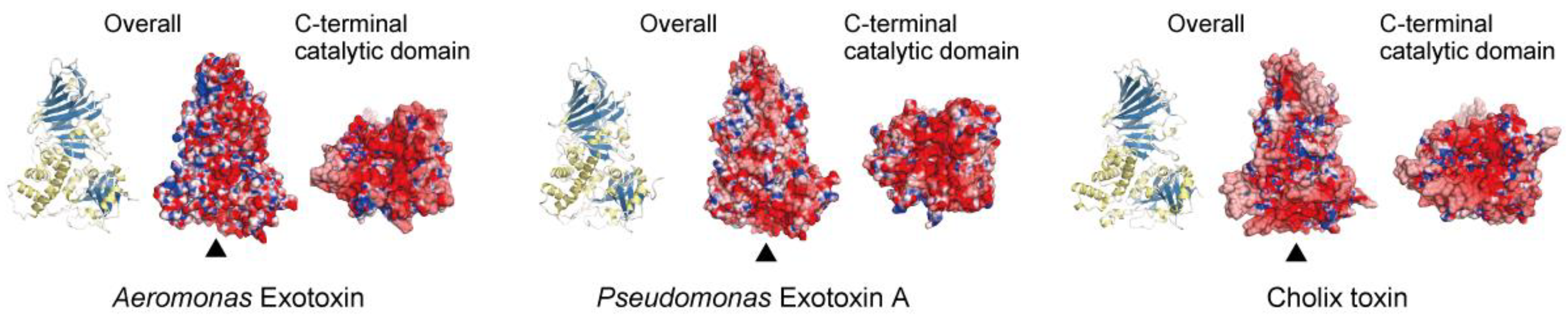
3. Structural Insights
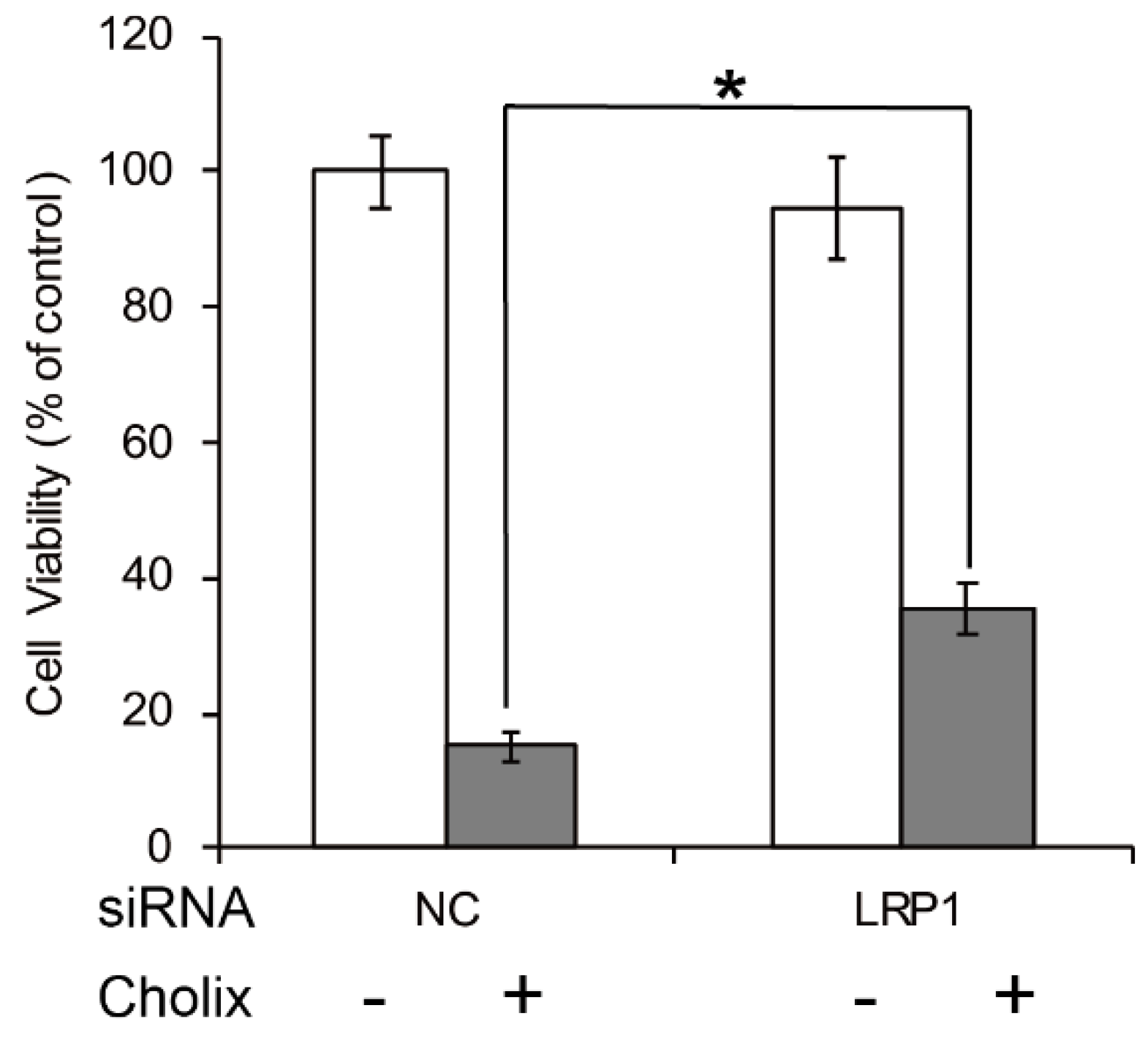
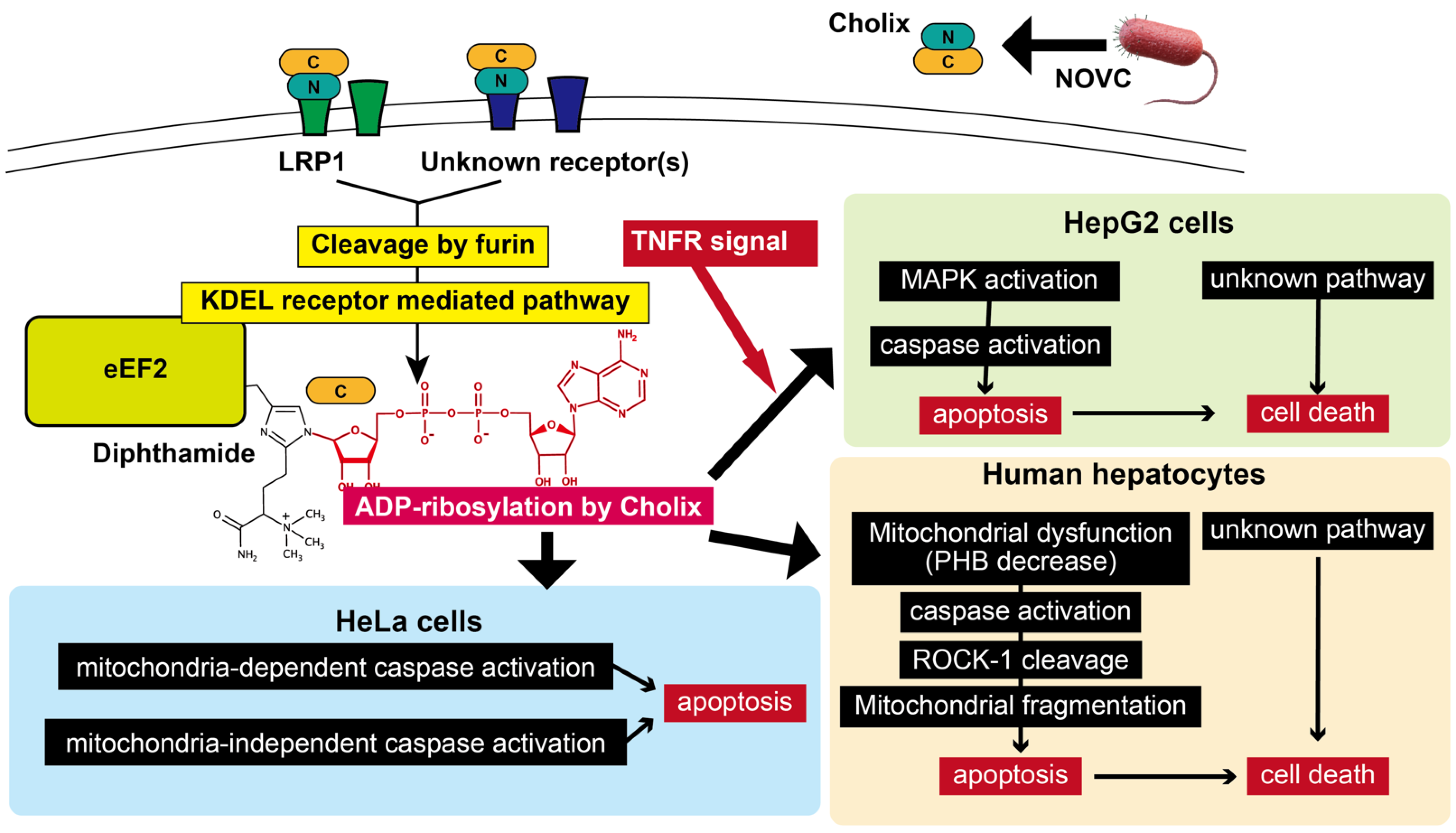
4. Cholix Receptor
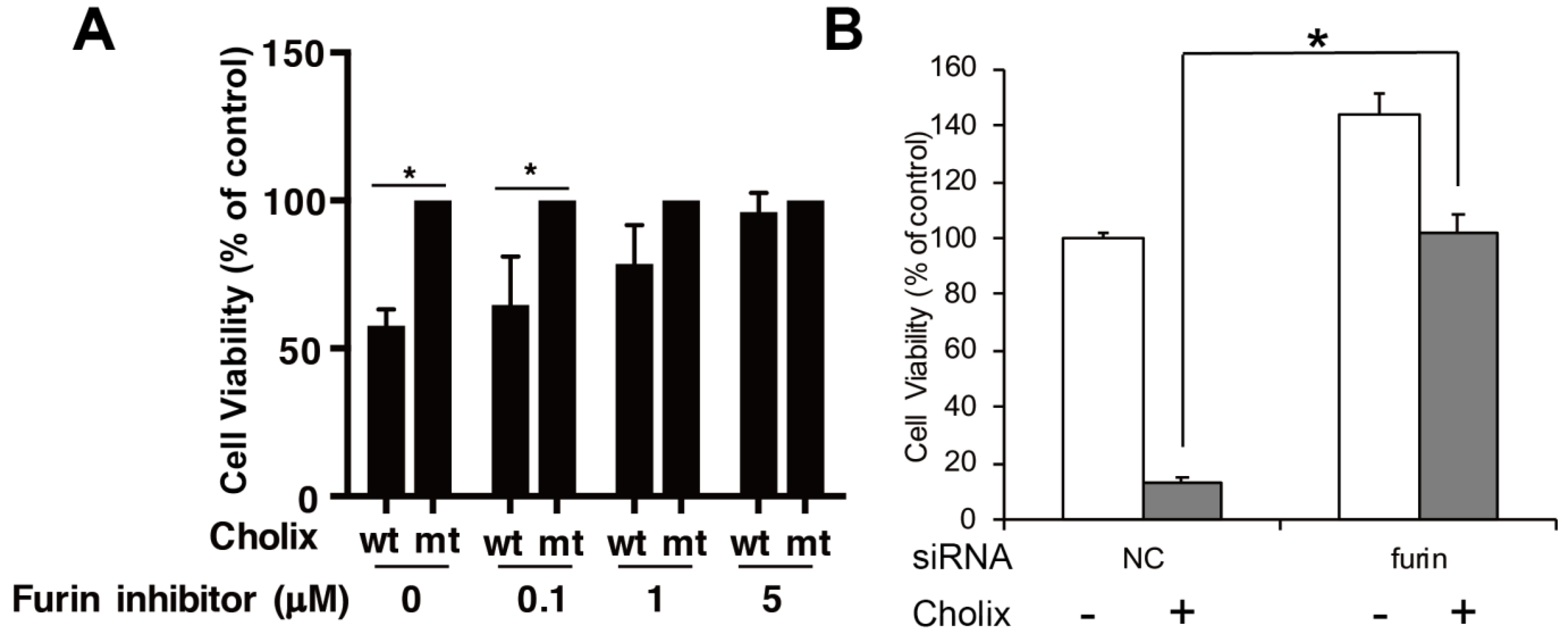
5. Translocation of Cholix in Host Cells
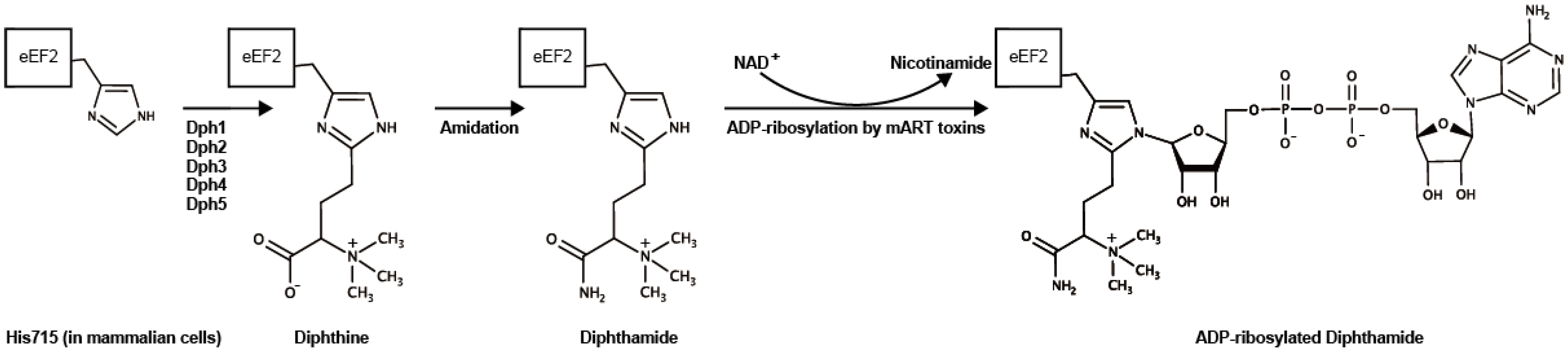
6. ADP-Ribosylation of eEF2 by Cholix
7. Liver Damage Induced by Cholix
8. Other Cell Death Mechanisms
9. Prohibitin Binding and Mitochondrial Dysfunction
10. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| eEF2 | Eukaryotic elongation factor 2 |
| Cholix | Cholix toxin |
| PEA | Pseudomonas exotoxin A |
| CT | Cholera toxin |
| mART | Mono-ADP-ribosyltransferase |
| TNF-α | Tumor necrosis factor-α |
| TNFR | Tumor necrosis factor receptor |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| PKC | Protein kinase C |
| ROS | Reactive oxygen species |
| ROCK | Rho-associated, coiled coil-containing protein kinase |
| PHB | Prohibitin |
| cPARP | Cleaved poly (ADP-ribose) polymerase |
| LRP1 | Low-density lipoprotein receptor-related protein 1 |
References
- Morris, J.G. Cholera and other types of vibriosis: A story of human pandemics and oysters on the half shell. Clin. Infect. Dis. 2003, 37, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.D.; Nair, G.B.; Ahmed, T.; Qadri, F.; Holmgren, J. Cholera. Lancet 2017, 390, 1539–1549. [Google Scholar] [CrossRef]
- Colwell, R.R. Global climate and infectious disease: The cholera paradigm. Science 1996, 274, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.Z.; Farhana, I.; Tulsiani, S.M.; Begum, A.; Jensen, P.K.M. Transmission and Toxigenic Potential of Vibrio cholerae in Hilsha Fish (Tenualosa ilisha) for Human Consumption in Bangladesh. Front. Microbiol. 2018, 9, 222. [Google Scholar] [CrossRef]
- Halpern, M.; Izhaki, I. Fish as Hosts of Vibrio cholerae. Front. Microbiol. 2017, 8, 282. [Google Scholar] [CrossRef]
- Chowdhury, F.R.; Nur, Z.; Hassan, N.; Seidlein, L.; Dunachie, S. Pandemics, pathogenicity and changing molecular epidemiology of cholera in the era of global warming. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 10. [Google Scholar] [CrossRef]
- Vezzulli, L.; Baker-Austin, C.; Kirschner, A.; Pruzzo, C.; Martinez-Urtaza, J. Global emergence of environmental non-O1/O139 Vibrio cholerae infections linked with climate change: A neglected research field? Environ. Microbiol. 2020, 4342–4355. [Google Scholar] [CrossRef]
- Waldor, M.K.; Mekalanos, J.J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 1996, 272, 1910–1913. [Google Scholar] [CrossRef]
- Dalsgaard, A.; Serichantalergs, O.; Forslund, A.; Lin, W.; Mekalanos, J.; Mintz, E.; Shimada, T.; Wells, J.G. Clinical and environmental isolates of vibrio cholerae serogroup O141 carry the CTX phage and the genes encoding the toxin-coregulated pili. J. Clin. Microbiol. 2001, 39, 4086–4092. [Google Scholar] [CrossRef]
