Limnological Differences in a Two-Basin Lake Help to Explain the Occurrence of Anatoxin-a, Paralytic Shellfish Poisoning Toxins, and Microcystins
Abstract
1. Introduction
2. Results
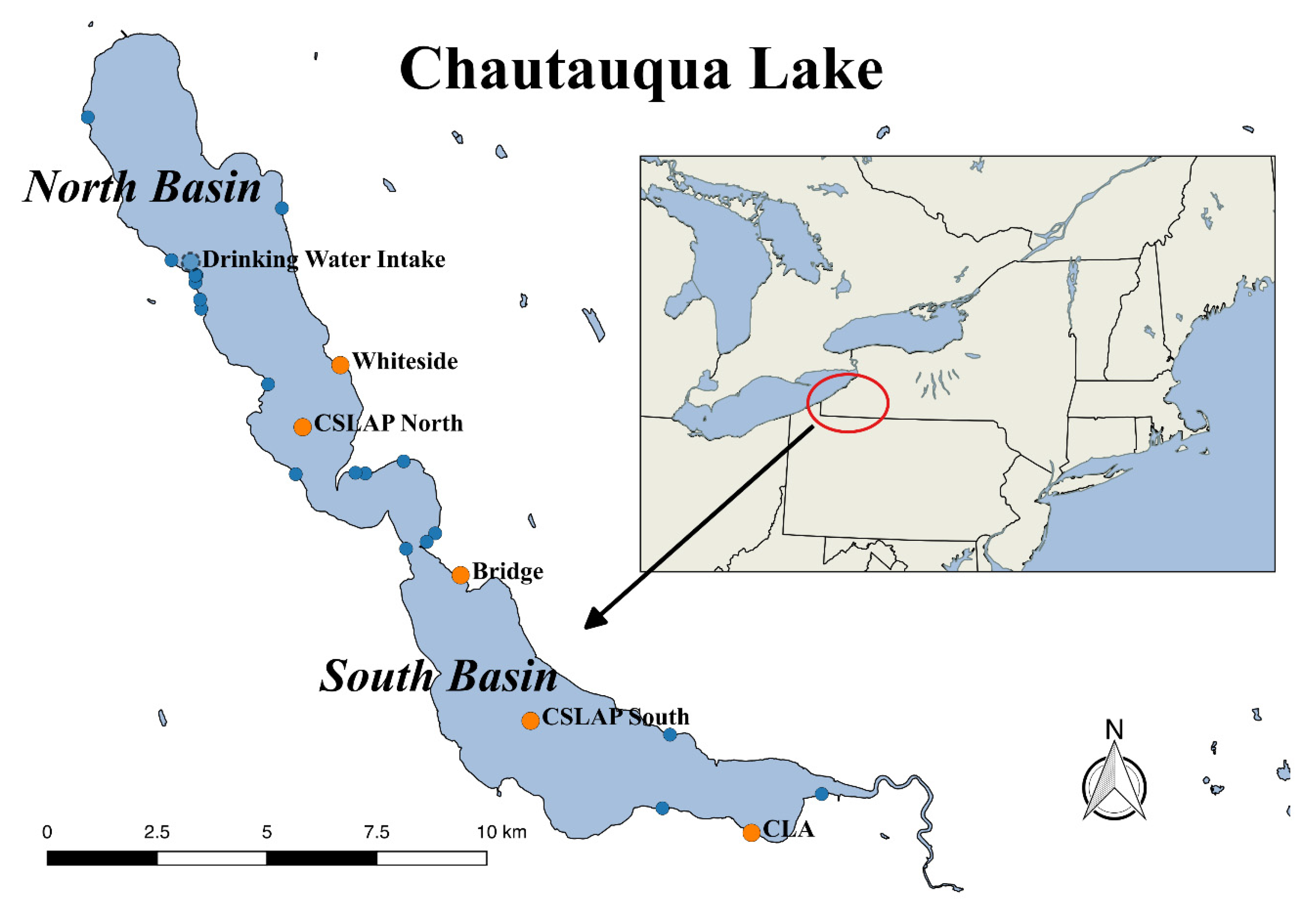
2.1. Chautauqua Lake Water Quality and Algal Bloom Monitoring
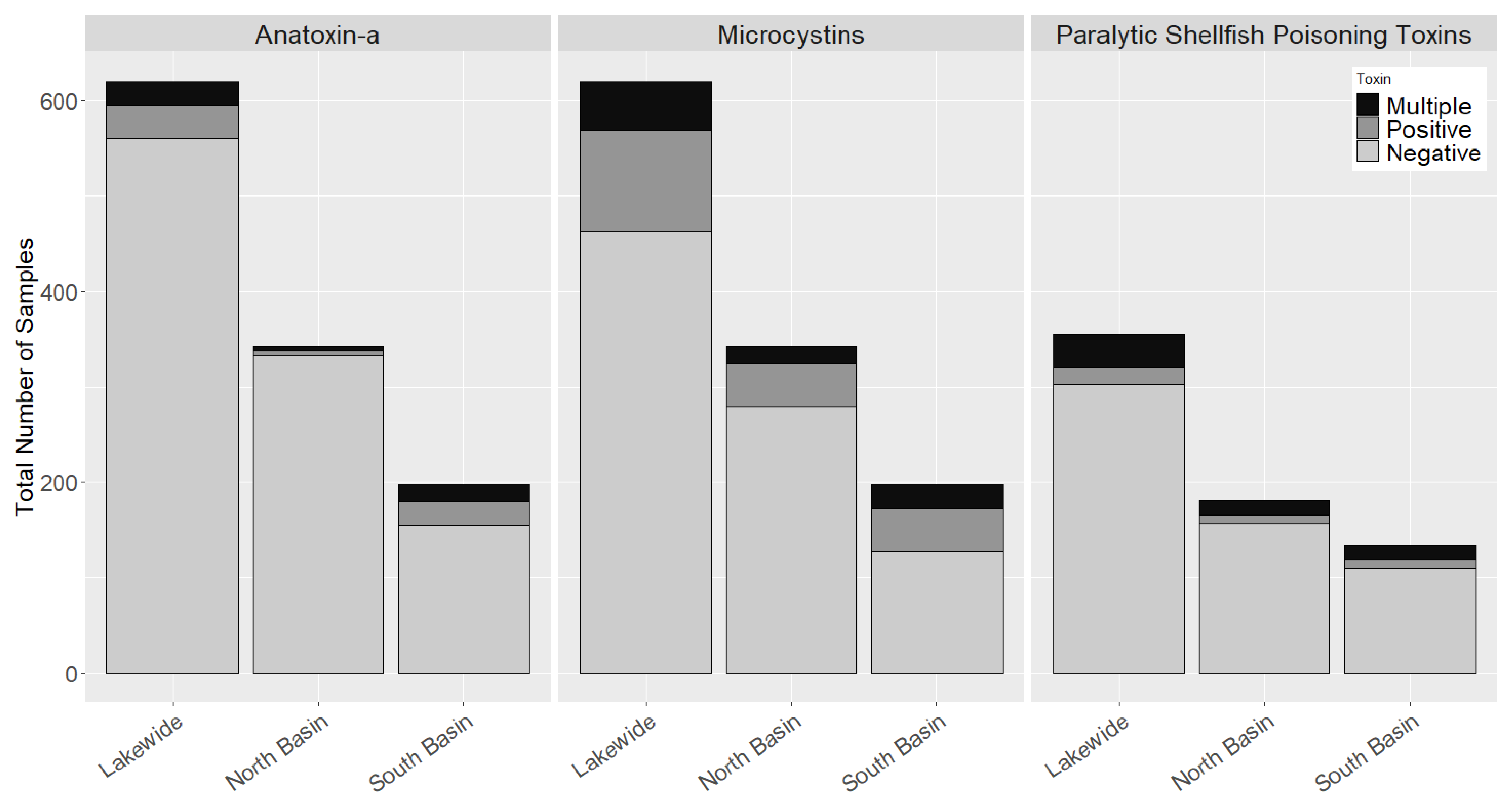
2.2. Occurrence of Cyanotoxins in Chautauqua Lake
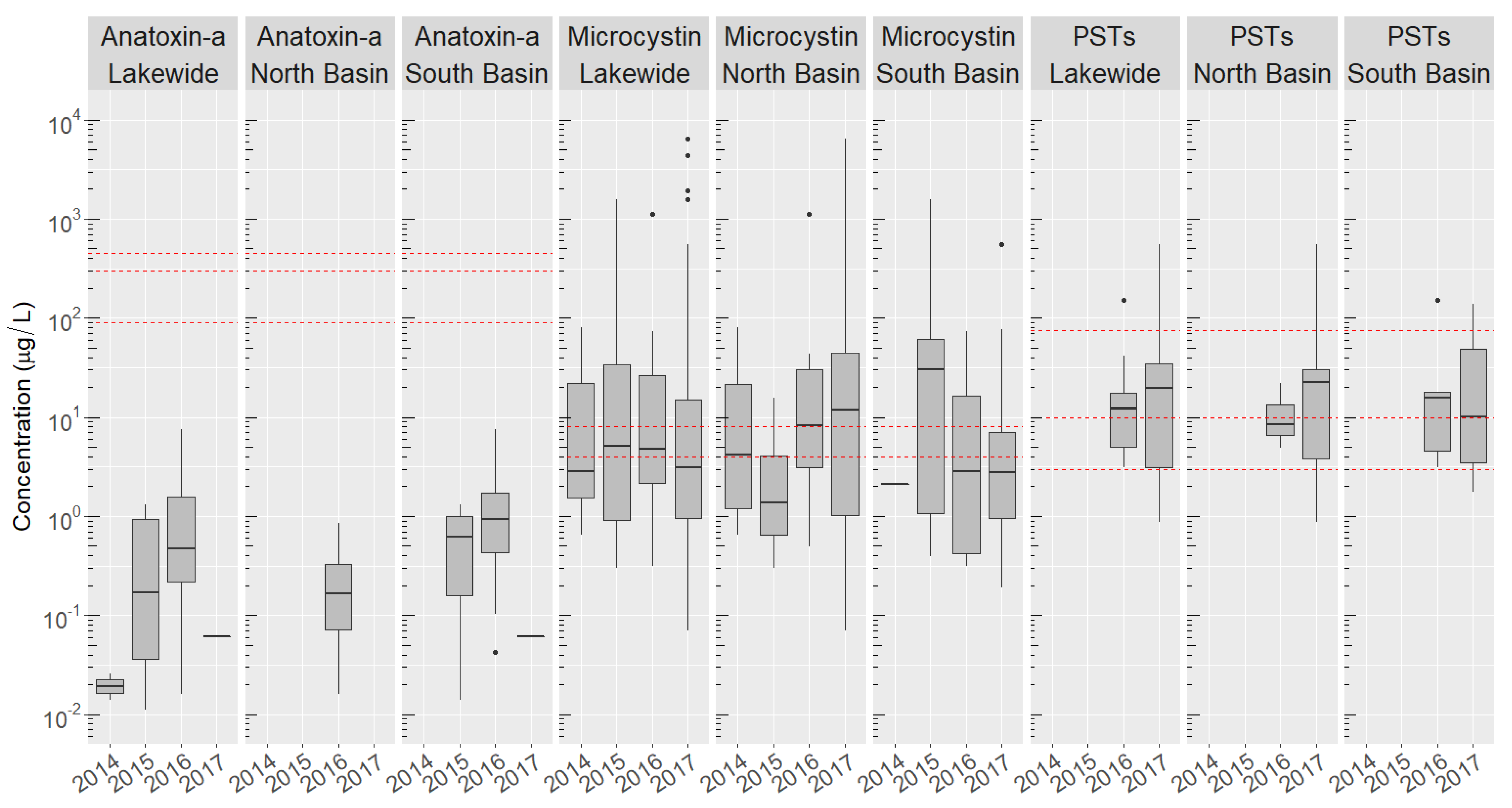
2.3. Basin-Wide Occurrence and Temporal Variation of Toxins
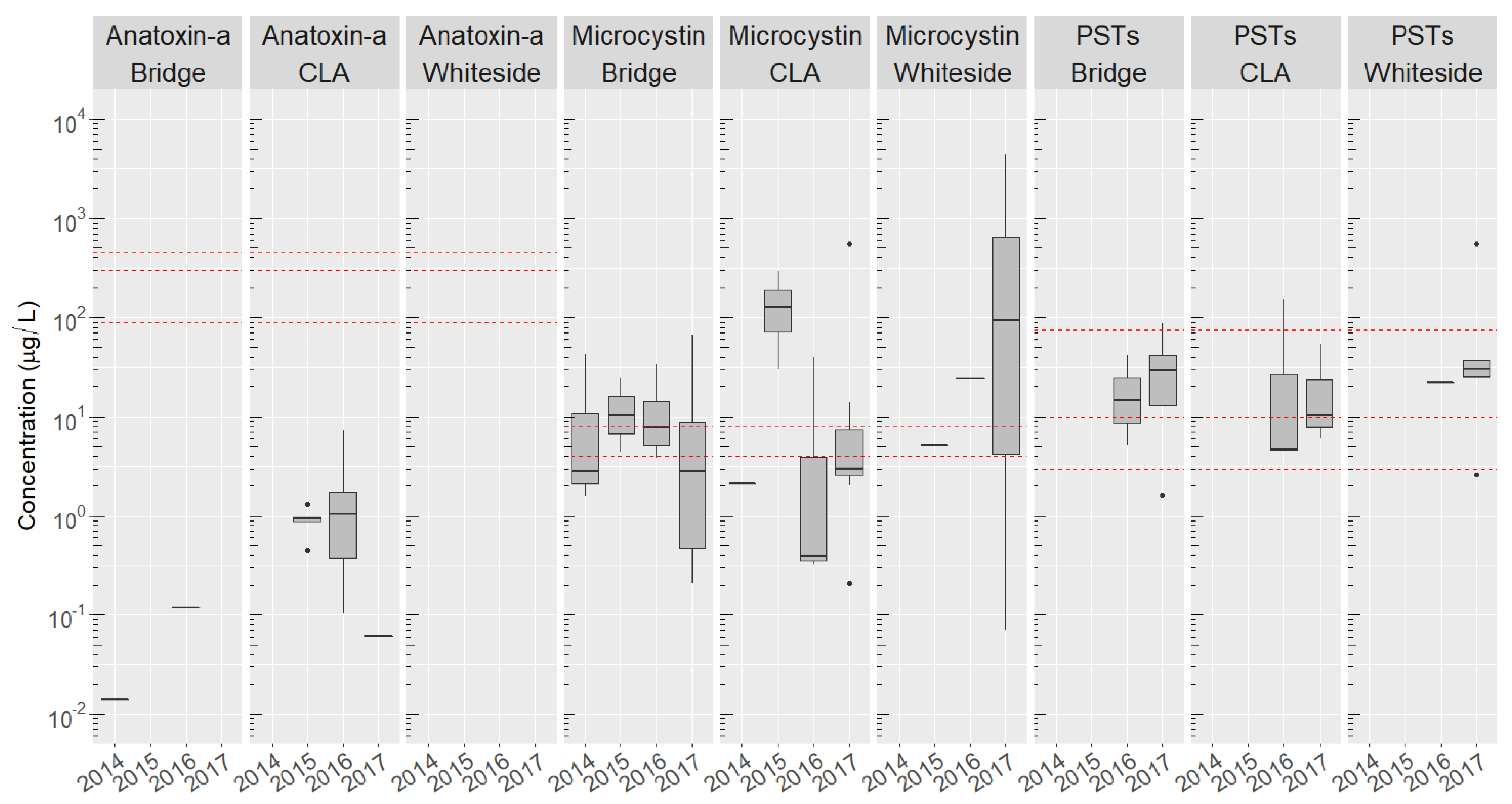
2.4. Site-Specific Occurrence of Toxins
2.5. Co-Occurrence of Multiple Cyanobacteria Toxins
2.6. Correlation of Cyanobacterial Chlorophyll to Environmental Variables
2.7. Comparison between Environmental Parameters for Cyanobacterial Chlorophyll and the Three Cyanotoxins
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Chlorophyll Analysis
4.2. Toxin Extraction and Analysis
4.3. Confirmation of PSTs Using the Saxitoxin Receptor Binding Assay and STX ELISA
4.4. Calculation of the Photosynthetically Active Radiation, Wind Speed, and Wind Direction
4.5. Model Simplification and Selection Approach for Blue-Green Algal Chlorophyll and Cyanotoxins
