Fraternine, a Novel Wasp Peptide, Protects against Motor Impairments in 6-OHDA Model of Parkinsonism
Abstract
1. Introduction
2. Results
2.1. Purification and Structural Analysis
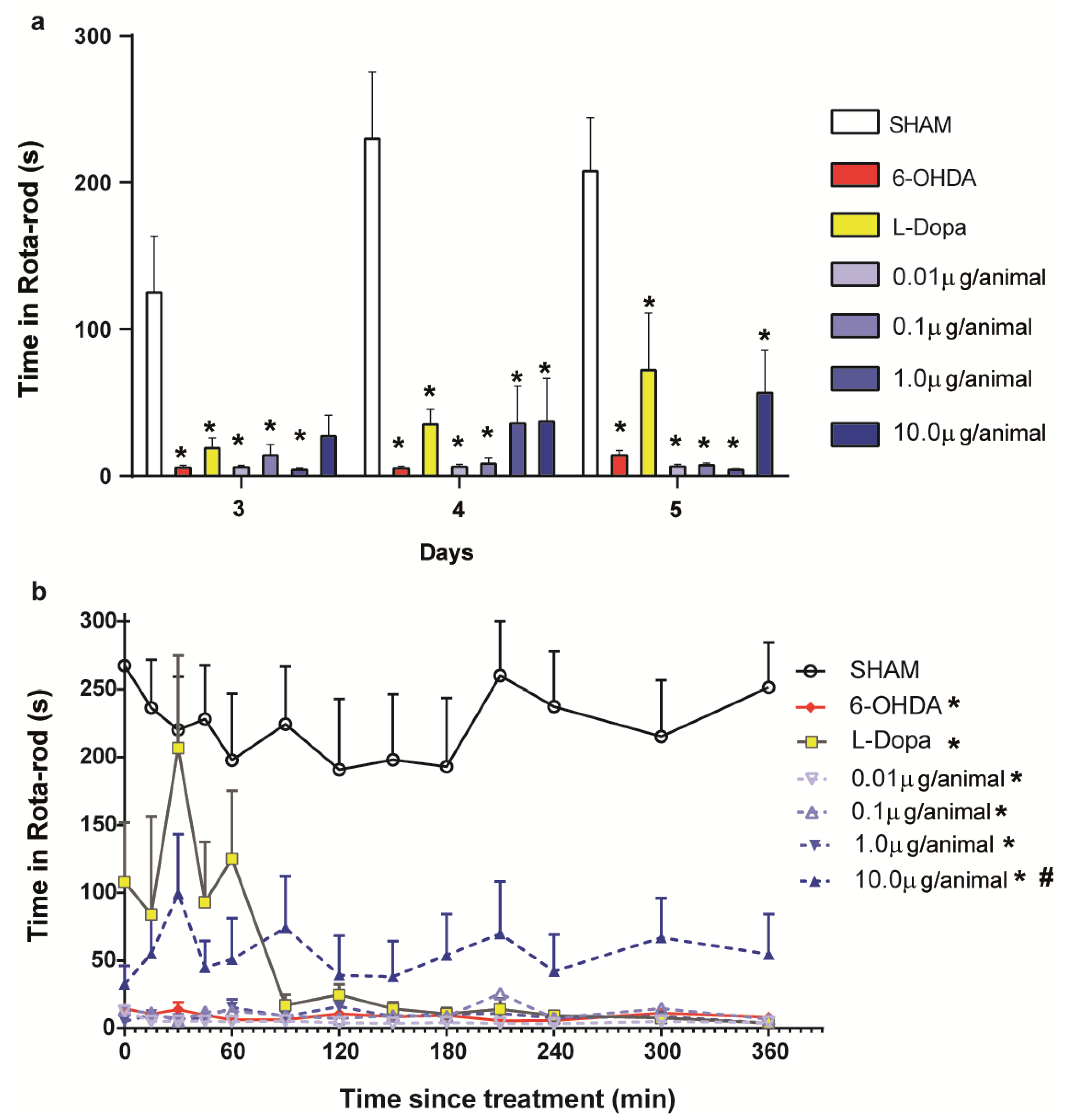
2.2. Evaluation of the Fraternine Peptide in the Murine Model of PD
2.3. Apomorphine-Induced Turning Behavior
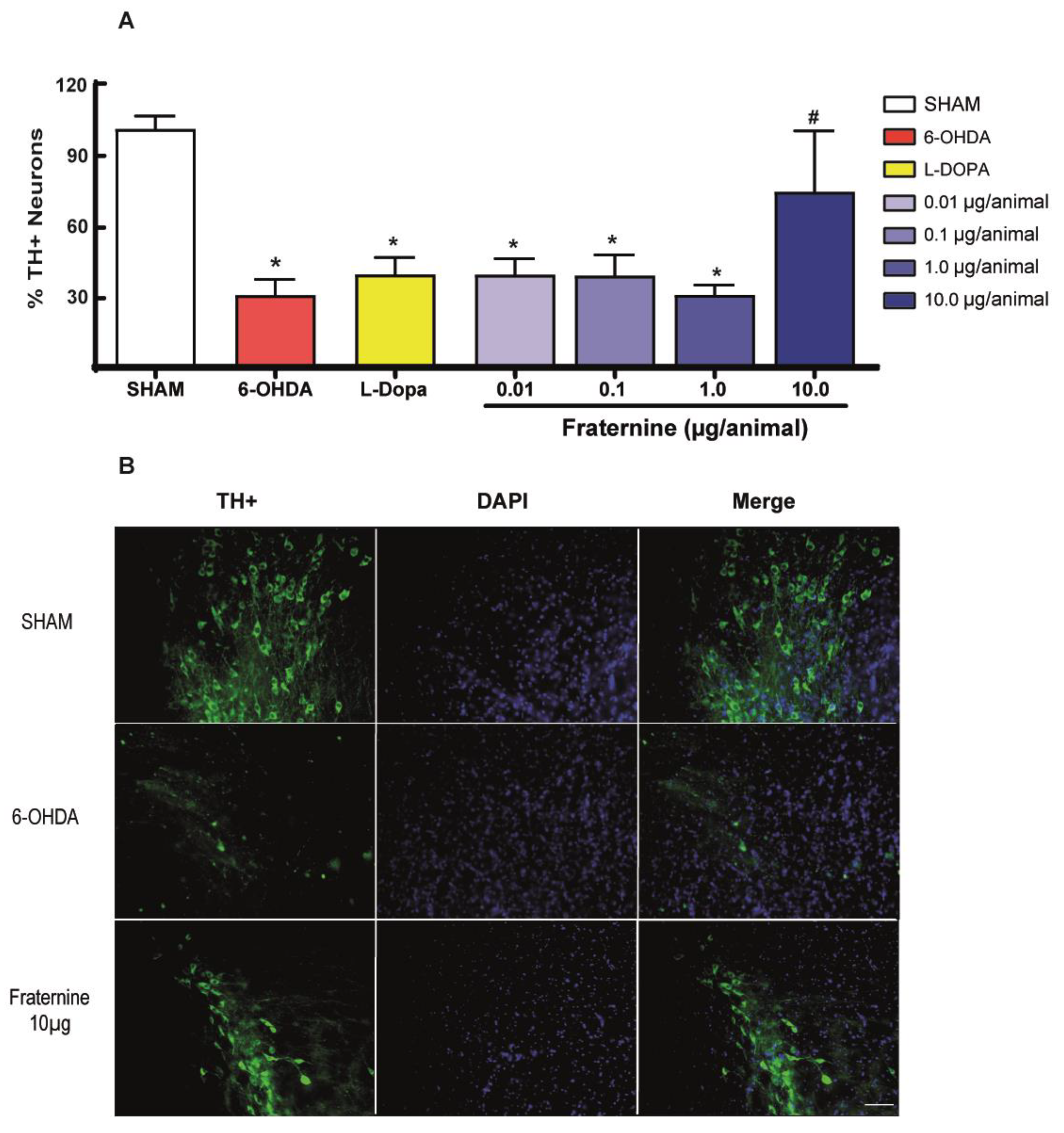
2.4. Quantification of Dopaminergic Neurons in Substantia Nigra
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Licenses, Permits and Ethical Issues
5.2. Biological Material
5.3. Purification and Identification of the Peptide
5.4. Experimental Animals
5.5. Surgical Procedure and Treatments
5.6. Rotarod Motor Coordination Tests
5.7. Apomorphine-Induced Turning Behavior
5.8. Immunohistochemistry
5.9. Statistical Analysis
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mazzoni, P.; Shabbott, B.; Cortes, J.C. Motor Control Abnormalities in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Marsh, L.; Schrag, A. Neuropsychiatric symptoms in Parkinson’s disease. Mov. Disord. 2009, 24, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Safarpour, D.; Willis, A.W. Clinical Epidemiology, Evaluation, and Management of Dementia in Parkinson Disease. Am. J. Alzheimer’s Dis. Other Dement. 2016, 31, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Tatton, W.G. Etiology and pathogenesis of Parkinson´s disease. Annu. Rev. Neurosci. 1999, 22, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Przedborski, S. Genetic clues to the pathogenesis of Parkinson’s disease. Nat. Med. 2004, 10, S58–S62. [Google Scholar] [CrossRef]
- Klingelhoefer, L.; Reichmann, H. Pathogenesis of Parkinson disease—The gut-brain axis and environmental factors. Nat. Rev. Neurol. 2015, 11, 625–636. [Google Scholar] [CrossRef]
- Youdim, M.B.H.; Geldenhuys, W.J.; Van der Schyf, C.J. Why should we use multifunctional neuroprotective and neurorestorative drugs for Parkinson’s disease? Park. Relat. Disord. 2007, 13. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Bezard, E.; Brotchie, J.; Calon, F.; Collingridge, G.L.; Ferger, B.; Hengerer, B.; Hirsch, E.; Jenner, P.; Le Novère, N.; et al. Novel pharmacological targets for the treatment of Parkinson’s disease. Nat. Rev. Drug Discov. 2006, 5, 845–854. [Google Scholar] [CrossRef]
