Staphylococcal Enterotoxin C—An Update on SEC Variants, Their Structure and Properties, and Their Role in Foodborne Intoxications
Abstract
1. Introduction
2. SEC in Food Intoxication
SEC in Milk and Dairy Products
3. Superantigenic Activity of SEC
4. Physical and Chemical SEC Protein Properties
5. Genetic Localization and Regulation of SEC
6. Challenges and Future Prospects
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Food-Borne Disease Burden Epidemiology Reference Group. WHO Estimates of the Global Burden of Foodborne Diseases; WHO Press: Geneva, Switzerland, 2015. [Google Scholar]
- EFSA. The European Union One Health 2018 Zoonoses Report; EFSA Journal: Parma, Italy, 2019; Volume 17. [Google Scholar]
- Le Loir, Y.; Baron, F.; Gautier, M. Staphylococcus aureus and food poisoning. Genet. Mol. Res. 2003, 2, 63–76. [Google Scholar] [PubMed]
- Argudín, M.Á.; Mendoza, M.C.; Rodicio, M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Thomer, L.; Schneewind, O.; Missiakas, D. Pathogenesis of Staphylococcus aureus Bloodstream Infections. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 343–364. [Google Scholar] [CrossRef]
- Mccormick, J.K.; Yarwood, J.M.; Schlievert, P.M. Toxic shock syndrome and bacterial superantigens: An Update. Ann. Rev. Microbiol. 2001, 55, 77–104. [Google Scholar] [CrossRef]
- Junecko, J.M.; Zielinska, A.K.; Mrak, L.N.; Ryan, D.C.; Graham, J.W.; Smeltzer, M.S.; Lee, C.Y. Transcribing virulence in Staphylococcus aureus. World J. Clin. Infect. Dis. 2012, 2, 63. [Google Scholar] [CrossRef]
- Kane, T.L.; Carothers, K.E.; Lee, S.W. Virulence factor targeting of the bacterial pathogen Staphylococcus aureus for vaccine and therapeutics. Curr. Drug Targets 2018, 176, 139–148. [Google Scholar] [CrossRef]
- Massey, R.C.; Horsburgh, M.J.; Lina, G.; Höök, M.; Recker, M. The evolution and maintenance of virulence in Staphylococcus aureus: A role for host-to-host transmission? Nat. Rev. Microbiol. 2006, 4, 953–958. [Google Scholar] [CrossRef]
- Lacey, K.A.; Geoghegan, J.A.; McLoughlin, R.M. The role of Staphylococcus aureus virulence factors in skin infection and their potential as vaccine antigens. Pathogens 2016, 5, 22. [Google Scholar] [CrossRef]
- Fetsch, A.; Johler, S. Staphylococcus aureus as a foodborne pathogen. Curr. Clin. Microbiol. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Benkerroum, N. Staphylococcal enterotoxins and enterotoxin-like toxins with special reference to dairy products: An overview. Crit. Rev. Food Sci. Nutr. 2018, 58, 1943–1970. [Google Scholar] [CrossRef]
- Kong, C.; Neoh, H.M.; Nathan, S. Targeting Staphylococcus aureus toxins: A potential form of anti-virulence therapy. Toxins 2016, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Lina, G.; Bohach, G.A.; Nair, S.P.; Hiramatsu, K.; Jouvin-Marche, E.; Mariuzza, R. Standard nomenclature for the superantigens expressed by Staphylococcus. J. Infect. Dis. 2004, 189, 2334–2336. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Staphylococcal enterotoxins. Toxins 2010, 2, 2177–2197. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kotzin, B.; Herront, L.; Callahan, J.; Marrack, P.; Kappler, J. Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc. Natl. Acad. Sci. USA 1989, 86, 8941–8945. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Bohach, G.A.; Ohlendorf, D.H.; Stauffacher, C.V.; Leung, D.Y.M.; Murray, D.L.; Earhart, C.A.; Jablonski, L.M.; Hoffmann, M.L.; Chi, Y.I. Molecular structure of Staphylococcus and Streptococcus superantigens. J. Clin. Immunol. 1995, 15, 4–10. [Google Scholar] [CrossRef]
- Casman, E.P. Further serological studies of staphylococcal enterotoxin. J. Bacteriol. 1960, 79, 849–856. [Google Scholar] [CrossRef]
- Bergdoll, M.S.; Sugiyama, H.; Dack, G.M. Staphylococcal enterotoxin. I. Purification. Arch. Biochem. Biophys. 1959, 85, 62–69. [Google Scholar] [CrossRef]
- Bergdoll, M.S.; Borja, C.R.; Avena, R.M. Identification of a new enterotoxin as enterotoxin C. J. Bacteriol. 1965, 90, 1481–1485. [Google Scholar] [CrossRef]
- Casman, E.P.; Bergdoll, M.S.; Robinson, J. Designation of staphylococcal enterotoxins. J. Bacteriol. 1963, 85, 715–716. [Google Scholar] [CrossRef]
- Bergdoll, M.S. Monkey feeding test for staphylococcal enterotoxin. Methods Enzymol. 1988, 165, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Omoe, K.; Hu, D.-L.; Ono, H.K.; Shimizu, S.; Takahashi-Omoe, H.; Nakane, A.; Uchiyama, T.; Shinagawa, K.; Imanishi, K. Emetic Potentials of Newly Identified Staphylococcal Enterotoxin-Like Toxins. Infect. Immun. 2013, 81, 3627–3631. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Tamate, N.; Yamaguchi, K.; Makino, S.I. Mass outbreak of food poisoning disease caused by small amounts of staphylococcal enterotoxins A and H. Appl. Environ. Microbiol. 2005, 71, 2793–2795. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.J.; Mathisen, T.; Løvseth, A.; Omoe, K.; Qvale, K.S.; Loncarevic, S. An outbreak of staphylococcal food poisoning caused by enterotoxin H in mashed potato made with raw milk. FEMS Microbiol. Lett. 2005, 252, 267–272. [Google Scholar] [CrossRef]