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pelaez, M.; Antoniou, M.G.; He, X.; Dionysiou, D.D.; de la Cruz, A.A.; Tsimeli, K.; Triantis, T.; Hiskia, A.; Kaloudis, T.; Williams, C.; et al. Sources and Occurrence of Cyanotoxins Worldwide. In Xenobiotics in the Urban Water Cycle; Fatta-Kassinos, D., Bester, K., Kümmerer, K., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 16, pp. 101–127. ISBN 978-90-481-3508-0. [Google Scholar]
- Sivonen, K. Cyanobacterial Toxins. In Encyclopedia of Microbiology; Elsevier: Oxford, UK, 2009; pp. 290–307. [Google Scholar]
- Carmichael, W.W. Health effects of toxin-producing cyanobacteria: “The CyanoHABs”. Hum. Ecol. Risk Assess. Int. J. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Carmichael, W.W. Cyanobacteria secondary metabolites—The cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A review of microcystin detections in Estuarine and Marine waters: Environmental implications and human health risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef]
- Hawkins, P.R.; Novic, S.; Cox, P.; Neilan, B.A.; Burns, B.P.; Shaw, G.; Wickramasinghe, W.; Peerapornpisal, Y.; Ruangyuttikarn, W.; Itayama, T.; et al. A review of analytical methods for assessing the public health risk from microcystin in the aquatic environment. J. Water Supply Res. Technol. Aqua 2005, 54, 509–518. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef]
- Roegner, A.F.; Brena, B.; González-Sapienza, G.; Puschner, B. Microcystins in potable surface waters: Toxic effects and removal strategies: Persistence of microcystins in drinking water. J. Appl. Toxicol. 2014, 34, 441–457. [Google Scholar] [CrossRef]