- Amaral, H.O.; Monge-Fuentes, V.; Biolchi, A.M.; Campos, G.A.A.; Lopes, K.S.; Camargo, L.C.; Schwartz, M.F.; Galante, P.; Mortari, M.R. Animal venoms: Therapeutic tools for tackling Parkinson’s disease. Drug Discov. Today 2019, 24, 2202–2211. [Google Scholar] [CrossRef]
- Zambelli, V.O.; Pasqualoto, K.F.M.; Picolo, G.; Chudzinski-Tavassi, A.M.; Cury, Y. Harnessing the knowledge of animal toxins to generate drugs. Pharmacol. Res. 2016, 112, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The Toxicogenomic Multiverse: Convergent Recruitment of Proteins into Animal Venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Bertilsson, G.; Patrone, C.; Zachrisson, O.; Andersson, A.; Dannaeus, K.; Heidrich, J.; Wikström, L. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J. Neurosci. Res. 2008, 86, 326–338. [Google Scholar] [CrossRef]
- Harkavyi, A.; Abuirmeileh, A.; Lever, R.; Kingsbury, A.E.; Biggs, C.S.; Whitton, P.S. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J. Neuroinflamm. 2008, 5, 1–9. [Google Scholar] [CrossRef]
- Kim, S.; Moon, M.; Park, S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J. Endocrinol. 2009, 202, 431–439. [Google Scholar] [CrossRef]
- Doo, A.-R.; Kim, S.-T.; Kim, S.-N.; Moon, W.; Yin, C.S.; Chae, Y.; Park, H.-K.; Lee, H.; Park, H.-J. Neuroprotective effects of bee venom pharmaceutical acupuncture in acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Neurol. Res. 2010, 32 (Suppl. 1), 88–91. [Google Scholar] [CrossRef]
- Cho, S.Y.; Shim, S.R.; Rhee, H.Y.; Park, H.J.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H.; Park, S.U. Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson’s disease. Park. Relat. Disord. 2012, 18, 948–952. [Google Scholar] [CrossRef]
- Dias, N.B.; de Souza, B.M.; Gomes, P.C.; Brigatte, P.; Palma, M.S. Peptidome profiling of venom from the social wasp Polybia paulista. Toxicon 2015, 107, 290–303. [Google Scholar] [CrossRef]
- Baptista-Saidemberg, N.B.; Saidemberg, D.M.; Palma, M.S. Profiling the peptidome of the venom from the social wasp agelaia pallipes pallipes. J. Proteom. 2011, 74, 2123–2137. [Google Scholar] [CrossRef]