- Aydanian, A.; Tang, L.; Morris, J.G.; Johnson, J.A.; Stine, O.C. Genetic diversity of O-antigen biosynthesis regions in Vibrio cholerae. Appl. Environ. Microbiol. 2011, 77, 2247–2253. [Google Scholar] [CrossRef]
- Tobin-D’Angelo, M.; Smith, A.R.; Bulens, S.N.; Thomas, S.; Hodel, M.; Izumiya, H.; Arakawa, E.; Morita, M.; Watanabe, H.; Marin, C.; et al. Severe diarrhea caused by cholera toxin-producing Vibrio cholerae serogroup O75 infections acquired in the southeastern United States. Clin. Infect. Dis. 2008, 47, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Reytor, D.; Jaña, V.; Pavez, L.; Navarrete, P.; García, K. Accessory toxins of vibriopathogens and their role in epithelial disruption during infection. Front. Microbiol. 2018, 9, 2248. [Google Scholar] [CrossRef] [PubMed]
- Shanley, J.; Kanj, A.; El Zein, S.; Tabaja, H.; Trzcinski, B.; Horman, J.; Salimnia, H.; Fairfax, M.; Singh, M. Non-O1, non-O139 Vibrio cholerae bacteremia in an urban academic medical center in the United States. IDCases 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, Y.; Qian, H.; Liu, G.; Mei, Y.; Jin, F.; Xia, W.; Ni, F. Non-O1, Non-O139 Vibrio cholerae (NOVC) Bacteremia: Case Report and Literature Review, 2015–2019. Infect. Drug Resist. 2020, 13, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Takeda, T.; Ogawa, A. The Gene Encoding the Heat-Stable Enterotoxin of Vibrio cholerae is Flanked by 123-Base Pair Direct Repeats. Microbiol. Immunol. 1993, 37, 774–777. [Google Scholar] [CrossRef]
- Cinar, H.N.; Kothary, M.; Datta, A.R.; Tall, B.D.; Sprando, R.; Bilecen, K.; Yildiz, F.; McCardell, B. Vibrio cholerae Hemolysin Is Required for Lethality, Developmental Delay, and Intestinal Vacuolation in Caenorhabditis elegans. PLoS ONE 2010, 5, e11558. [Google Scholar] [CrossRef]
- Dziejman, M.; Serruto, D.; Tam, V.C.; Sturtevant, D.; Diraphat, P.; Faruque, S.M.; Rahman, M.H.; Heidelberg, J.F.; Decker, J.; Li, L.; et al. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc. Natl. Acad. Sci. USA 2005, 102, 3465–3470. [Google Scholar] [CrossRef]
- Tam, V.C.; Serruto, D.; Dziejman, M.; Brieher, W.; Mekalanos, J.J. A Type III Secretion System in Vibrio cholerae Translocates a Formin/Spire Hybrid-like Actin Nucleator to Promote Intestinal Colonization. Cell Host Microbe 2007, 1, 95–107. [Google Scholar] [CrossRef]
- Satchell, K.J.F. Multifunctional-autoprocessing repeats-in-toxin (MARTX) Toxins of Vibrios. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Fasano, A.; Baudry, B.; Pumplin, D.W.; Wasserman, S.S.; Tall, B.D.; Ketley, J.M.; Kaper, J.B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. USA 1991, 88, 5242–5246. [Google Scholar] [CrossRef]