- Catherine, A.; Bernard, C.; Spoof, L.; Bruno, M. Microcystins and Nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 107–126. ISBN 978-1-119-06876-1. [Google Scholar]
- Loftin, K.A.; Graham, J.L.; Hilborn, E.D.; Lehmann, S.C.; Meyer, M.T.; Dietze, J.E.; Griffith, C.B. Cyanotoxins in inland lakes of the United States: Occurrence and potential recreational health risks in the EPA National Lakes Assessment 2007. Harmful Algae 2016, 56, 77–90. [Google Scholar] [CrossRef]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef]
- Boyer, G.; Kishbaugh, S.; Perkins, M.; Mueller, N. The New York State citizen-based monitoring program for cyanobacteria toxins. In Proceedings of the 16th International Conference on Harmful Algae, Wellington, New Zealand, 27–31 October 2014; pp. 250–253. [Google Scholar]
- Shoemaker, J.A.; Tettenhorst, D.R.; Delacruz, A. Determination of Microcystins and Nodularin in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS); U.S. Environmental Protection Agency: Washington, DC, USA, 2015; pp. 1–40.
- U.S. EPA. Method 546: Determination of Total Microcystins and Nodularins in Drinking Water and Ambient Water by Adda Enzyme-Linked Immunosorbent Assay; U.S. EPA: Washington, DC, USA, 2016; pp. 1–24.