- do Couto, L.L.; dos Anjos, L.C.; Araujo, M.A.F.; Mourão, C.A.; Schwartz, C.A.; Ferreira, L.B.; Mortari, M.R. Anticonvulsant and anxiolytic activity of the peptide fraction isolated from the venom of the social wasp Polybia paulista. Pharmacogn. Mag. 2012, 8, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; do Couto, L.L.; de Oliveira Amaral, H.; Gomes, F.M.M.; Campos, G.A.A.; Silva, L.P.; Mortari, M.R. Neuropolybin: A new antiseizure peptide obtained from wasp venom. Biochem. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Gomes, F.M.M.; Campos, G.A.A.; de Castro, S.J.; Biolchi, A.M.; dos Anjos, L.C.; Gonçalves, J.C.; Lopes, K.S.; Mortari, M.R. Neuroactive compounds obtained from arthropod venoms as new therapeutic platforms for the treatment of neurological disorders. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Monge-Fuentes, V.; Gomes, F.; Lopes, K.; dos Anjos, L.; Campos, G.; Arenas, C.; Biolchi, A.; Gonçalves, J.; Galante, P.; et al. Pharmacological alternatives for the treatment of neurodegenerative disorders: Wasp and bee venoms and their components as new neuroactive tools. Toxins 2015, 7, 3179–3209. [Google Scholar] [CrossRef]
- Gonçalves, J.; Rangel, M.; Biolchi, A.; Alves, E.; Moreira, K.; Silva, L.; Mortari, M. Antinociceptive properties of the mastoparan peptide Agelaia-MPI isolated from social wasps. Toxicon 2016, 120, 15–21. [Google Scholar] [CrossRef]
- Mendes, M.A.; De Souza, B.M.; Marques, M.R.; Palma, M.S. Structural and biological characterization of two novel peptides from the venom of the neotropical social wasp Agelaia pallipes pallipes. Toxicon 2004, 44, 67–74. [Google Scholar] [CrossRef]
- Fadel, V.; Martins, D.B.; Dos Santos Cabrera, M.P. PDB entry-6N68-NMR solution structure of Protonectin (Agelaia pallipes pallipes) interacting with SDS micelles: An antimicrobial peptide with anticancer activity on breast cancer cells. Available online: https://www.rcsb.org/structure/6N68 (accessed on 5 August 2020). [CrossRef]
- Baptista-Saidemberg, N.B.; Saidemberg, D.M.; Ribeiro, R.A.; Arcuri, H.A.; Palma, M.S.; Carneiro, E.M. Agelaia MP-I: A peptide isolated from the venom of the social wasp, Agelaia pallipes pallipes, enhances insulin secretion in mice pancreatic islets. Toxicon 2012, 60, 596–602. [Google Scholar] [CrossRef]
- Dohtsu, K.; Okumura, K.; Hagiwara, K.; Palma, M.S.; Nakajima, T. Isolation and sequence analysis of peptides from the venom of Protonectarina sylveirae (hymenoptera-vespidae). Nat. Toxins 1993, 1, 271–276. [Google Scholar] [CrossRef]