- Hu, D.; Omoe, K.; Shimoda, Y.; Nakane, A.; Shinagawa, K. Induction of emetic response to staphylococcal enterotoxins in the house musk shrew (Suncus murinus). Infect. Immun. 2003, 71, 567–570. [Google Scholar] [CrossRef]
- Wright, A.; Andrews, P.L.R.; Titball, R.W. Induction of emetic, pyrexic, and behavioral effects of Staphylococcus aureus enterotoxin B in the ferret. Infect. Immun. 2000, 68, 2386–2389. [Google Scholar] [CrossRef]
- Johler, S.; Sihto, H.-M.; Macori, G.; Stephan, R. Sequence variability in staphylococcal enterotoxin genes seb, sec, and sed. Toxins 2016, 8, 169. [Google Scholar] [CrossRef]
- Marr, J.C.; Lyon, J.D.; Roberson, J.R.; Lupher, M.; Davis, W.C.; Bohach, G.A. Characterization of novel type C staphylococcal enterotoxins: Biological and evolutionary implications. Infect. Immun. 1993, 61, 4254–4262. [Google Scholar] [CrossRef]
- Fang, R.; Cui, J.; Cui, T.; Guo, H.; Ono, H.K.; Park, C.H.; Okamura, M.; Nakane, A.; Hu, D.L. Staphylococcal enterotoxin C is an important virulence factor for mastitis. Toxins 2019, 11, 141. [Google Scholar] [CrossRef]
- Niskanen, A.; Koranen, L.; Roine, K. Staphylococcal enterotoxin and thermonuclease production during induced bovine mastitis and the clinical reaction of enterotoxin in udders. Infect. Immun. 1978, 19, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.R. Livestock-associated Staphylococcus aureus: Origin, evolution and public health threat. Trends Microbiol. 2012, 20, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Orden, J.A.; Goyache, J.; Hernández, J.; Doménech, A.; Suárez, G.; Gómez-Lucia, E. Detection of enterotoxins and TSST-1 secreted by Staphylococcus aureus isolated from ruminant mastitis. Comparison of ELISA and immunoblot. J. Appl. Bacteriol. 1992, 72, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Franck, K.T.; Gumpert, H.; Olesen, B.; Larsen, A.R.; Petersen, A.; Bangsborg, J.; Albertsen, P.; Westh, H.; Bartels, M.D. Staphylococcal aureus Enterotoxin C and enterotoxin-like L associated with post-partum mastitis. Front. Microbiol. 2017, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Kinney, K.J.; Tran, P.M.; Gibson-Corley, K.N.; Forsythe, A.N.; Kulhankova, K.; Salgado-Pabón, W. Staphylococcal enterotoxin C promotes Staphylococcus aureus infective endocarditis independent of superantigen activity. bioRxiv 2019. [Google Scholar] [CrossRef]
- Bunikowski, R.; Mielke, M.E.A.; Skarabis, H.; Worm, M.; Anagnostopoulos, I.; Kolde, G.; Wahn, U.; Renz, H. Evidence for a disease-promoting effect of Staphylococcus aureus-derived exotoxins in atopic dermatitis. J. Allergy Clin. Immunol. 2000, 105, 814–819. [Google Scholar] [CrossRef]
- Spaulding, A.R.; Salgado-Pabón, W.; Kohler, P.L.; Horswill, A.R.; Leung, D.Y.M.M.; Schlievert, P.M. Staphylococcal and streptococcal superantigen exotoxins. Clin. Microbiol. Rev. 2013, 26, 422–447. [Google Scholar] [CrossRef]
- Edwards, V.M.; Deringer, J.R.; Callantine, S.D.; Deobald, C.F.; Berger, P.H.; Kapur, V.; Stauffacher, C.V.; Bohach, G.A. Characterization of the canine type C enterotoxin produced by Staphylococcus intermedius pyoderma isolates. Infect. Immun. 1997, 65, 2346–2352. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Cunha, M.L.R.S. Staphylococcal enterotoxins: Molecular aspects and detection methods. J. Public Health Epidemiol. 2010, 2, 29–42. [Google Scholar]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef]
- Fries, B.C.; Varshney, A.K. Bacterial toxins—Staphylococcal enterotoxin B. Microb. Spectr. 2013, 1, 1–21. [Google Scholar] [CrossRef]
- Krakauer, T.; Stiles, B.G. The staphylococcal enterotoxin (SE) family: SEB and siblings. Virulence 2013, 4, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Susilo, Y.B. Staphylococcus aureus Toxins—Influence on Food Safety and Animal Health. Ph.D. Thesis, Lund University, Lund, Sweden, 2016. [Google Scholar]
- Fisher, E.L.; Otto, M.; Cheung, G.Y.C.C. Basis of virulence in enterotoxin-mediated staphylococcal food poisoning. Front. Microbiol. 2018, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Betley, M.J.; Mekalanos, J.J. Staphylococcal enterotoxin A is encoded by phage. Science 1985, 229, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Betley, M.J.; Mekalanos, J.J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J. Bacteriol. 1988, 170, 34–41. [Google Scholar] [CrossRef]