- Carmichael, W.W.; An, J. Using an enzyme linked immunosorbent assay (ELISA) and a protein phosphatase inhibition assay (PPIA) for the detection of microcystins and nodularins. Nat. Toxins 1999, 7, 377–385. [Google Scholar] [CrossRef]
- Yilmaz, M.; Foss, A.J.; Miles, C.O.; Özen, M.; Demir, N.; Balcı, M.; Beach, D.G. Comprehensive multi-technique approach reveals the high diversity of microcystins in field collections and an associated isolate of Microcystis aeruginosa from a Turkish lake. Toxicon 2019, 167, 87–100. [Google Scholar] [CrossRef]
- Carmichael, W.; Biggs, D.; Gorham, P. Toxicology and pharmacological action of Anabaena flos-aquae toxin. Science 1975, 187, 542–544. [Google Scholar] [CrossRef]
- Aráoz, R.; Molgó, J.; Tandeau de Marsac, N. Neurotoxic cyanobacterial toxins. Toxicon 2010, 56, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.P.; Edwards, O.E.; Gorham, P.R.; Hunter, N.R.; Pike, R.K.; Stavric, B. Anatoxin-a, a toxic alkaloid from Anabaena flos-aquae NRC-44h. Can. J. Chem. 1977, 55, 1367–1371. [Google Scholar] [CrossRef]
- Skulberg, O.M.; Skulberg, R.; Carmichael, W.W.; Andersen, R.A.; Matsunaga, S.; Moore, R.E. Investigations of a neurotoxic oscillatorialean strain (Cyanophyceae) and its toxin. Isolation and characterization of homoanatoxin-a. Environ. Toxicol. Chem. 1992, 11, 321–329. [Google Scholar] [CrossRef]
- Smith, R.A.; Lewis, D. A rapid analysis of water for anatoxin a, the unstable toxic alkaloid from Anabaena flos-aquae, the stable non-toxic alkaloids left after bioreduction and a related amine which may be nature’s precursor to anatoxin a. Vet. Hum. Toxicol. 1987, 29, 153–154. [Google Scholar] [PubMed]
- Harada, K.; Nagai, H.; Kimura, Y.; Suzuki, M.; Park, H.-D.; Watanabe, M.F.; Luukkainen, R.; Sivonen, K.; Carmichael, W.W. Liquid chromatography/mass spectrometric detection of anatoxin-a, a neurotoxin from cyanobacteria. Tetrahedron 1993, 49, 9251–9260. [Google Scholar] [CrossRef]
- James, K.J.; Furey, A.; Sherlock, I.R.; Stack, M.A.; Twohig, M.; Caudwell, F.B.; Skulberg, O.M. Sensitive determination of anatoxin-a, homoanatoxin-a and their degradation products by liquid chromatography with fluorimetric detection. J. Chromatogr. A 1998, 798, 147–157. [Google Scholar] [CrossRef]
- Wood, S.A.; Biessy, L.; Puddick, J. Anatoxins are consistently released into the water of streams with Microcoleus autumnalis-dominated (cyanobacteria) proliferations. Harmful Algae 2018, 80, 88–95. [Google Scholar] [CrossRef]
- Namikoshi, M.; Murakami, T.; Watanabe, M.F.; Oda, T.; Yamada, J.; Tsujimura, S.; Nagai, H.; Oishi, S. Simultaneous production of homoanatoxin-a, anatoxin-a, and a new non-toxic 4-hydroxyhomoanatoxin-a by the cyanobacterium Raphidiopsis mediterranea Skuja. Toxicon 2003, 42, 533–538. [Google Scholar] [CrossRef]
- U.S. EPA. Method 545: Determination of Cylindrospermopsin and Anatoxin-a in Drinking Water by Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry (LC/ESI-MS/MS), EPA 815-R-15-009; U.S. EPA: Washington, DC, USA, 2015; pp. 1–27.
- Quiñones-Reyes, G.; Agulló, C.; Mercader, J.V.; Abad-Somovilla, A.; Abad-Fuentes, A. Synthetic haptens and monoclonal antibodies to the cyanotoxin anatoxin-a. Angew. Chem. Int. Ed. 2019, 58, 9134–9139. [Google Scholar] [CrossRef] [PubMed]
- Terlau, H.; Heinemann, S.H.; Stühmer, W.; Pusch, M.; Conti, F.; Imoto, K.; Numa, S. Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett. 1991, 293, 93–96. [Google Scholar] [CrossRef]
- Walker, J.R.; Novick, P.A.; Parsons, W.H.; McGregor, M.; Zablocki, J.; Pande, V.S. Marked difference in saxitoxin and tetrodoxin affinity for the human nociceptive voltage-gated sodium channel (Nav1.7). Proc. Natl. Acad. Sci. USA 2012, 109, 18102–18107. [Google Scholar] [CrossRef] [PubMed]
- Trainer, V.L.; Baden, D.G. High affinity binding of red tide neurotoxins to marine mammal brain. Aquat. Toxicol. 1999, 46, 139–148. [Google Scholar] [CrossRef]
- Negri, A.P.; Jones, G.J.; Hindmarsh, M. Sheep mortality associated with paralytic shellfish poisons from the cyanobacterium Anabaena circinalis. Toxicon 1995, 33, 1321–1329. [Google Scholar] [CrossRef]
- McGregor, G.B.; Sendall, B.C. Phylogeny and toxicology of Lyngbya wollei (Cyanobacteria, Oscillatoriales) from north-eastern Australia, with a description of Microseira gen. nov. J. Phycol. 2015, 51, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Lagos, N.; Onodera, H.; Zagatto, P.A.; Andrinolo, D.; Azevedo, S.M.F.Q.; Oshima, Y. The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil. Toxicon 1999, 37, 1359–1373. [Google Scholar] [CrossRef]
- Ohio Algae Information for Recreational Waters. Available online: http://epa.ohio.gov/habalgae.aspx#147744471-publications-and-helpful-links (accessed on 20 November 2015).
- Onodera, H.; Satake, M.; Oshima, Y.; Yasumoto, T.; Carmichael, W.W. New saxitoxin analogues from the freshwater filamentous cyanobacterium Lyngbya wollei. Nat. Toxins 1997, 5, 146–151. [Google Scholar] [CrossRef]
- Mahmood, N.A.; Carmichael, W.W. Paralytic shellfish poisons produced by the freshwater cyanobacterium Aphanizomenon flos-aquae NH-5. Toxicon 1986, 24, 175–186. [Google Scholar] [CrossRef]
- Foss, A.J.; Phlips, E.J.; Yilmaz, M.; Chapman, A. Characterization of paralytic shellfish toxins from Lyngbya wollei dominated mats collected from two Florida springs. Harmful Algae 2012, 16, 98–107. [Google Scholar] [CrossRef]
- Smith, Z.J.; Martin, R.M.; Wei, B.; Wilhelm, S.W.; Boyer, G.L. Spatial and temporal variation in paralytic shellfish toxin production by benthic Microseira (Lyngbya) wollei in a freshwater New York lake. Toxins 2019, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Evans, W.R.; Yin, Q.Q.; Bell, P.; Moczydlowski, E. Evidence for paralytic shellfish poisons in the freshwater cyanobacterium Lyngbya wollei (Farlow ex Gomont) comb. nov. Appl. Environ. Microbiol. 1997, 63, 3104–3110. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Segura, P.A.; Gélinas, M.; Hudon, C.; Thomas, K.; Quilliam, M.A.; Gagnon, C. Detection and confirmation of saxitoxin analogues in freshwater benthic Lyngbya wollei algae collected in the St. Lawrence River (Canada) by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1219, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Ballot, A.; Fastner, J.; Wiedner, C. Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in northeast Germany. Appl. Environ. Microbiol. 2010, 76, 1173–1180. [Google Scholar] [CrossRef]
- Ledreux, A.; Thomazeau, S.; Catherine, A.; Duval, C.; Yéprémian, C.; Marie, A.; Bernard, C. Evidence for saxitoxins production by the cyanobacterium Aphanizomenon gracile in a French recreational water body. Harmful Algae 2010, 10, 88–97. [Google Scholar] [CrossRef]