- Schwarting, R.K.W.; Huston, J.P. Unilateral 6-hydroxydopamine lesions of meso-striatal dopamine neurons and their physiological sequelae. Prog. Neurobiol. 1996, 49, 215–266. [Google Scholar] [CrossRef]
- Youdim, M.B.H.; Kupershmidt, L.; Amit, T.; Weinreb, O. Promises of novel multi-target neuroprotective and neurorestorative drugs for Parkinson’s disease. Park. Relat. Disord. 2014, 20, S132–S136. [Google Scholar] [CrossRef]
- Lewis, R.J.; Garcia, M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Olmos, I.; Dickson, J.; Kefalopoulou, Z.; Djamshidian, A.; Ell, P.; Soderlund, T.; Whitton, P.; Wyse, R.; Isaacs, T.; Lees, A.; et al. Exenatide and the treatment of patients with Parkinson’s disease. J. Clin. Investig. 2013, 123, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Olmos, I.; Dickson, J.; Kefalopoulou, Z.; Djamshidian, A.; Kahan, J.; Ell, P.; Whitton, P.; Wyse, R.; Isaacs, T.; Lees, A.; et al. Motor and Cognitive Advantages Persist 12 Months after Exenatide Exposure in Parkinson’s Disease. J. Parkinsons. Dis. 2015, 4, 337–344. [Google Scholar] [CrossRef]
- Athauda, D.; Maclagan, K.; Skene, S.S.; Bajwa-Joseph, M.; Letchford, D.; Chowdhury, K.; Hibbert, S.; Budnik, N.; Zampedri, L.; Dickson, J.; et al. Exenatide once weekly versus placebo in Parkinson’s disease: A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1664–1675. [Google Scholar] [CrossRef]
- Yun, S.P.; Kam, T.I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.S.; Kwon, S.H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018. [Google Scholar] [CrossRef]
- Dong, J.; Cui, Y.; Li, S.; Le, W. Current pharmaceutical treatments and alternative therapies of Parkinson’s Disease. Curr. Neuropharmacol. 2016, 14, 339–355. [Google Scholar] [CrossRef]
- Grealish, S.; Mattsson, B.; Draxler, P.; Björklund, A. Characterisation of behavioural and neurodegenerative changes induced by intranigral 6-hydroxydopamine lesions in a mouse model of Parkinson’s disease. Eur. J. Neurosci. 2010, 31, 2266–2278. [Google Scholar] [CrossRef]
- Kim, M.E.; Lee, J.Y.; Lee, K.M.; Park, H.R.; Lee, E.; Lee, Y.; Lee, J.S.; Lee, J. Neuroprotective effect of bee venom is mediated by reduced astrocyte activation in a subchronic MPTP-induced model of Parkinson’s disease. Arch. Pharm. Res. 2016, 39, 1160–1170. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7. [Google Scholar] [CrossRef]