- Di Pierro, M.; Lu, R.; Uzzau, S.; Wang, W.; Margaretten, K.; Pazzani, C.; Maimone, F.; Fasano, A. Zonula occludens toxin structure-function analysis: Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J. Biol. Chem. 2001, 276, 19160–19165. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, R.; Purdy, A.E.; Fieldhouse, R.J.; Kimber, M.S.; Bartlett, D.H.; Merrill, A.R. Cholix toxin, a novel ADP-ribosylating factor from Vibrio cholerae. J. Biol. Chem. 2008, 283, 10671–10678. [Google Scholar] [CrossRef] [PubMed]
- Purdy, A.E.; Balch, D.; Lizárraga-Partida, M.L.; Islam, M.S.; Martinez-Urtaza, J.; Huq, A.; Colwell, R.R.; Bartlett, D.H. Diversity and distribution of cholix toxin, a novel ADP-ribosylating factor from Vibrio cholerae. Environ. Microbiol. Rep. 2010, 2, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bravo, A.; Kilgore, P.B.; Andersson, J.A.; Blears, E.; Figueras, M.J.; Hasan, N.A.; Colwell, R.R.; Sha, J.; Chopra, A.K. T6SS and ExoA of flesh-eating Aeromonas hydrophila in peritonitis and necrotizing fasciitis during mono- And polymicrobial infections. Proc. Natl. Acad. Sci. USA 2019, 116, 24084–24092. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J. Understanding the mode of action of diphtheria toxin: A perspective on progress during the 20th century. Toxicon 2001, 39, 1793–1803. [Google Scholar] [CrossRef]
- Honjo, T.; Nishizuka, Y.; Hayaishi, O. Adenosine diphosphoribosylation of aminoacyl transferase II by diphtheria toxin. Cold Spring Harb. Symp. Quant. Biol. 1969, 34, 603–608. [Google Scholar] [CrossRef]
- Jørgensen, R.; Merrill, A.R.; Andersen, G.R. The life and death of translation elongation factor 2. Biochem. Soc. Trans. 2006, 34, 1. [Google Scholar] [CrossRef]
- Awasthi, S.P.; Asakura, M.; Chowdhury, N.; Neogi, S.B.; Hinenoya, A.; Golbar, H.M.; Yamate, J.; Arakawa, E.; Tada, T.; Ramamurthy, T.; et al. Novel cholix toxin variants, ADP-ribosylating toxins in Vibrio cholerae non-O1/non-O139 strains, and their pathogenicity. Infect. Immun. 2013, 81, 531–541. [Google Scholar] [CrossRef]
- Schirmeister, F.; Dieckmann, R.; Bechlars, S.; Bier, N.; Faruque, S.M.; Strauch, E. Genetic and phenotypic analysis of Vibrio cholerae non-O1, non-O139 isolated from German and Austrian patients. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 767–778. [Google Scholar] [CrossRef]
- Schwartz, K.; Hammerl, J.A.; Göllner, C.; Strauch, E. Environmental and Clinical Strains of Vibrio cholerae Non-O1, Non-O139 from Germany Possess Similar Virulence Gene Profiles. Front. Microbiol. 2019, 10, 733. [Google Scholar] [CrossRef]