- Pereira, P.; Li, R.; Carmichael, W.; Dias, E.; Franca, S. Taxonomy and production of paralytic shellfish toxins by the freshwater cyanobacterium Aphanizomenon gracile LMECYA40. Eur. J. Phycol. 2004, 39, 361–368. [Google Scholar] [CrossRef]
- Belykh, O.I.; Tikhonova, I.V.; Kuzmin, A.V.; Sorokovikova, E.G.; Fedorova, G.A.; Khanaev, I.V.; Sherbakova, T.A.; Timoshkin, O.A. First detection of benthic cyanobacteria in Lake Baikal producing paralytic shellfish toxins. Toxicon 2016, 121, 36–40. [Google Scholar] [CrossRef]
- Smith, F.M.J.; Wood, S.A.; van Ginkel, R.; Broady, P.A.; Gaw, S. First report of saxitoxin production by a species of the freshwater benthic cyanobacterium, Scytonema Agardh. Toxicon 2011, 57, 566–573. [Google Scholar] [CrossRef]
- Gessner, B.D.; Middaugh, J.P. Paralytic shellfish poisoning in alaska: A 20-Year retrospective analysis. Am. J. Epidemiol. 1995, 141, 766–770. [Google Scholar] [CrossRef]
- Gessner, B.D.; Middaugh, J.P.; Doucette, G.J. Paralytic shellfish poisoning in Kodiak, Alaska. West. J. Med. 1997, 167, 351–353. [Google Scholar]
- García, C.; del Carmen Bravo, M.; Lagos, M.; Lagos, N. Paralytic shellfish poisoning: Post-mortem analysis of tissue and body fluid samples from human victims in the Patagonia fjords. Toxicon 2004, 43, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, L.E.; Dodd, M.J.; Robertson, A.; Ericson, G.; de Koning, C.; Negri, A.P. Post-mortem analysis of samples from a human victim of a fatal poisoning caused by the xanthid crab, Zosimus aeneus. Toxicon 2002, 40, 1463–1469. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Tsuchiya, S.; Yoshioka, R.; Omura, T.; Konoki, K.; Oshima, Y.; Yotsu-Yamashita, M. The presence of 12β-deoxydecarbamoylsaxitoxin in the Japanese toxic dinoflagellate Alexandrium determined by simultaneous analysis for paralytic shellfish toxins using HILIC-LC–MS/MS. Harmful Algae 2015, 49, 58–67. [Google Scholar] [CrossRef]
- D’Agostino, P.M.; Boundy, M.J.; Harwood, T.D.; Carmichael, W.W.; Neilan, B.A.; Wood, S.A. Re-evaluation of paralytic shellfish toxin profiles in cyanobacteria using hydrophilic interaction liquid chromatography-tandem mass spectrometry. Toxicon 2019, 158, 1–7. [Google Scholar] [CrossRef]
- JOINT FAO/WHO Toxicity Equivalency Factors for Marine Biotoxins Associated with Bivalve Molluscs. 2016. Available online: http://www.fao.org/3/a-i5970e.pdf (accessed on 22 September 2019).
- Van Dolah, F.M.; Fire, S.E.; Leighfield, T.A.; Mikulski, C.M.; Doucette, G.J. Determination of paralytic shellfish toxins in shellfish by receptor binding assay: Collaborative study. J. AOAC Int. 2012, 95, 795–812. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C. Quantitative determination of paralytic shellfish poisoning toxins in shellfish using prechromatographic oxidation and liquid chromatography with fluorescence detection: Collaborative study. J. AOAC Int. 2005, 88, 1714–1732. [Google Scholar] [CrossRef]
- van de Riet, J.; Gibbs, R.S.; Muggah, P.M.; Rourke, W.A.; MacNeil, J.D.; Quilliam, M.A. Liquid chromatography post-column oxidation (PCOX) method for the determination of paralytic shellfish toxins in mussels, clams, oysters, and scallops: Collaborative study. J. AOAC Int. 2011, 94, 1154–1176. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; Fong, S.Y.T.; Hungerford, J.; McNabb, P.S.; Boundy, M.J.; Harwood, D.T. Ultrahigh-performance hydrophilic interaction liquid chromatography with tandem mass spectrometry method for the determination of paralytic shellfish toxins and tetrodotoxin in mussels, oysters, clams, cockles, and scallops: Collaborative study. J. AOAC Int. 2019, 103, 1–29. [Google Scholar] [CrossRef]
- Dell’Aversano, C.; Hess, P.; Quilliam, M.A. Hydrophilic interaction liquid chromatography—Mass spectrometry for the analysis of paralytic shellfish poisoning (PSP) toxins. J. Chromatogr. A 2005, 1081, 190–201. [Google Scholar] [CrossRef]
- Usleber, E.; Dietrich, R.S.; Buerk, C.; Schneider, E.A.; Märtlbauer, E. Immunoassay methods for paralytic shellfish poisoning toxins. J. AOAC Int. 2001, 84, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- State of Emergency Declared in Toledo Area. Available online: http://www.toledoblade.com/local/2014/08/02/City-of-Toledo-issues-do-no-drink-water-advisery/stories/20140802085 (accessed on 5 October 2019).
- Coin, G. State: No Algae Toxins Found in Anybody’s Tap Water from Skaneateles Lake. Available online: https://www.syracuse.com/news/2017/09/state_no_algae_toxins_found_in_anybodys_tap_water_from_skaneateles_lake.html (accessed on 7 October 2019).
- Onondaga County Water Authority. Important Updates re: Skaneateles Lake Algae Issue. 2017. Available online: https://www.ocwa.org/2017/09/20/important-updates-re-skaneateles-lake-algae-issue/ (accessed on 7 October 2019).
- Update: Cayuga County Finds Low Level Toxins in Auburn, Owasco Drinking Water—Syracuse.com. Available online: https://www.syracuse.com/news/2016/10/update_cayuga_county_finds_low_level_toxins_in_auburn_owasco_drinking_water.html (accessed on 19 February 2020).
- Brown, K. Recreational and Do-Not-Drink Advisories for Detroit Lake, Oregon. 2018. Available online: https://www.oregon.gov/oha/PH/HEALTHYENVIRONMENTS/DRINKINGWATER/PREPAREDNESS/Documents/FAQ-Recreational-and-Drinking-Water-Advisories.pdf (accessed on 6 October 2019).
- Governor Cuomo Announces Action Plans to Combat Harmful Algal Blooms. Governor Andrew M. Cuomo. Available online: https://www.governor.ny.gov/news/governor-cuomo-announces-action-plans-combat-harmful-algal-blooms (accessed on 19 February 2020).
- U.S. EPA. Toxicological Review of Cyanobacterial Toxins: Microcystins Lr, Rr, Yr and La (External Review Draft); EPA/600/R-06/139; U.S. Environmental Protection Agency: Washington, DC, USA, 2006. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NCEA&dirEntryId=160548 (accessed on 29 August 2020).
- U.S. EPA. Toxicological Review of Cyanobacterial Toxins: Anatoxin-a (External Review Draft). EPA/600/R-06/137; U.S. Environmental Protection Agency: Washington, DC, USA, 2006. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=160546 (accessed on 29 August 2020).
- Wood, S.A.; Hawes, I.; McBride, G.; Truman, P.; Dietrich, D. Advice to Inform the Development of a Benthic Cyanobacteria Attribute; Cawthron Institute Report No 2752; Ministry for the Environment: Nelson, New Zealand, 2015; pp. 1–105.
- Canada, H. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Cyanobacterial Toxins. Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-cyanobacterial-toxins-document.html (accessed on 24 September 2019).