- Kuroda, M.; Ohta, T.; Uchiyama, I.; Baba, T.; Yuzawa, H.; Kobayashi, I.; Cui, L.; Oguchi, A.; Aoki, K.; Nagai, Y.; et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 2001, 357, 1225–1240. [Google Scholar] [CrossRef]
- Borst, D.W.; Betley, M.J. Phage-associated differences in staphylococcal enterotoxin A gene (sea) expression correlate with sea allele class. Infect. Immun. 1994, 62, 113–118. [Google Scholar] [CrossRef]
- Shafer, W.M.; Iandolo, J.J. Chromosomal locus for staphylococcal enterotoxin B. Infect. Immun. 1978, 20, 273–278. [Google Scholar] [CrossRef]
- Stevens, M.J.A.; Stephan, R.; Johler, S. Complete and assembled genome sequence of Staphylococcus aureus RKI4, a food-poisoning strain exhibiting a novel S. aureus pathogenicity island carrying seb. Genome Announc. 2015, 3, 2015. [Google Scholar] [CrossRef]
- Shalita, Z.; Hertman, I.; Sarid, S. Isolation and characterization of a plasmid involved with enterotoxin B production in Staphylococcus aureus. J. Bacteriol. 1977, 129, 317–325. [Google Scholar] [CrossRef]
- Altboum, Z.; Hertman, I.; Sarid, S. Penicillinase plasmid-linked genetic determinants for enterotoxins B and C1 production in Staphylococcus aureus. Infect. Immun. 1985, 47, 514–521. [Google Scholar] [CrossRef]
- Sato’o, Y.; Omoe, K.; Ono, H.K.; Nakane, A.; Hu, D.-L. A novel comprehensive analysis method for Staphylococcus aureus pathogenicity islands. Microbiol. Immunol. 2013, 57, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Betley, M.J.; Bergdoll, M.S. Staphylococcal enterotoxin type C genes not associated with extrachromosomal DNA. In Proceedings of the Annual Meeting ASM, Dallas, TX, USA, 1–6 March 1981; Volume 38, p. 49. [Google Scholar]
- Novick, R.P.; Christie, G.E.; Penades, J.R.; Penadés, J.R. The phage-related chromosomal islands of Gram-positive bacteria. Nat. Rev. Microbiol. 2010, 8, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.R.; Monday, S.R.; Foster, T.J.; Bohach, G.A.; Hartigan, P.J.; Meaney, W.J.; Smyth, C.J. Characterization of a putative pathogenicity island from bovine Staphylococcus aureus encoding multiple superantigens. J. Bacteriol. 2001, 183, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Bergdoll, M.S.; Robbins, R.N.; Weiss, K.; Borja, C.R.; Huang, Y.; Chu, F.S. The staphylococcal enterotoxins: Similarities. Contrib. Microbiol. Immunol. 1973, 1, 390–396. [Google Scholar]
- Carolyn, J.H.; Gregory, A.; Bohach, S.P.H. Nucleotide sequence of the staphlyococcal enterotoxin C3 gene: Sequence comparison of all three Type C staphylococcal enterotoxins. Mol. Gen. Genet. 1990, 3, 329–333. [Google Scholar]
- Reiser, R.F.; Robbins, R.N.; Noleto, A.L.; Khoe, G.P.; Bergdoll, M.S. Identification, purification, and some physicochemical properties of staphylococcal enterotoxin C3. Infect. Immun. 1984, 45, 625–630. [Google Scholar] [CrossRef]
- Baba, T.; Takeuchi, F.; Kuroda, M.; Yuzawa, H.; Aoki, K.I.; Oguchi, A.; Nagai, Y.; Iwama, N.; Asano, K.; Naimi, T.; et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 2002, 359, 1819–1827. [Google Scholar] [CrossRef]
- Viana, D.; Blanco, J.; Tormo-Más, M.Á.; Selva, L.; Guinane, C.M.; Baselga, R.; Corpa, J.M.; Lasa, Í.; Novick, R.P.; Fitzgerald, J.R.; et al. Adaptation of Staphylococcus aureus to ruminant and equine hosts involves SaPI-carried variants of von Willebrand factor-binding protein. Mol. Microbiol. 2010, 77, 1583–1594. [Google Scholar] [CrossRef]
- Hajek, V. Identification of enterotoxigenic staphylococci from sheep and sheep cheese. Appl. Environ. Microbiol. 1978, 35, 264–268. [Google Scholar] [CrossRef]
- Casman, E.P.; Bennett, R.W.; Dorsey, A.E.; Issa, J.A. Identification of a fourth staphylococcal enterotoxin, enterotoxin D. J. Bacteriol. 1967, 94, 1875–1882. [Google Scholar] [CrossRef]
- Bayles, K.W.; Iandolo, J.J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J. Bacteriol. 1989, 171, 4799–4806. [Google Scholar] [CrossRef]
- Chang, H.C.; Bergdoll, M.S. Purification and some physicochemical properties of staphylococcal enterotoxin D. Biochemistry 1979, 18, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Bergdoll, M.S.; Borja, C.R.; Robbins, R.N.; Weiss, K.F. Identification of enterotoxin E. Infect. Immun. 1971, 4, 593–595. [Google Scholar] [CrossRef]
- Couch, J.L.; Soltis, M.T.; Betley, M.J. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J. Bacteriol. 1988, 170, 2954–2960. [Google Scholar] [CrossRef] [PubMed]
- Jarraud, S.; Peyrat, M.A.; Lim, A.; Tristan, A.; Bes, M.; Mougel, C.; Etienne, J.; Vandenesch, F.; Bonneville, M.; Lina, G. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 2001, 166, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Jarraud, S.; Peyrat, M.A.; Lim, A.; Tristan, A.; Bes, M.; Mougel, C.; Etienne, J.; Vandenesch, F.; Bonneville, M.; Lina, G. Correction. J. Immunol. 2001, 166, 4260. [Google Scholar]