- Hackbusch, S.; Wichels, A.; Gimenez, L.; Döpke, H.; Gerdts, G. Potentially human pathogenic Vibrio spp. in a coastal transect: Occurrence and multiple virulence factors. Sci. Total Environ. 2020, 707, 136113. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, N.; Kappe, E.; Gangl, A.; Schwartz, K.; Mayer-Scholl, A.; Hammerl, J.A.; Strauch, E. Phenotypic and genotypic properties of vibrio cholerae non-o1, non-o139 isolates recovered from domestic ducks in germany. Microorganisms 2020, 8, 1104. [Google Scholar] [CrossRef] [PubMed]
- Tangestani, M.G.; Alinezhad, J.; Khajeian, A.; Gharibi, S.; Haghighi, M.A. Identification of cholix toxin gene in vibrio cholerae non-O1/non-O139 isolated from diarrhea patients in Bushehr, Iran. Iran. J. Microbiol. 2020, 12, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Saidi, S.M.; Chowdhury, N.; Awasthi, S.P.; Asakura, M.; Hinenoya, A.; Iijima, Y.; Yamasaki, S. Prevalence of Vibrio cholerae O1 El Tor variant in a cholera-endemic zone of Kenya. J. Med. Microbiol. 2014, 63, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Villicaña, C.; Amarillas, L.; Soto-Castro, L.; Gómez-Gil, B.; Lizárraga-Partida, M.L.; León-Félix, J. Occurrence and abundance of pathogenic Vibrio species in raw oysters at retail seafood markets in northwestern Mexico. J. Food Prot. 2019, 82, 2094–2099. [Google Scholar] [CrossRef]
- Purdy, A.; Rohwer, F.; Edwards, R.; Azam, F.; Bartlett, D.H. A glimpse into the expanded genome content of Vibrio cholerae through identification of genes present in environmental strains. J. Bacteriol. 2005, 187, 2992–3001. [Google Scholar] [CrossRef]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef]
- Lugo, M.R.; Merrill, A.R. The Father, Son and Cholix Toxin: The Third Member of the DT Group Mono-ADP-Ribosyltransferase Toxin Family. Toxins 2015, 7, 2757–2772. [Google Scholar] [CrossRef]
- Fieldhouse, R.J.; Jørgensen, R.; Lugo, M.R.; Merrill, A.R. The 1.8 Å cholix toxin crystal structure in complex with NAD+ and evidence for a new kinetic model. J. Biol. Chem. 2012, 287, 21176–21188. [Google Scholar] [CrossRef]
- Turgeon, Z.; Jørgensen, R.; Visschedyk, D.; Edwards, P.R.; Legree, S.; McGregor, C.; Fieldhouse, R.J.; Mangroo, D.; Schapira, M.; Merrill, A.R. Newly discovered and characterized antivirulence compounds inhibit bacterial mono-ADP-ribosyltransferase toxins. Antimicrob. Agents Chemother. 2011, 55, 983–991. [Google Scholar] [CrossRef]
- Lugo, M.R.; Merrill, A.R. Pocket analysis of the full-length cholix toxin. An assessment of the structure-dynamics of the apo catalytic domain. J. Biomol. Struct. Dyn. 2015, 33, 2452–2468. [Google Scholar] [CrossRef] [PubMed]
- Lugo, M.R.; Merrill, A.R. A comparative structure-function analysis of active-site inhibitors of Vibrio cholerae cholix toxin. J. Mol. Recognit. 2015, 28, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Masuyer, G. Crystal structure of exotoxin a from aeromonas pathogenic species. Toxins 2020, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef]