- Chorus, I. Current Approaches to Cyanotoxin Risk Assessment, Risk Management and Regulations in Different Countries; Umwelt Bundesamt: Dessau-Roßlau, Germany, 2012; pp. 1–151. [Google Scholar]
- Ohio Microcystin, Anatoxin-a, and Saxitoxins Sampling and Water Quality Guidelines and Public Water Supply Response Strategy. Available online: https://epa.ohio.gov/Portals/28/documents/habs/2019-PWS-HAB-Response-Strategy.pdf (accessed on 17 September 2019).
- Washington State Anatoxin-a and Microcystin Guidelines. Available online: https://www.doh.wa.gov/Portals/1/Documents/4400/334-177-recguide.pdf (accessed on 17 September 2019).
- California State Anatoxin-a and Microcystin Guidelines. Available online: https://www.waterboards.ca.gov/water_issues/programs/peer_review/docs/calif_cyanotoxins/cyanotoxins053112.pdf (accessed on 17 September 2019).
- Farrer, D.; Counter, M.; Hillwig, R.; Cude, C. Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins 2015, 7, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Boyer, G.L. Cyanobacterial toxins in New York and the lower Great Lakes ecosystems. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: Berlin/Heidelberg, Germany, 2008; pp. 153–165. [Google Scholar]
- Yang, X. The Occurrence of the Cyanobacterial Neurotoxin, Anatoxin-a, in New York State Waters. Ph.D. Thesis, State University of New York College of Environmental Science and Forestry, Syracuse, NY, USA, 2007; pp. 1–232. [Google Scholar]
- Arnich, N.; Thébault, A. Dose-response modelling of paralytic shellfish poisoning (PSP) in humans. Toxins 2018, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Bouma-Gregson, K.; Kudela, R.M.; Power, M.E. Widespread anatoxin-a detection in benthic cyanobacterial mats throughout a river network. PLoS ONE 2018, 13, e197669. [Google Scholar] [CrossRef]
- Fetscher, A.E.; Howard, M.D.A.; Stancheva, R.; Kudela, R.M.; Stein, E.D.; Sutula, M.A.; Busse, L.B.; Sheath, R.G. Wadeable streams as widespread sources of benthic cyanotoxins in California, USA. Harmful Algae 2015, 49, 105–116. [Google Scholar] [CrossRef]
- Foss, A.J.; Butt, J.; Aubel, M.T. Benthic periphyton from Pennsylvania, USA is a source for both hepatotoxins (microcystins/nodularin) and neurotoxins (anatoxin-a/homoanatoxin-a). Toxicon 2018, 150, 13–16. [Google Scholar] [CrossRef]
- Ruiz, M.; Galanti, L.; Ruibal, A.L.; Rodriguez, M.I.; Wunderlin, D.A.; Amé, M.V. First report of microcystins and anatoxin-a co-occurrence in San Roque reservoir (Córdoba, Argentina). Water Air Soil Pollut. 2013, 224, 1–17. [Google Scholar] [CrossRef]
- Czyżewska, W.; Piontek, M.; Łuszczyńska, K. The Occurrence of Potential Harmful Cyanobacteria and Cyanotoxins in the Obrzyca River (Poland), a Source of Drinking Water. Toxins 2020, 12, 284. [Google Scholar] [CrossRef]
- Christophoridis, C.; Zervou, S.-K.; Manolidi, K.; Katsiapi, M.; Moustaka-Gouni, M.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. Occurrence and diversity of cyanotoxins in Greek lakes. Sci. Rep. 2018, 8, 17877. [Google Scholar] [CrossRef]
- Minasyan, A.; Christophoridis, C.; Wilson, A.E.; Zervou, S.-K.; Kaloudis, T.; Hiskia, A. Diversity of cyanobacteria and the presence of cyanotoxins in the epilimnion of Lake Yerevan (Armenia). Toxicon 2018, 150, 28–38. [Google Scholar] [CrossRef]
- Sabart, M.; Crenn, K.; Perrière, F.; Abila, A.; Leremboure, M.; Colombet, J.; Jousse, C.; Latour, D. Co-occurrence of microcystin and anatoxin-a in the freshwater lake Aydat (France): Analytical and molecular approaches during a three-year survey. Harmful Algae 2015, 48, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Mantzouki, E.; Lürling, M.; Fastner, J.; de Senerpont Domis, L.; Wilk-Wo’zniak, E. Temperature effects explain continental scale distribution of cyanobacterial toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Al-Sammak, M.A.; Hoagland, K.D.; Cassada, D.; Snow, D.D. Co-occurrence of the cyanotoxins BMAA, DABA and anatoxin-a in Nebraska reservoirs, fish, and aquatic plants. Toxins 2014, 6, 488–508. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Banack, S.A.; Lindsay, J.; Morrison, L.F.; Cox, P.A.; Codd, G.A. Co-occurrence of β-N-methylamino-l-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990–2004. Environ. Microbiol. 2008, 10, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Beversdorf, L.J.; Rude, K.; Weirich, C.A.; Bartlett, S.L.; Seaman, M.; Kozik, C.; Biese, P.; Gosz, T.; Suha, M.; Stempa, C.; et al. Analysis of cyanobacterial metabolites in surface and raw drinking waters reveals more than microcystin. Water Res. 2018, 140, 280–290. [Google Scholar] [CrossRef]
- Dietrich, D.R.; Fischer, A.; Michel, C.; Hoeger, S. Toxin mixture in cyanobacterial blooms—A critical comparison of reality with current procedures employed in human health risk assessment. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; Volume 619, pp. 885–912. ISBN 978-0-387-75864-0. [Google Scholar]
- Miller, T.; Beversdorf, L.; Weirich, C.; Bartlett, S. Cyanobacterial toxins of the Laurentian Great Lakes, their toxicological effects, and numerical limits in drinking water. Mar. Drugs 2017, 15, 160. [Google Scholar] [CrossRef]
- Tressler, W.L.; Bere, R., VIII. A Limnological study of Chautauqua Lake. A Biological Survey of the Allegheny and Chemung Watersheds; Supplemental to the Twenty-Seventh Annual Report; Albany, J. B. Lyon Company: Albany, NY, USA, 1938; Volume 12. [Google Scholar]
- New York Department of Environmental Conservation. Chautauqua Lake CSLAP Sampling and Condition Information 2017. Available online: https://www.dec.ny.gov/docs/water_pdf/cslrpt17chautauqualn.pdf (accessed on 9 October 2019).
- Wigdahl-Perry, C. (State University of New York at Fredonia, Fredonia, NY, USA). Personal Communication, 2019.
- Conroe, D. (Chautauqua Lake Association, Chautauqua, NY, USA). Personal Communication, 2019.
- Miller, T. (University of Wisconsin-Milwaukee, Milwaukee, WI, USA). Personal Communication, 2019.
- Oshima, Y. Postcolumn derivatization liquid chromatographic method for paralytic shellfish toxins. J. AOAC Int. 1995, 78, 528–532. [Google Scholar] [CrossRef]
- Smith, Z. Freshwater Paralytic Shellfish Poisoning Toxins and other Cyanobacterial Neurotoxins in New York Lakes. Ph.D. Thesis, State University of New York College of Environmental Science and Forestry, Syracuse, NY, USA, 2019; pp. 1–385. Available online: https://digitalcommons.esf.edu/etds/122 (accessed on 29 August 2020).