- Sumby, P.; Waldor, M.K. Transcription of the toxin genes present within the staphylococcal phage φSa3ms is intimately linked with the phage’s life cycle. J. Bacteriol. 2003, 185, 6841–6851. [Google Scholar] [CrossRef]
- Abe, J.; Ito, Y.; Onimaru, M.; Kohsaka, T.; Takeda, T. Characterization and distribution of a new enterotoxin-related superantigen produced by Staphylococcus aureus. Microbiol. Immunol. 2000, 44, 79–88. [Google Scholar] [CrossRef]
- Noto, M.J.; Archer, G.L. A subset of Staphylococcus aureus strains harboring staphylococcal cassette chromosome mec (SCCmec) type IV is deficient in CcrAB-mediated SCCmec excision. Antimicrob. Agents Chemother. 2006, 50, 2782–2788. [Google Scholar] [CrossRef]
- Su, Y.C.; Wong, A.C. Identification and purification of a new staphylococcal enterotoxin, H. Appl. Environ. Microbiol. 1995, 61, 1438–1443. [Google Scholar] [CrossRef]
- Ren, K.; Bannan, J.D.; Pancholi, V.; Cheung, A.L.; Robbins, J.C.; Fischetti, V.A.; Zabriskie, J.B. Characterization and biological properties of a new staphylococcal exotoxin. J. Exp. Med. 1994, 180, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Munson, S.H.; Tremaine, M.T.; Betley, M.J.; Welch, R.A. Identification and characterization of staphylococcal enterotoxin types G and I from Staphylococcus aureus. Infect. Immun. 1998, 66, 3337–3348. [Google Scholar] [CrossRef] [PubMed]
- Omoe, K.; Hu, D.L.; Takahashi-Omoe, H.; Nakane, A.; Shinagawa, K. Identification and characterization of a new staphylococcal enterotoxin-related putative toxin encoded by two kinds of plasmids. Infect. Immun. 2003, 71, 6088–6094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Iandolo, J.J.; Stewart, G.C. The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej). FEMS Microbiol. Lett. 1998, 168, 227–233. [Google Scholar] [CrossRef]
- Orwin, P.M.; Leung, D.Y.M.; Donahue, H.L.; Novick, R.P.; Schlievert, P.M. Biochemical and biological properties of staphylococcal enterotoxin K. Infect. Immun. 2001, 69, 360–366. [Google Scholar] [CrossRef]
- Ono, H.K.; Hirose, S.; Naito, I.; Sato’o, Y.; Asano, K.; Hu, D.-L.; Omoe, K.; Nakane, A. The emetic activity of staphylococcal enterotoxins, SEK, SEL, SEM, SEN and SEO in a small emetic animal model, the house musk shrew. Microbiol. Immunol. 2017, 61, 12–16. [Google Scholar] [CrossRef]
- Orwin, P.M.; Fitzgerald, J.R.; Leung, D.Y.M.; Gutierrez, J.A.; Bohach, G.A.; Schlievert, P.M. Characterization of Staphylococcus aureus enterotoxin L. Infect. Immun. 2003, 71, 2916–2919. [Google Scholar] [CrossRef]
- Yarwood, J.M.; McCormick, J.K.; Paustian, M.L.; Orwin, P.M.; Kapur, V.; Schlievert, P.M. Characterization and expression analysis of Staphylococcus aureus pathogenicity island 3. Implications for the evolution of staphylococcal pathogenicity islands. J. Biol. Chem. 2002, 277, 13138–13147. [Google Scholar] [CrossRef]
- Omoe, K.; Imanishi, K.; Hu, D.-L.L.; Kato, H.; Fugane, Y.; Abe, Y.; Hamaoka, S.; Watanabe, Y.; Nakane, A.; Uchiyama, T.; et al. Characterization of novel staphylococcal enterotoxin-like toxin type P. Infect. Immun. 2005, 73, 5540–5546. [Google Scholar] [CrossRef]
- Hu, D.-L.; Ono, H.K.; Isayama, S.; Okada, R.; Okamura, M.; Lei, L.C.; Liu, Z.S.; Zhang, X.-C.; Liu, M.Y.; Cui, J.C.; et al. Biological characteristics of staphylococcal enterotoxin Q and its potential risk for food poisoning. J. Appl. Microbiol. 2017, 122, 1672–1679. [Google Scholar] [CrossRef]
- Orwin, P.M.; Leung, D.Y.; Tripp, T.J.; Bohach, G.A.; Earhart, C.A.; Ohlendorf, D.H.; Schlievert, P.M. Characterization of a novel staphylococcal enterotoxin-like superantigen, a member of the group V subfamily of pyrogenic toxins. Biochemistry 2002, 41, 14033–14040. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.K.; Omoe, K.; Imanishi, K.; Iwakabe, Y.; Hu, D.L.; Kato, H.; Saito, N.; Nakane, A.; Uchiyama, T.; Shinagawa, K. Identification and characterization of two novel staphylococcal enterotoxins, types S and T. Infect. Immun. 2008, 76, 4999–5005. [Google Scholar] [CrossRef] [PubMed]
- Letertre, C.; Perelle, S.; Dilasser, F.; Fach, P. Identification of a new putative enterotoxin SEU encoded by the egc cluster of Staphylococcus aureus. J. Appl. Microbiol. 2003, 95, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.Y.; Jarraud, S.; Lemercier, B.; Cozon, G.; Echasserieau, K.; Etienne, J.; Gougeon, M.-L.; Lina, G.; Vandenesch, F. Staphylococcal enterotoxin-like toxins U2 and V, two new staphylococcal superantigens arising from recombination within the enterotoxin gene cluster. Infect. Immun. 2006, 74, 4724–4734. [Google Scholar] [CrossRef]