- Kounnas, M.Z.; Morris, R.E.; Thompson, M.R.; FitzGerald, D.J.; Strickland, D.K.; Saelinger, C.B. The alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein binds and internalizes Pseudomonas exotoxin A. J. Biol. Chem. 1992, 267, 12420–12423. [Google Scholar] [PubMed]
- Yahiro, K.; Ogura, K.; Terasaki, Y.; Satoh, M.; Miyagi, S.; Terasaki, M.; Yamasaki, E.; Moss, J. Cholix toxin, an eukaryotic elongation factor 2 ADP-ribosyltransferase, interacts with Prohibitins and induces apoptosis with mitochondrial dysfunction in human hepatocytes. Cell. Microbiol. 2019, 21, e13033. [Google Scholar] [CrossRef]
- Ogura, K.; Yahiro, K.; Tsutsuki, H.; Nagasawa, S.; Yamasaki, S.; Moss, J.; Noda, M. Characterization of cholix toxin-induced apoptosis in HeLa cells. J. Biol. Chem. 2011, 286. [Google Scholar] [CrossRef]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef]
- Ogata, M.; Chaudhary, V.K.; Pastan, I.; FitzGerald, D.J. Processing of Pseudomonas exotoxin by a cellular protease results in the generation of a 37,000-Da toxin fragment that is translocated to the cytosol. J. Biol. Chem. 1990, 265, 20678–20685. [Google Scholar]
- Morlon-Guyot, J.; Méré, J.; Bonhoure, A.; Beaumelle, B. Processing of Pseudomonas aeruginosa exotoxin A is dispensable for cell intoxication. Infect. Immun. 2009, 77, 3090–3099. [Google Scholar] [CrossRef]
- Mckee, M.L.; Fitzgerald, D.J. Reduction of Furin-Nicked Pseudomonas Exotoxin A: An Unfolding Story. Biochemistry 1999, 38, 16507–16513. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Spooner, R.A.; Watson, P.D.; Murray, J.L.; Hodge, T.W.; Amessou, M.; Johannes, L.; Lord, J.M.; Roberts, L.M. Internalized pseudomonas exotoxin A can exploit multiple pathways to reach the endoplasmic reticulum. Traffic 2006, 7, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Michalska, M.; Wolf, P. Pseudomonas Exotoxin A: Optimized by evolution for effective killing. Front. Microbiol. 2015, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Hessler, J.L.; Kreitman, R.J. An early step in Pseudomonas exotoxin action is removal of the terminal lysine residue, which allows binding to the KDEL receptor. Biochemistry 1997, 36, 14577–14582. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Pastan, I. Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Biochem. J. 1995, 307, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.E.; Simpson, J.C.; Girod, A.; Pepperkok, R.; Roberts, L.M.; Lord, J.M. The KDEL retrieval system is exploited by Pseudomonas exotoxin A, but not by Shiga-like toxin-1, during retrograde transport from the Golgi complex to the endoplasmic reticulum. J. Cell Sci. 1999, 112 Pt 4, 467–475. [Google Scholar]