- Harmful Blue-green Algae Blooms at New York State Regulated Beaches. Available online: https://www.health.ny.gov/environmental/water/drinking/bluegreenalgae/bga_bathingbeaches.htm (accessed on 15 September 2019).
- U.S. EPA. Recommended Human Health Recreational Ambient Water Quality Criteria or Swimming Advisories for Microcystins and Cylindrospermopsin. Available online: https://www.epa.gov/wqc/recommended-human-health-recreational-ambient-water-quality-criteria-or-swimming-advisories (accessed on 3 June 2020).
- Washington State Cylindrospermopsin and Saxitoxin Guidelines. Available online: https://www.doh.wa.gov/Portals/1/Documents/4400/332-118-CylindroSax%20Report.pdf (accessed on 6 September 2016).
- Brazil Ministry of Health Brazil Saxitoxin Guidelines Ordinance No. 2914. 2011. Available online: http://site.sabesp.com.br/site/uploads/file/asabesp_doctos/PortariaMS291412122011.pdf (accessed on 24 September 2019).
- Conroe, D. (Chautauqua Lake Association, Chautauqua, NY, USA). Personal Communication, 2020.
- Håkanson, L. The Importance of Lake Morphometry for the Structureand Function of Lakes. Int. Rev. Hydrobiol. 2005, 90, 433–461. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Davis, T.W.; Doherty, O.M.; Gobler, C.J. The Role of Surface Water Warming in the Timing of the Microcystis-Dominated Cyanobacterial Blooms in Western Lake Erie. In Proceedings of the 10th US Symposium on Harmful Algae, Orange Beach, AL, USA, 3–9 November 2019. [Google Scholar]
- Stoner, S. New York State Nutrient Standards Plan. Available online: https://www.dec.ny.gov/docs/water_pdf/nutrientstds2011.pdf.13 (accessed on 21 November 2019).
- Wang, G.X.; Zhang, L.M.; Chua, H.; Li, X.D.; Xia, M.F.; Pu, P.M. A mosaic community of macrophytes for the ecological remediation of eutrophic shallow lakes. Ecol. Eng. 2009, 35, 582–590. [Google Scholar] [CrossRef]
- Schindler, D.W. Eutrophication and recovery in experimental lakes: Implications for lake management. Science 1974, 184, 897–899. [Google Scholar] [CrossRef]
- Boyer, G. Cyanobacteria and their Toxins in NY and Great Lakes Waters. Available online: https://www.neiwpcc.org/neiwpcc_docs/2Boyer-CyanobacteriaToxinsinNewYorkState.pdf (accessed on 24 November 2019).
- Westrick, J.A.; Szlag, D.C.; Southwell, B.J.; Sinclair, J. A review of cyanobacteria and cyanotoxins removal/inactivation in drinking water treatment. Anal. Bioanal. Chem. 2010, 397, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Pouria, S.; de Andrade, A.; Barbosa, J.; Cavalcanti, R.; Barreto, V.; Ward, C.; Preiser, W.; Poon, G.K.; Neild, G.; Codd, G. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 1998, 352, 21–26. [Google Scholar] [CrossRef]
- Yu, S.-Z. Drinking water and primary liver cancer. In Primary Liver Cancer; Tang, Z.-Y., Wu, M.-C., Xia, S.-S., Eds.; Springer: Berlin/Heidelberg, Germany; China Academic Publications: Beijing, China, 1989; pp. 30–37. [Google Scholar]
- Svircev, Z.; Krstic, S.; Miladinov-Mikov, M.; Baltic, V.; Vidovic, M. Freshwater cyanobacterial blooms and primary liver cancer epidemiological studies in serbia. J. Environ. Sci. Health Part C 2009, 27, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.C.B.; Lürling, M.; Dorr, F.; Pinto, E.; Marinho, M.M. Combined Effect of Light and Temperature on the Production of Saxitoxins in Cylindrospermopsis raciborskii Strains. Toxins 2019, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Rinta-Kanto, J.M.; Wilhelm, S.W. Diversity of Microcystin-Producing Cyanobacteria in Spatially Isolated Regions of Lake Erie. AEM 2006, 72, 5083–5085. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.K.; Krieger, R.I. Stability studies on the cyanobacterial nicotinic alkaloid snatoxin-A. Toxicon 1991, 29, 167–179. [Google Scholar] [CrossRef]
- Kaminski, A.; Bober, B.; Lechowski, Z.; Bialczyk, J. Determination of anatoxin-a stability under certain abiotic factors. Harmful Algae 2013, 28, 83–87. [Google Scholar] [CrossRef]
- Doucette, G.J.; Logan, M.M.; Ramsdell, J.S.; Van Dolah, F.M. Development and preliminary validation of a microtiter plate-based receptor binding assay for paralytic shellfish poisoning toxins. Toxicon 1997, 35, 625–636. [Google Scholar] [CrossRef]
- Laycock, M.; Kralovec, J.; Richards, R. Some In vitro chemical interconversions of paralytic shellfish poisoning PSP toxins useful in the preperation of analytic standards.pdf. J. Mar. Biotechnol. 1994, 3, 121–125. [Google Scholar]
- CSLAP Training Materials and Forms—NYS Department of Environmental Conservation. Available online: https://www.dec.ny.gov/chemical/81849.html (accessed on 16 April 2016).
- CSLAP Report Search. Available online: https://nysfola.org/cslap-report-search/ (accessed on 6 August 2020).
- Catherine, A.; Escoffier, N.; Belhocine, A.; Nasri, A.B.; Hamlaoui, S.; Yéprémian, C.; Bernard, C.; Troussellier, M. On the use of the FluoroProbe®, a phytoplankton quantification method based on fluorescence excitation spectra for large-scale surveys of lakes and reservoirs. Water Res. 2012, 46, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Kring, S.A.; Figary, S.E.; Boyer, G.L.; Watson, S.B.; Twiss, M.R. Rapid in situ measures of phytoplankton communities using the bbe FluoroProbe: Evaluation of spectral calibration, instrument intercompatibility, and performance range. Can. J. Fish. Aquat. Sci. 2014, 71, 1087–1095. [Google Scholar] [CrossRef]
- Beutler, M.; Wiltshire, K.H.; Meyer, B.; Moldaenke, C.; Lüring, C.; Meyerhöfer, M.; Hansen, U.-P.; Dau, H. A fluorometric method for the differentiation of algal populations in vivo and in situ. Photosynth. Res. 2002, 72, 39–53. [Google Scholar] [CrossRef]
- Boyer, G.L. LCMS-SOP Determination of Microcystins in Water Samples by High Performance Liquid Chromatography (HPLC) with Single Quadrupole Mass Spectrometry (MS). Limnol. Oceanogr. 2020. [Google Scholar] [CrossRef]
- Matson, P.G.; Boyer, G.L.; Bridgeman, T.B.; Bullerjahn, G.S.; Kane, D.D.; McKay, R.M.L.; McKindles, K.M.; Raymond, H.A.; Snyder, B.K.; Stumpf, R.P.; et al. Physical drivers facilitating a toxigenic cyanobacterial bloom in a major Great Lakes tributary. Limnol. Oceanogr. 2020, 1–17. [Google Scholar] [CrossRef]
- Furey, A.; Crowley, J.; Lehane, M.; James, K.J. Liquid chromatography with electrospray ion-trap mass spectrometry for the determination of anatoxins in cyanobacteria and drinking water. Rapid. Commun. Mass. Spectrom. 2003, 17, 583–588. [Google Scholar] [CrossRef]
- Van de Riet, J.M.; Gibbs, R.S.; Chou, F.W.; Muggah, P.M.; Rourke, W.A.; Burns, G.; Thomas, K.; Quilliam, M.A. Liquid chromatographic post-column oxidation method for analysis of paralytic shellfish toxins in mussels, clams, scallops, and oysters: Single-laboratory validation. J. AOAC Int. 2009, 92, 1690–1704. [Google Scholar]
- Lage, S.; Burian, A.; Rasmussen, U.; Costa, P.R.; Annadotter, H.; Godhe, A.; Rydberg, S. BMAA extraction of cyanobacteria samples: Which method to choose? Environ. Sci. Pollut. Res. 2016, 23, 338–350. [Google Scholar] [CrossRef]
- NSRDB Data Viewer. Available online: https://maps.nrel.gov/nsrdb-viewer/?aL=0&bL=groad&cE=0&lR=0&mC=43.02472955416351%2C-74.652099609375&zL=7 (accessed on 10 April 2018).