- Okumura, K.; Shimomura, Y.; Murayama, S.; Yagi, J.; Ubukata, K.; Kirikae, T.; Miyoshi-Akiyama, T. Evolutionary paths of streptococcal and staphylococcal superantigens. BMC Genom. 2012, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.J.; Seo, K.S.; Cartwright, R.A.; Connelley, T.; Chuang-Smith, O.N.; Merriman, J.A.; Guinane, C.M.; Park, J.Y.; Bohach, G.A.; Schlievert, P.M.; et al. A novel core genome-encoded superantigen contributes to lethality of community-associated MRSA necrotizing pneumonia. PLoS Pathog. 2011, 7, e1002271. [Google Scholar] [CrossRef]
- Ono, H.K.; Sato’o, Y.; Narita, K.; Naito, I.; Hirose, S.; Hisatsune, J.; Asano, K.; Hu, D.L.; Omoe, K.; Sugai, M.; et al. Identification and characterization of a novel staphylococcal emetic toxin. Appl. Environ. Microbiol. 2015, 81, 7034–7040. [Google Scholar] [CrossRef]
- Wilson, G.J.; Tuffs, S.W.; Wee, B.A.; Seo, K.S.; Park, N.; Connelley, T.; Guinane, C.M.; Morrison, W.I.; Fitzgerald, J.R. Bovine Staphylococcus aureus superantigens stimulate the entire T cell repertoire of cattle. Infect. Immun. 2018, 86, 1–16. [Google Scholar] [CrossRef]
- Novick, R.P.; Ram, G. Staphylococcal pathogenicity islands—Movers and shakers in the genomic firmament. Curr. Opin. Microbiol. 2017, 38, 197–204. [Google Scholar] [CrossRef]
- Tuffs, S.W.; Haeryfar, S.M.M.; McCormick, J.K. Manipulation of innate and adaptive immunity by staphylococcal superantigens. Pathogens 2018, 7, 53. [Google Scholar] [CrossRef]
- Maina, E.K.; Hu, D.L.; Tsuji, T.; Omoe, K.; Nakane, A. Staphylococcal enterotoxin A has potent superantigenic and emetic activities but not diarrheagenic activity. Int. J. Med. Microbiol. 2012, 302, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S. Studies on the relationship between gastric acidity and the development of MRSA. Especially for the prevention of MRSA enterocolitis. J. Nippon Med. Sch. 1994, 61, 563–571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Acton, D.S.; Tempelmans Plat-Sinnige, M.J.; Van Wamel, W.; De Groot, N.; Van Belkum, A. Intestinal carriage of Staphylococcus aureus: How does its frequency compare with that of nasal carriage and what is its clinical impact? Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.F.; Kellum, M.E.; Porter, S.S.; Bell, M.; Schaffner, W. An outbreak of community-acquired foodborne illness caused by methicillin-resistant Staphylococcus aureus. Emerg. Infect. Dis. 2002, 8, 82–84. [Google Scholar] [CrossRef]
- Veras, J.F.; do Carmo, L.S.; Tong, L.C.; Shupp, J.W.; Cummings, C.; dos Santos, D.A.; Cerqueira, M.M.O.P.; Cantini, A.; Nicoli, J.R.; Jett, M. A study of the enterotoxigenicity of coagulase-negative and coagulase-positive staphylococcal isolates from food poisoning outbreaks in Minas Gerais, Brazil. Int. J. Infect. Dis. 2008, 12, 410–415. [Google Scholar] [CrossRef]
- Wieneke, A.A.; Roberts, D.; Gilbert, R.J. Staphylococcal food poisoning in the United Kingdom, 1969–1990. Epidemiol. Infect. 1993, 110, 519–531. [Google Scholar] [CrossRef]
- Schelin, J.; Wallin-Carlquist, N.; Cohn, M.T.; Lindqvist, R.; Barker, G.C.; Rådström, P. The formation of Staphylococcus aureus enterotoxin in food environments and advances in risk assessment. Virulence 2011, 2, 580–592. [Google Scholar] [CrossRef]
- Hu, D.L.; Nakane, A. Mechanisms of staphylococcal enterotoxin-induced emesis. Eur. J. Pharmacol. 2014, 722, 95–107. [Google Scholar] [CrossRef]
- Edwards, L.A.; O’Neill, C.; Furman, M.A.; Hicks, S.; Torrente, F.; Pérez-Machado, M.; Wellington, E.M.; Phillips, A.D.; Murch, S.H. Enterotoxin-producing staphylococci cause intestinal inflammation by a combination of direct epithelial cytopathy and superantigen-mediated T-cell activation. Inflamm. Bowel Dis. 2012, 18, 624–640. [Google Scholar] [CrossRef]
- Ono, H.K.; Nishizawa, M.; Yamamoto, Y.; Hu, D.L.; Nakane, A.; Shinagawa, K.; Omoe, K. Submucosal mast cells in the gastrointestinal tract are a target of staphylococcal enterotoxin type A. FEMS Immunol. Med. Microbiol. 2012, 64, 392–402. [Google Scholar] [CrossRef]
- Ono, H.K.; Hirose, S.; Narita, K.; Sugiyama, M.; Asano, K.; Hu, D.L.; Nakane, A. Histamine release from intestinal mast cells induced by staphylococcal enterotoxin a (SEA) evokes vomiting reflex in common marmoset. PLoS Pathog. 2019, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wells, U.; Ravenscroft, M.; Bhandari, P.; Andrews, P.L.R. Serotonin and serotonergic drugs in emesis. In Serotonin: From Cell Biology to Pharmacology and Therapeutics; Vanhoutte, P.M., Saxena, P.R., Paoletti, R., Brunello, N., Jackson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 179–186. ISBN 978-94-011-1920-7. [Google Scholar]
- Sugiyama, H.; Hayama, T. Abdominal viscera as site of emetic action for staphylococcal enterotoxin in the monkey. J. Infect. Dis. 1965, 115, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Schlievert, P.M.; Jablonski, L.M.; Roggiani, M.; Sadler, I.; Callantine, S.; Mitchell, D.T.; Ohlendorf, D.H.; Bohach, G.A. Pyrogenic toxin superantigen site specificity in toxic shock syndrome and food poisoning in animals. Infect. Immun. 2000, 68, 3630–3634. [Google Scholar] [CrossRef] [PubMed]