- Ogata, M.; Fryling, C.M.; Pastan, I.; FitzGerald, D.J. Cell-mediated cleavage of Pseudomonas exotoxin between Arg279 and Gly280 generates the enzymatically active fragment which translocates to the cytosol. J. Biol. Chem. 1992, 267, 25396–25401. [Google Scholar]
- Theuer, C.P.; Buchner, J.; FitzGerald, D.; Pastan, I. The N-terminal region of the 37-kDa translocated fragment of Pseudomonas exotoxin A aborts translocation by promoting its own export after microsomal membrane insertion. Proc. Natl. Acad. Sci. USA 1993, 90, 7774–7778. [Google Scholar] [CrossRef]
- Nowakowska-Gołacka, J.; Sominka, H.; Sowa-Rogozińska, N.; Słomińska-Wojewódzka, M. Toxins utilize the endoplasmic reticulum-associated protein degradation pathway in their intoxication process. Int. J. Mol. Sci. 2019, 20, 1307. [Google Scholar] [CrossRef]
- London, E.; Luongo, C.L. Domain-specific bias in arginine/lysine usage by protein toxins. Biochem. Biophys. Res. Commun. 1989, 160, 333–339. [Google Scholar] [CrossRef]
- Semenza, J.C.; Hardwick, K.G.; Dean, N.; Pelham, H.R.B. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell 1990, 61, 1349–1357. [Google Scholar] [CrossRef]
- Lewis, M.J.; Sweet, D.J.; Pelham, H.R.B. The ERD2 gene determines the specificity of the luminal ER protein retention system. Cell 1990, 61, 1359–1363. [Google Scholar] [CrossRef]
- Vitale, A.; Ceriotti, A.; Denecke, J. The role of the endoplasmic reticulum in protein synthesis, modification and intracellular transport. J. Exp. Bot. 1993, 44, 1417–1444. [Google Scholar] [CrossRef]
- Lewis, M.J.; Pelham, H.R.B. A human homologue of the yeast HDEL receptor. Nature 1990, 348, 162–163. [Google Scholar] [CrossRef]
- Raykhel, I.; Alanen, H.; Salo, K.; Jurvansuu, J.; Van, D.N.; Latva-Ranta, M.; Ruddock, L. A molecular specificity code for the three mammalian KDEL receptors. J. Cell Biol. 2007, 179, 1193–1204. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Lajoie, G.; Merrill, A.R. The role of the diphthamide-containing loop within eukaryotic elongation factor 2 in ADP-ribosylation by Pseudomonas aeruginosa exotoxin A. Biochem. J. 2008, 413, 163–174. [Google Scholar] [CrossRef]
- Su, X.; Lin, Z.; Lin, H. The biosynthesis and biological function of diphthamide. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 515–521. [Google Scholar] [CrossRef]
- Proud, C.G. Peptide-chain elongation in eukaryotes. Mol. Biol. Rep. 1994, 19, 161–170. [Google Scholar] [CrossRef]
- Uthman, S.; Bär, C.; Scheidt, V.; Liu, S.; ten Have, S.; Giorgini, F.; Stark, M.J.R.; Schaffrath, R. The Amidation Step of Diphthamide Biosynthesis in Yeast Requires DPH6, a Gene Identified through Mining the DPH1-DPH5 Interaction Network. PLoS Genet. 2013, 9, e1003334. [Google Scholar] [CrossRef]