- Alados, I.; Foyo-Moreno, I.; Alados-Arboledas, L. Photosynthetically active radiation: Measurements and modelling. Agric. For. Meteorol. 1996, 78, 121–131. [Google Scholar] [CrossRef]
- Abram, S.V.; Helwig, N.E.; Moodie, C.A.; DeYoung, C.G.; MacDonald, A.W.I.; Waller, N.G. Bootstrap enhanced penalized regression for variable selection with neuroimaging data. Front. Neurosci. 2016, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Bunea, F.; She, Y.; Ombao, H.; Gongvatana, A.; Devlin, K.; Cohen, R. Penalized least squares regression methods and applications to neuroimaging. Neuroimage 2011, 55, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
| Basin | North Basin (Mean ± SD) | South Basin (Mean ± SD) | Paired t-test Difference in Means (p-Value) |
|---|---|---|---|
| TP (μg P/L) | 41.7 ± 18.4 | 68.9 ± 35.1 | 27.1 (<0.0001) |
| TN (mg N/L) | 0.57 ± 0.68 | 0.74 ± 0.33 | 0.166 (0.15) |
| Ammonia (mg N/L) | 0.046 ± 0.057 | 0.034 ± 0.032 | 0.012 (0.29) |
| NO2− + NO3− (mg N/L) | 0.029 ± 0.028 | 0.026 ± 0.038 | 0.004 (0.66) |
| Water Temp (°C) | 22.4 ± 2.3 | 23.5 ± 2.6 | 0.66 (0.039) |
| pH | 7.93 ± 0.39 | 8.07 ± 0.66 | 0.13 (0.23) |
| Secchi disk depth (m) | 2.29 ± 0.95 | 1.09 ± 0.70 | 1.17 (<0.0001) |
| Conductivity (μS) | 190.4 ± 33.3 | 193 ± 31.7 | 3.35 (0.57) |
| Total chlorophyll (µg/L) | 2426 ± 16,780 | 700 ± 3370 | NA * |
| Cyanobacterial chlorophyll (µg/L) | 2408 ± 16,780 | 635 ± 3080 | NA * |
| ln(Total chlorophyll + 1) * | 3.1 ± 2.3 | 3.8 ± 2.0 | 0.70 (<0.001) * |
| ln(Cyanobacterial chlorophyll + 1) * | 2.5 ± 2.6 | 3.3 ± 2.3 | 0.78 (<0.001) * |
| Basin | Number of Samples | Range (µg/L) | Mean ± SD (µg/L) | Median (µg/L) |
|---|---|---|---|---|
| North | 86 | 32.4–210,100 | 9590 ± 32,610 | 212 |
| South | 49 | 97.4–29,700 | 2490 ± 5830 | 373 |
| Year | Number of Samples Collected | Date Range | Microcystins | Anatoxins | Paralytic Shellfish Poisoning Toxins | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | SD | Number of Toxic Samples | Median | Mean | SD | Number of Toxic Samples | Median | Mean | SD | Number of Toxic Samples | |||
| 2014 | 137 | 22 June–13 October | 2.8 | 17 | 25 | 11 | 0.88 | 1.2 | 1.2 | 12 | NA | NA | NA | NA |
| 2015 | 128 | 07 June–19 October | 5.1 | 101 | 325 | 23 | 0.058 | 0.34 | 0.46 | 23 | NA | NA | NA | NA |
| 2016 | 144 | 29 May–17 October | 4.8 | 54 | 211 | 28 | 0.36 | 0.91 | 1.5 | 53 | 8.3 | 17 | 25 | 14 |
| 2017 | 211 | 29 May–28 November | 3.1 | 192 | 841 | 95 | 0.033 | 0.052 | 0.075 | 7 | 24 | 46 | 98 | 32 |
| Location | Model Predictors | |||
|---|---|---|---|---|
| Cyanobacterial Chlorophyll (Chl) | Microcystins | Anatoxins | Paralytic Shellfish Poisoning Toxins | |
| Lakewide | pH, TP, TN | None | Chl, PAR, AWS * | Chl, AWS, conductivity |
| North Basin | pH, TP, Secchi | Chl, TP, water temp, rainfall | - | AWS, rainfall, Secchi |
| South Basin | None | Chl, PAR, AWD | Chl, AWD, water temp, Secchi | Chl, AWS, rainfall, Secchi |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, Z.J.; Conroe, D.E.; Schulz, K.L.; Boyer, G.L. Limnological Differences in a Two-Basin Lake Help to Explain the Occurrence of Anatoxin-a, Paralytic Shellfish Poisoning Toxins, and Microcystins. Toxins 2020, 12, 559. https://doi.org/10.3390/toxins12090559
Smith ZJ, Conroe DE, Schulz KL, Boyer GL. Limnological Differences in a Two-Basin Lake Help to Explain the Occurrence of Anatoxin-a, Paralytic Shellfish Poisoning Toxins, and Microcystins. Toxins. 2020; 12(9):559. https://doi.org/10.3390/toxins12090559
Chicago/Turabian StyleSmith, Zacharias J., Douglas E. Conroe, Kimberly L. Schulz, and Gregory L. Boyer. 2020. "Limnological Differences in a Two-Basin Lake Help to Explain the Occurrence of Anatoxin-a, Paralytic Shellfish Poisoning Toxins, and Microcystins" Toxins 12, no. 9: 559. https://doi.org/10.3390/toxins12090559
APA StyleSmith, Z. J., Conroe, D. E., Schulz, K. L., & Boyer, G. L. (2020). Limnological Differences in a Two-Basin Lake Help to Explain the Occurrence of Anatoxin-a, Paralytic Shellfish Poisoning Toxins, and Microcystins. Toxins, 12(9), 559. https://doi.org/10.3390/toxins12090559