- Aragon-Alegro, L.C.; Konta, E.M.; Suzuki, K.; Silva, M.G.; Júnior, A.F.; Rall, R.; Rall, V.L.M. Occurrence of coagulase-positive Staphylococcus in various food products commercialized in Botucatu, SP, Brazil and detection of toxins from food and isolated strains. Food Control 2007, 18, 630–634. [Google Scholar] [CrossRef]
- Lindqvist, R.; Sylve, S. Quantitative microbial risk assessment exemplified by Staphylococcus aureus in unripened cheese made from raw milk. Int. J. Food Microbiol. 2002, 78, 155–170. [Google Scholar] [CrossRef]
- Scherrer, D.; Corti, S.; Muehlherr, J.E.; Zweifel, C.; Stephan, R. Phenotypic and genotypic characteristics of Staphylococcus aureus isolates from raw bulk-tank milk samples of goats and sheep. Vet. Microbiol. 2004, 101, 101–107. [Google Scholar] [CrossRef]
- Rosengren, Å.; Fabricius, A.; Guss, B.; Sylvén, S.; Lindqvist, R. Occurrence of foodborne pathogens and characterization of Staphylococcus aureus in cheese produced on farm-dairies. Int. J. Food Microbiol. 2010, 144, 263–269. [Google Scholar] [CrossRef]
- Artursson, K.; Schelin, J.; Thisted Lambertz, S.; Hansson, I.; Olsson Engvall, E. Foodborne pathogens in unpasteurized milk in Sweden. Int. J. Food Microbiol. 2018, 284, 120–127. [Google Scholar] [CrossRef]
- Valihrach, L.; Alibayov, B.; Demnerova, K. Production of staphylococcal enterotoxin C in milk. Int. Dairy J. 2013, 30, 103–107. [Google Scholar] [CrossRef]
- Necidova, L.; Bogdanovicova, K.; Harustiakova, D.; Bartova, K. Short communication: Pasteurization as a means of inactivating staphylococcal enterotoxins A, B, and C in milk. J. Dairy Sci. 2016, 99, 8638–8643. [Google Scholar] [CrossRef]
- Necidová, L.; Bursová, Š.; Haruštiaková, D.; Bogdanovičová, K.; Lačanin, I. Effect of heat treatment on activity of staphylococcal enterotoxins of type A, B, and C in milk. J. Dairy Sci. 2019, 102, 3924–3932. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.; Butler, F.; Jordan, K. Modelling production of S. aureus enterotoxin Cbovine in milk, and its production during cheesemaking. Dairy Sci. Technol. 2015, 95, 747–757. [Google Scholar] [CrossRef]
- Silva, G.O.; Castro, R.D.; Oliveira, L.G.; Sant’Anna, F.M.; Barbosa, C.D.; Sandes, S.H.C.; Silva, R.S.; Resende, M.F.S.; Lana, A.M.Q.; Nunes, A.C.; et al. Viability of Staphylococcus aureus and expression of its toxins (SEC and TSST-1) in cheeses using Lactobacillus rhamnosus D1 or Weissella paramesenteroides GIR16L4 or both as starter cultures. J. Dairy Sci. 2020, 103, 4100–4108. [Google Scholar] [CrossRef] [PubMed]
- Johler, S.; Zurfluh, K.; Stephan, R. Tracing and inhibiting growth of Staphylococcus aureus in barbecue cheese production after product recall. J. Dairy Sci. 2016, 99, 3345–3350. [Google Scholar] [CrossRef] [PubMed]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and staphylococcal food-borne disease: An ongoing challenge in public health. Biomed Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef]
- Jie, K.G.; Jiang, H.; Sun, L.; Wang, H.R.; Zheng, Y.L.; Li, Y.; Jiang, Y.Q. The pilot study of anti-tumor effects versus immunosuppression of staphylococcal enterotoxin C. Cancer Biol. Ther. 2007, 6, 1584–1591. [Google Scholar] [CrossRef][Green Version]
- Cho, S.; Swaminathan, C.P.; Yang, J.; Kerzic, M.C.; Guan, R.; Kieke, M.C.; Kranz, D.M.; Mariuzza, R.A.; Sundberg, E.J. Structural basis of affinity maturation and intramolecular cooperativity in a protein-protein interaction. Structure 2005, 13, 1775–1787. [Google Scholar] [CrossRef]
- Deringer, J.R.; Ely, R.J.; Stauffacher, C.V.; Bohach, G.A. Subtype-specific interactions of type C staphylococcal enterotoxins with the T-cell receptor. Mol. Microbiol. 1996, 22, 523–534. [Google Scholar] [CrossRef]
- Langley, R.J.; Fraser, J.D.; Proft, T. Bacterial Superantigens and Superantigen-Like Toxins; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9780128005897. [Google Scholar]
- Fields, B.A.; Malchiodi, E.L.; Li, H.; Ysern, X.; Stauffacher, C.V.; Schlievert, P.M.; Karjalainen, K.; Mariuzza, R.A. Crystal structure of a T-cell receptor β-chain complexed with a superantigen. Nature 1996, 384, 188–192. [Google Scholar] [CrossRef]
- Deringer, J.R.; Ely, R.J.; Monday, S.R.; Stauffacher, C.V.; Bohach, G.A. Vβ-dependent stimulation of bovine and human T cells by host-specific staphylococcal enterotoxins. Infect. Immun. 1997, 65, 4048–4054. [Google Scholar] [CrossRef]
- Lamphear, J.G.; Bohach, G.A.; Rich, R.R. Structural dichotomy of staphylococcal enterotoxin C superantigens leading to MHC class II-independent activation of T lymphocytes. J. Immunol. 1998, 160, 2107–2114. [Google Scholar]