- Ogura, K.; Terasaki, Y.; Miyoshi-Akiyama, T.; Terasaki, M.; Moss, J.; Noda, M.; Yahiro, K. Vibrio cholerae Cholix toxin-induced HepG2 cell death is enhanced by tumor necrosis factor-alpha through ROS and intracellular signal-regulated kinases. Toxicol. Sci. 2017, 156. [Google Scholar] [CrossRef]
- Iglewski, B.H.; Liu, P.V.; Kabat, D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect. Immun. 1977, 15, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Schümann, J.; Angermüller, S.; Bang, R.; Lohoff, M.; Tiegs, G. Acute hepatotoxicity of Pseudomonas aeruginosa exotoxin A in mice depends on T cells and TNF. J. Immunol. 1998, 161, 5745–5754. [Google Scholar] [PubMed]
- Schümann, J.; Mühlen, K.; Kiemer, A.K.; Vollmar, A.M.; Tiegs, G. Parenchymal, but not leukocyte, TNF receptor 2 mediates T cell-dependent hepatitis in mice. J. Immunol. 2003, 170, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Mühlen, K.A.; Schümann, J.; Wittke, F.; Stenger, S.; Van Rooijen, N.; Van Kaer, L.; Tiegs, G. NK cells, but not NKT cells, are involved in Pseudomonas aeruginosa exotoxin A-induced hepatotoxicity in mice. J. Immunol. 2004, 172, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Dhar, R.; Ghafoor, M.A.; Nasralah, A.Y. Unusual non-serogroup O1 Vibrio cholerae bacteremia associated with liver disease. J. Clin. Microbiol. 1989, 27, 2853–2855. [Google Scholar] [CrossRef] [PubMed]
- Petsaris, O.; Nousbaum, J.B.; Quilici, M.L.; Le Coadou, G.; Payan, C.; Abalain, M.L. Non-O1, non-O139 Vibrio cholerae bacteraemia in a cirrhotic patient. J. Med. Microbiol. 2010, 59, 1260–1262. [Google Scholar] [CrossRef][Green Version]
- Taverner, A.; MacKay, J.; Laurent, F.; Hunter, T.; Liu, K.; Mangat, K.; Song, L.; Seto, E.; Postlethwaite, S.; Alam, A.; et al. Cholix protein domain I functions as a carrier element for efficient apical to basal epithelial transcytosis. Tissue Barriers 2020, 8. [Google Scholar] [CrossRef]
- Adams, K.W.; Cooper, G.M. Rapid turnover of Mcl-1 couples translation to cell survival and apoptosis. J. Biol. Chem. 2007, 282, 6192–6200. [Google Scholar] [CrossRef]
- Peng, Y.T.; Chen, P.; Ouyang, R.Y.; Song, L. Multifaceted role of prohibitin in cell survival and apoptosis. Apoptosis 2015, 20, 1135–1149. [Google Scholar] [CrossRef]