- Kaempfer, R.; Arad, G.; Levy, R.; Hillman, D.; Nasie, I.; Rotfogel, Z. CD28: Direct and critical receptor for superantigen toxins. Toxins 2013, 5, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Arad, G.; Levy, R.; Nasie, I.; Hillman, D.; Rotfogel, Z.; Barash, U.; Supper, E.; Shpilka, T.; Minis, A.; Kaempfer, R. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol. 2011, 9, e1001149. [Google Scholar] [CrossRef] [PubMed]
- Shands, K.N.; Schmid, G.P.; Dan, B.B.; Blum, D.; Guidotti, R.J.; Hargrett, N.T.; Anderson, R.L.; Hill, D.L.; Broome, C.V.; Band, J.D.; et al. Toxic-Shock Syndrome in menstruating women. N. Engl. J. Med. 1980, 303, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Rizkallah, M.F.; Tolaymat, A.; Martinez, J.S.; Schlievert, P.M.; Ayoub, E.M. Toxic shock syndrome caused by a strain of Staphylococcus aureus that produces enterotoxin C but not toxic shock syndrome toxin-1. Am. J. Dis. Child. 1989, 143, 848–849. [Google Scholar] [CrossRef]
- Bartlett, A.H.; Hulten, K.G. Staphylococcus aureus pathogenesis: Secretion systems, adhesins, and invasins. Pediatr. Infect. Dis. J. 2010, 29, 860–861. [Google Scholar] [CrossRef]
- Christianson, K.K.; Tweten, R.K.; Iandolo, J.J. Transport and processing of staphylococcal enterotoxin A. Appl. Environ. Microbiol. 1985, 50, 696–697. [Google Scholar] [CrossRef]
- Tweten, R.K.; Iandolo, J.J. Purification and partial characterization of a putative precursor to staphylococcal enterotoxin B. Infect. Immun. 1981, 34, 900–907. [Google Scholar] [CrossRef]
- Tweten, R.K.; Iandolo, J.J. Transport and processing of staphylococcal enterotoxin B. J. Bacteriol. 1983, 153, 297–303. [Google Scholar] [CrossRef]
- Papageorgiou, A.C.; Acharya, K.R.; Shapiro, R.; Passalacqua, E.F.; Brehm, R.D.; Tranter, H.S. Crystal structure of the superantigen enterotoxin C2 from Staphylococcus aureus reveals a zinc-binding site. Structure 1995, 3, 769–779. [Google Scholar] [CrossRef]
- Baker, M.; Gutman, D.M.; Papageorgiou, A.C.; Collins, C.M.; Acharya, K.R. Structural features of a zinc binding site in the superantigen strepococcal pyrogenic exotoxin A (SpeA1): Implications for MHC class II recognition. Protein Sci. 2001, 10, 1268–1273. [Google Scholar] [CrossRef]
- Papageorgiou, A.C.; Baker, M.D.; McLeod, J.D.; Goda, S.K.; Manzotti, C.N.; Sansom, D.M.; Tranter, H.S.; Acharya, K.R. Identification of a secondary zinc-binding site in staphylococcal enterotoxin C2. J. Biol. Chem. 2004, 279, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.-I.; Sadler, I.; Jablonski, L.M.; Callantine, S.D.; Deobald, C.F.; Stauffacher, C.V.; Bohach, G.A. Zinc-mediated dimerization and its effect on activity and conformation of staphylococcal enterotoxin type C. J. Biol. Chem. 2002, 277, 22839–22846. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, T.; Accolla, R.S.; MacDonald, H.R. Different staphylococcal enterotoxins bind preferentially to distinct major histocompatibility complex class II isotypes. Eur. J. Immunol. 1989, 19, 2171–2174. [Google Scholar] [CrossRef] [PubMed]
- Jardetzky, T.S.; Brown, J.H.; Gorga, J.C.; Stern, L.J.; Urban, R.G.; Chi, Y.; Stauffacher, C.; Strominger, J.L.; Wiley, D.C. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature 1994, 368, 711–718. [Google Scholar] [CrossRef]
- Thomas, D.; Chou, S.; Dauwalder, O.; Lina, G. Diversity in Staphylococcus aureus enterotoxins. In Superantigens and Superallergens; Karger: Basel, Switzerland, 2007; Volume 93, pp. 24–41. [Google Scholar]
- Hovde, C.J.; Marr, J.C.; Hoffmann, M.L.; Hackett, S.P.; Chi, Y.Y.-I.; Crum, K.K.; Stevens, D.L.; Stauffacher, C.V.; Bohach, G.A. Investigation of the role of the disulphide bond in the activity and structure of staphylococcal enterotoxin C1. Mol. Microbiol. 1994, 13, 897–909. [Google Scholar] [CrossRef]
- Hoffman, M.; Tremaine, M.; Mansfield, J.; Betley, M. Biochemical and mutational analysis of the histidine residues of staphylococcal enterotoxin A. Infect. Immun. 1996, 64, 885–890. [Google Scholar] [CrossRef]
- Harris, T.O.; Grossman, D.; Kappler, J.W.; Marrack, P.; Rich, R.R.; Betley, M.J. Lack of complete correlation between emetic and T-cell-stimulatory activities of staphylococcal enterotoxins. Infect. Immun. 1993, 61, 3175–3183. [Google Scholar] [CrossRef]
- Maina, E.K.; Hu, D.L.; Asano, K.; Nakane, A. Inhibition of emetic and superantigenic activities of staphylococcal enterotoxin A by synthetic peptides. Peptides 2012, 38, 1–7. [Google Scholar] [CrossRef]
- Otero, A.; Garcia, M.L.C.; Garcia, M.L.C.; Moreno, B.; Bergdoll, M.S. Production of staphylococcal enterotoxins C1 and C2 and thermonuclease throughout the growth cycle. Appl. Environ. Microbiol. 1990, 56, 555–559. [Google Scholar] [CrossRef]