- Anderson, C.J.; Kahl, A.; Qian, L.; Stepanova, A.; Starkov, A.; Manfredi, G.; Iadecola, C.; Zhou, P. Prohibitin is a positive modulator of mitochondrial function in PC12 cells under oxidative stress. J. Neurochem. 2018, 146, 235–250. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, M.; Mao, C.; Yu, F.; Wang, Y.; Xiao, R.; Jiang, C.; Zheng, L.; Xu, Q.; Zheng, M.; et al. COMP-prohibitin 2 interaction maintains mitochondrial homeostasis and controls smooth muscle cell identity. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Artal-Sanz, M.; Tavernarakis, N. Prohibitin and mitochondrial biology. Trends Endocrinol. Metab. 2009, 20, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, M.; Shepherd, B.R.; Suarez, Y.; Fernandez-Hernando, C.; Yu, J.; Pan, Y.; Acevedo, L.M.; Shadel, G.S.; Sessa, W.C. Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. J. Cell Biol. 2008, 180, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Xu, F.; Hou, T.; Sun, T.; Li, J.; Cheng, H.; Wang, X. Deficiency of PHB complex impairs respiratory supercomplex formation and activates mitochondrial flashes. J. Cell Sci. 2017, 130, 2620–2630. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Long, J.; Wang, J.; Haudek, S.B.; Overbeek, P.; Chang, B.H.J.; Schumacker, P.T.; Danesh, F.R. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012, 15, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Xie, M.; Shah, V.R.; Schneider, M.D.; Entman, M.L.; Wei, L.; Schwartz, R.J. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc. Natl. Acad. Sci. USA 2006, 103, 14495–14500. [Google Scholar] [CrossRef]
| Characteristics | Pseudomonas Exotoxin A | Cholix Toxin |
|---|---|---|
| Target | Diphthamide on eEF2 | Diphthamide on eEF2 |
| Enzymatic activity Km(NAD+) (μm)/kcat (s−1) | Mono-ADP-ribosylation 121 ± 21/13 ± 2 | Mono-ADP-ribosylation 45 ± 3/10 ± 3 |
| Host receptor | LRP1 | LRP1 and/or unknown cell surface receptor(s) |
| Translocation | Furin protease-dependent KDEL motif (REDLK)-dependent | Furin protease-dependent KDEL motif (RKDELK)-dependent |
| Administration into mice | Liver damage | Liver damage |
| PHB1 expression | No change | Decrease |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogura, K.; Yahiro, K.; Moss, J. Cell Death Signaling Pathway Induced by Cholix Toxin, a Cytotoxin and eEF2 ADP-Ribosyltransferase Produced by Vibrio cholerae. Toxins 2021, 13, 12. https://doi.org/10.3390/toxins13010012
Ogura K, Yahiro K, Moss J. Cell Death Signaling Pathway Induced by Cholix Toxin, a Cytotoxin and eEF2 ADP-Ribosyltransferase Produced by Vibrio cholerae. Toxins. 2021; 13(1):12. https://doi.org/10.3390/toxins13010012
Chicago/Turabian StyleOgura, Kohei, Kinnosuke Yahiro, and Joel Moss. 2021. "Cell Death Signaling Pathway Induced by Cholix Toxin, a Cytotoxin and eEF2 ADP-Ribosyltransferase Produced by Vibrio cholerae" Toxins 13, no. 1: 12. https://doi.org/10.3390/toxins13010012
APA StyleOgura, K., Yahiro, K., & Moss, J. (2021). Cell Death Signaling Pathway Induced by Cholix Toxin, a Cytotoxin and eEF2 ADP-Ribosyltransferase Produced by Vibrio cholerae. Toxins, 13(1), 12. https://doi.org/10.3390/toxins13010012