- Regassa, L.B.; Betley, M.J. High sodium chloride concentrations inhibit staphylococcal enterotoxin C gene (sec) expression at the level of sec mRNA. Infect. Immun. 1993, 61, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
- Regassa, L.B.; Couch, J.L.; Betley, M.J. Steady-state staphylococcal enterotoxin type C mRNA is affected by a product of the accessory gene regulator (agr) and by glucose. Infect. Immun. 1991, 59, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.W.; Zhang, S.; Stewart, G.C. Accessory gene regulator control of staphyloccoccal enterotoxin D gene expression. J. Bacteriol. 2004, 186, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.Y.; Ching, W.T.; Stewart, G.C. Regulation of rot expression in Staphylococcus aureus. J. Bacteriol. 2008, 190, 546–554. [Google Scholar] [CrossRef]
- Novick, R.P.; Projan, S.J.; Kornblum, J.; Ross, H.F.; Ji, G.; Kreiswirth, B.; Vandenesch, F.; Moghazeh, S.; Novick, R.P. The agr P2 operon: An autocatalytic sensory transduction system in Staphylococcus aureus. MGG Mol. Gen. Genet. 1995, 248, 446–458. [Google Scholar] [CrossRef]
- Kusch, K.; Hanke, K.; Holtfreter, S.; Schmudde, M.; Kohler, C.; Erck, C.; Wehland, J.; Hecker, M.; Ohlsen, K.; Bröker, B.; et al. The influence of SaeRS and σB on the expression of superantigens in different Staphylococcus aureus isolates. Int. J. Med. Microbiol. 2011, 301, 488–499. [Google Scholar] [CrossRef]
- Liu, Q.; Yeo, W.S.; Bae, T. The SaeRS two-component system of Staphylococcus aureus. Genes 2016, 7, 81. [Google Scholar] [CrossRef]
- Genigeorgis, C.; Foda, M.S.; Mantis, A.; Sadler, W.W. Effect of sodium chloride and pH on enterotoxin C production. Appl. Microbiol. 1971, 21, 862–866. [Google Scholar] [CrossRef]
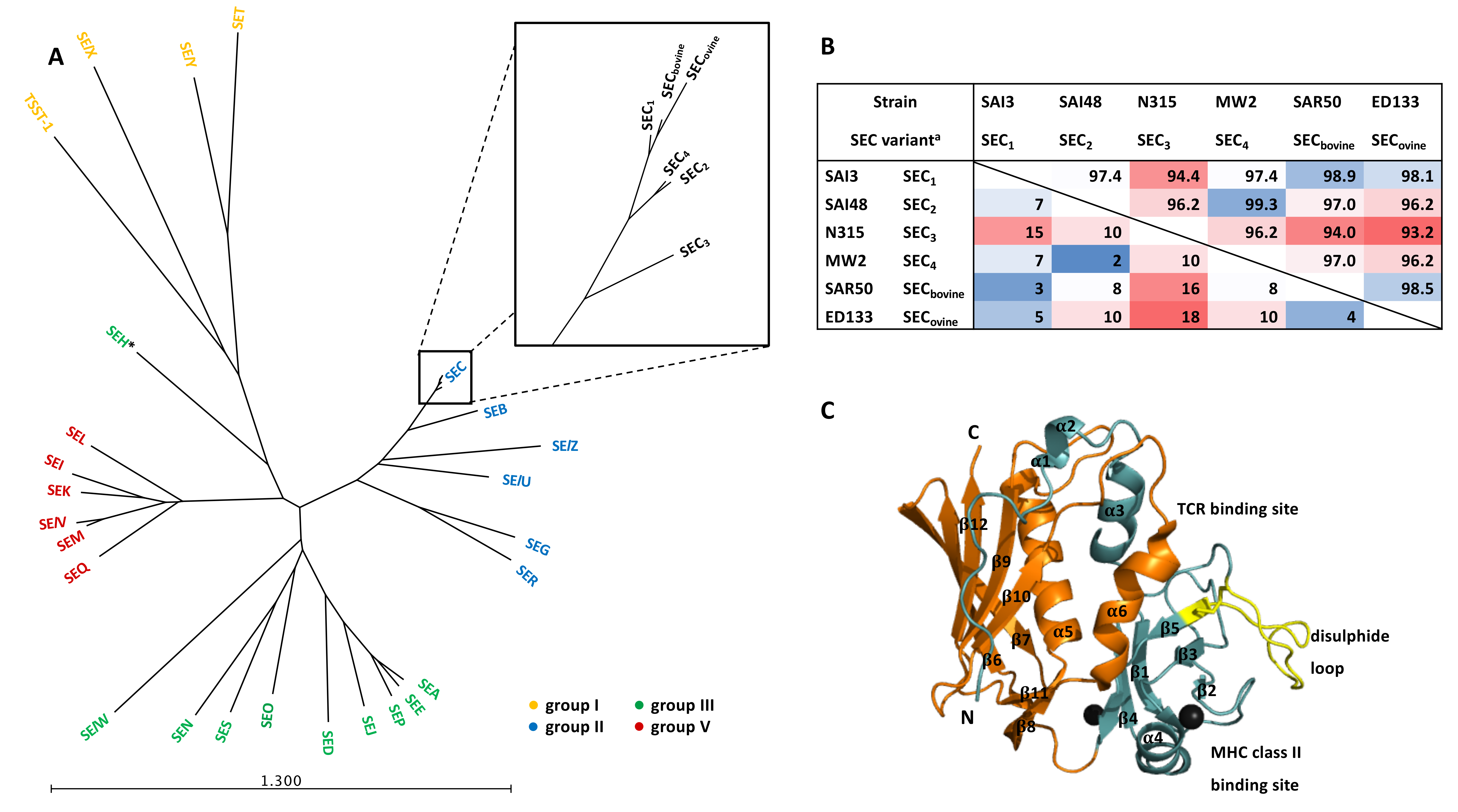
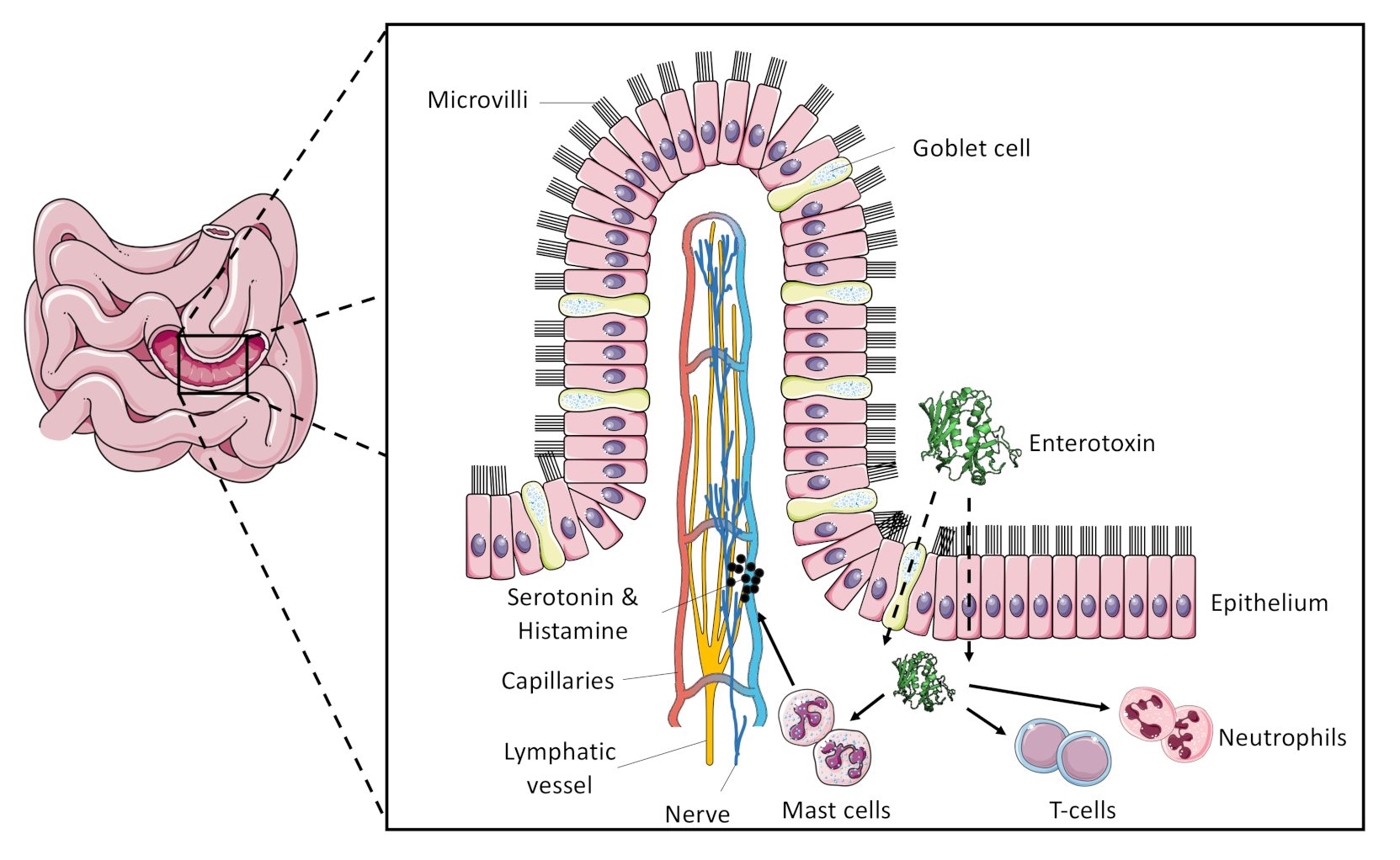
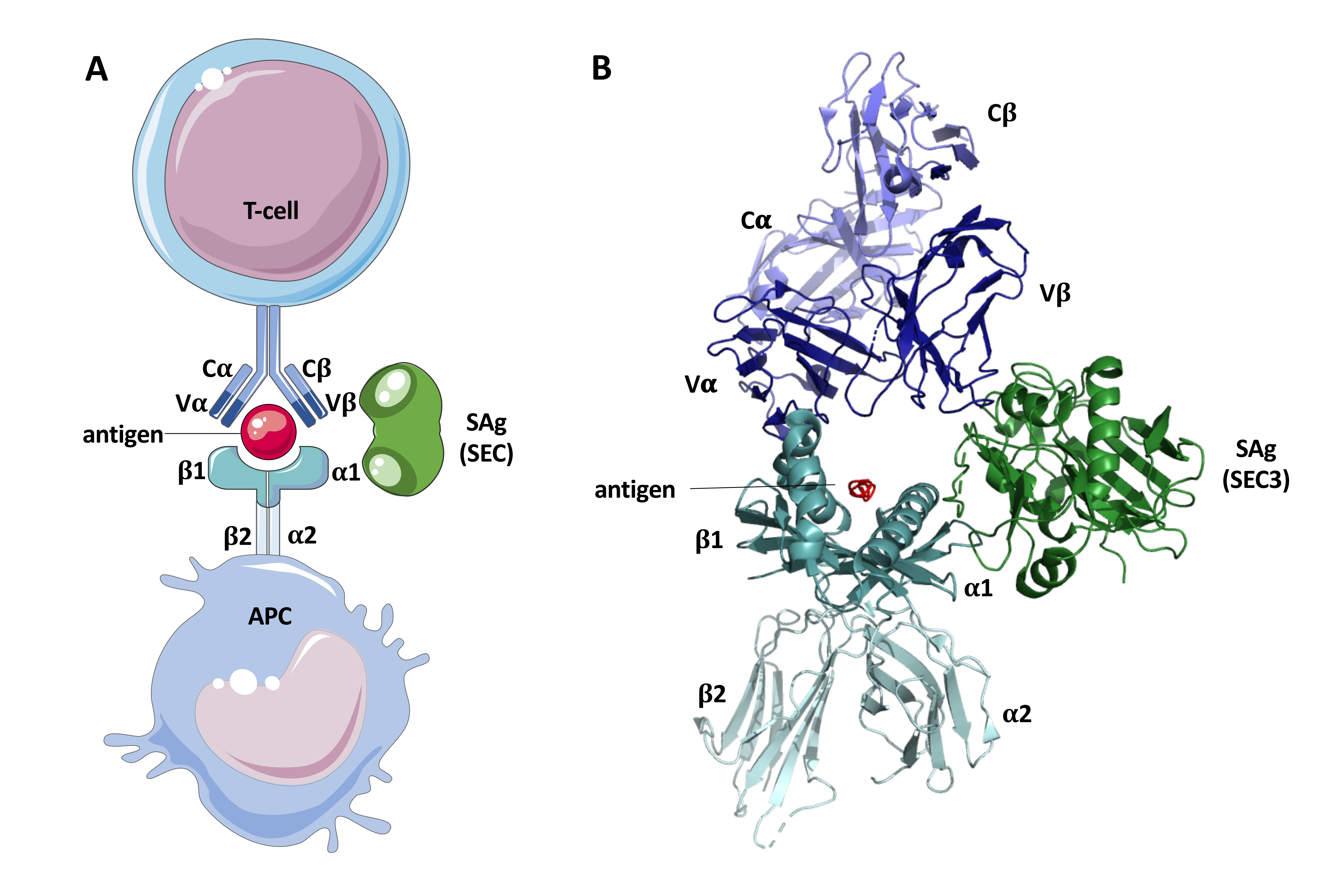
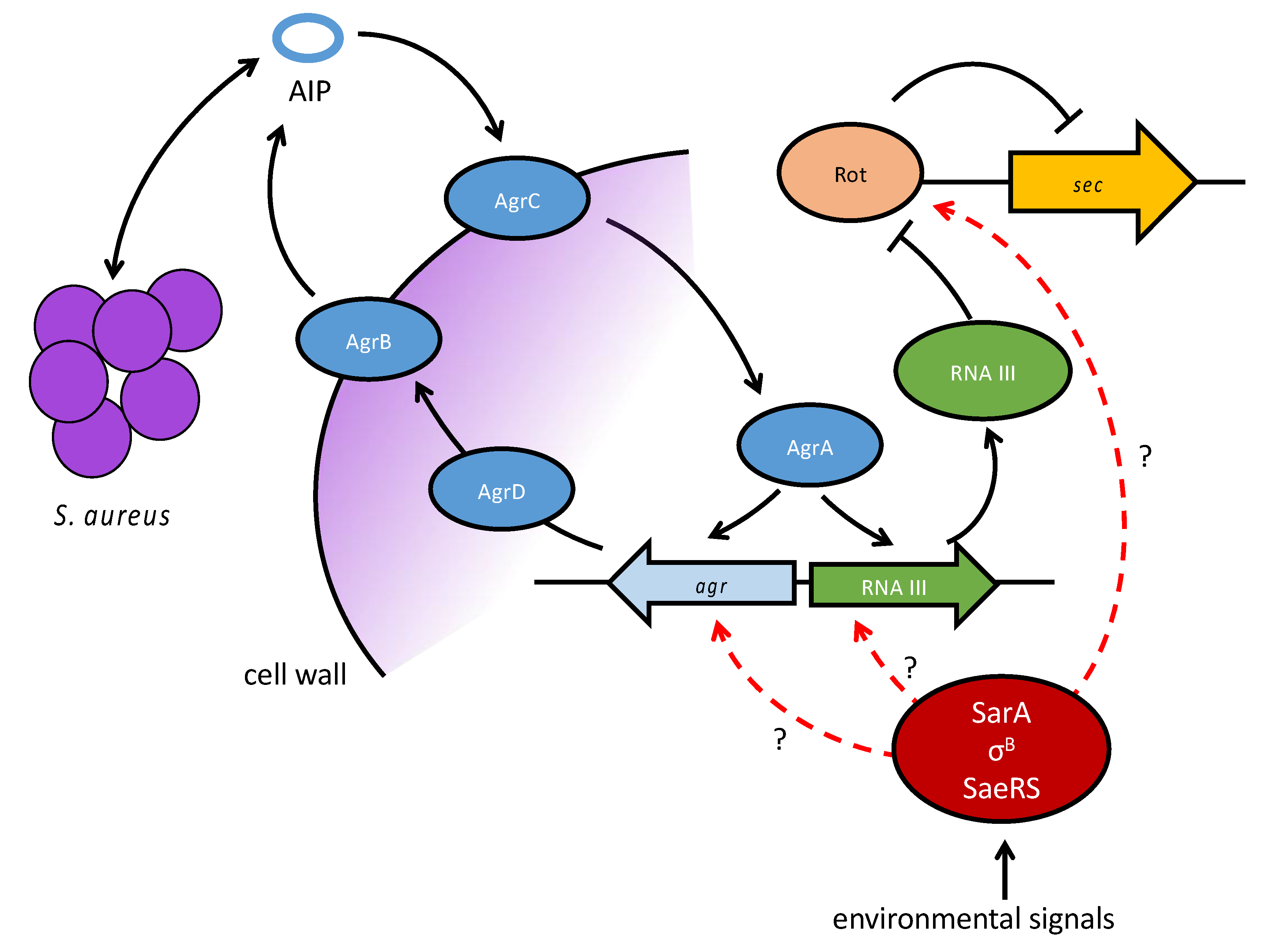
| Enterotoxin/Enterotoxin-Like SAg | Emetic Activity | Associated Genetic Element | References |
|---|---|---|---|
| SEA | + | Prophage (φSa3ms, φSa3mw, φ252B, φNM3, φMu50a) | [19,46,47,48,49] |
| SEB | + | SaPIs (SaPI1, SaPI2, SaPI3, SaPI4, SaPImw2, SaPIrki4) Plasmid (pZA10) | [50,51,52,53,54] |
| SEC | + | SaPIs Plasmid | [55,56,57] |
| SEC1 | + | SaPINuSAα2 a, pZA10 | [53] |
| SEC2 | + | SaPITokyo a | [58] |
| SEC3 | + | SaPIn1/SaPIm1 b | [59,60] |
| SEC4 | nd | SaPImw2 | [61] |
| SECbovine | nd | SaPIbov1 | [30,62] |
| SECovine | nd | SaPIbov5 a, SaPIov1 | [30,62,63] |
| SED | + | Plasmid (pIB485-like) | [64,65,66] |
| SEE | + | Prophage(hypothetical) | [67,68] |
| SEG | + | egc (egc1–4) Prophage (φSa3ms) | [61,69,70,71,72] |
| SEH | + | Transposon (MGEwm2/mssa476 seh/Δseo) | [61,73,74,75] |
| SEI | + | egc (egc1–3) | [69,70,76] |
| SElJ | nd | Plasmid (pIB485-like, pF5) | [27,77,78] |
| SEK | + | SaPIs (SaPIbov1, SaPI1, SaPI3, SaPI5) Prophage (φSa3ms, φSa3mw) | [24,61,71,79,80] |
| SEL | + | SaPIs (SaPIbov1, SaPI3, SaPIn1, SaPIm1, SaPImw2) | [24,57,80,81,82] |
| SEM | + | egc (egc1–2) | [24,69,70,80] |
| SEN | + | egc (egc1–4) | [24,69,70,80] |
| SEO | + | egc (egc1–4) Transposon (MGEwm2/mssa476 seh/Δseo) | [24,69,70,80] |
| SEP | + | Prophage (φSa3n, φN315, φMu3A) | [24,83] |
| SEQ | + | SaPIs (SaPI1, SaPI3, SaPI5) Prophage (φSa3ms, φSa3mw) | [24,84,85] |
| SER | + | Plasmid (pIB485-like, pF5) | [77,86] |
| SES | + | Plasmid (pF5) | [86] |
| SET | + | Plasmid (pF5) | [86] |
| SElU | nd | egc (egc2–3) | [87,88] |
| SElU2 c | nd | egc (egc4) | [88] |
| SElV | nd | egc (egc4) | [88] |
| SElW | nd | Chromosome | [89] |
| SElX | nd | Chromosome | [90] |
| SElY | nd | Chromosome | [91] |
| SElZ | nd | Chromosome | [92] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etter, D.; Schelin, J.; Schuppler, M.; Johler, S. Staphylococcal Enterotoxin C—An Update on SEC Variants, Their Structure and Properties, and Their Role in Foodborne Intoxications. Toxins 2020, 12, 584. https://doi.org/10.3390/toxins12090584
Etter D, Schelin J, Schuppler M, Johler S. Staphylococcal Enterotoxin C—An Update on SEC Variants, Their Structure and Properties, and Their Role in Foodborne Intoxications. Toxins. 2020; 12(9):584. https://doi.org/10.3390/toxins12090584
Chicago/Turabian StyleEtter, Danai, Jenny Schelin, Markus Schuppler, and Sophia Johler. 2020. "Staphylococcal Enterotoxin C—An Update on SEC Variants, Their Structure and Properties, and Their Role in Foodborne Intoxications" Toxins 12, no. 9: 584. https://doi.org/10.3390/toxins12090584
APA StyleEtter, D., Schelin, J., Schuppler, M., & Johler, S. (2020). Staphylococcal Enterotoxin C—An Update on SEC Variants, Their Structure and Properties, and Their Role in Foodborne Intoxications. Toxins, 12(9), 584. https://doi.org/10.3390/toxins12090584