- Lane, E.L.; Dunnet, S.B. Animal Models of Movement Disorders: Volume II, 2011 ed.; Humana Press: Totowa, NJ, USA, 2011; ISBN 9781617793004. [Google Scholar]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Shimohama, S.; Sawada, H.; Kitamura, Y.; Taniguchi, T. Disease model: Parkinson’s disease. Trends Mol. Med. 2003, 9, 360–365. [Google Scholar] [CrossRef]
- Williams, M.; Porsolt, R.D. CNS safety pharmacology. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–13. ISBN 978-0-08-055232-3. [Google Scholar]
- Ungerstedt, U. Postsynaptic Supersensitivity after 6-Hydroxy-dopamine Induced Degeneration of the Nigro-striatal Dopamine System. Acta Physiol. Scand. 1971, 82, 69–93. [Google Scholar] [CrossRef] [PubMed]




| Fraction a | MM | Peptide | Sequence | Effects |
|---|---|---|---|---|
| 3 | 1055.6 | unknown | -b | - |
| 4 | 1358.8 | unknown | -b | - |
| 5 | 1209.9 | protonectin | ILGTILGLLKGL-NH2 | chemotactic peptide, antimicrobial, anticancer activity on breast cancer cells [26,27] |
| 6 | 2748.5 | Fraternine | I/LSFQQVKEQVCKVEAQI/LGQQI/LPFC-NH2 | Neuroprotective effect (this study) |
| 7 | 1566.9 | Agelaia-MP | INWLKLGKAIIDAL–NH2 | broad-spectrum action against microorganisms, inhibitory effects against tumor proliferation, antinociceptive and stimulating serotonin release from platelets and mast cell degranulation [25,26,28] |
| Process | Theoretical Mass [M + H]+ | Experimental Mass [M + H]+ | Figure | Sequence |
|---|---|---|---|---|
| Red-Alkylation | 2864.59 | 2864.6 | Figure S3a,b | I/LSFQQVKEKVC (Acm)KVEAKI/LGKKI/LPFC (Acm) |
| GluC | - | - | Figure S4a | MS spectrum after 1 h |
| - | - | Figure S4b | MS spectrum after 1.5 h | |
| Figure S4c | MS spectrum after 3 h | |||
| Figure S4d | MS spectrum after 4.5 h | |||
| Figure S4e | MS spectrum after 5.5 h | |||
| GluC | 978.52 | 978.6 | Figure S5 | I/LSFQQVKE |
| Trypsin | - | - | Figure S6a | MS spectrum after 1 h |
| - | - | Figure S6b | MS spectrum after 3 h | |
| - | - | Figure S6c | MS spectrum after 5.5 h | |
| Trypsin | 849.48 | 849.5 | Figure S7a | I/LSFQQVK |
| 1106.62 | 1106.6 | Figure S7b | I/LSFQQVKEK | |
| 1493.81 | 1493.8 | Figure S7c | I/LSFQQVKEKVC(Acm)K | |
| 1921.06 | 1921.0 | Figure S6a * | I/LSFQQVKEKVC(Acm)KVEAK | |
| 2219.26 | 2219.2 | Figure S7d | I/LSFQQVKEKVC(Acm)KVEAKI/LGK | |
| 2347.37 | 2347.3 | Figure S6a * | I/LSFQQVKEKVC(Acm)KVEAKI/LGKK | |
| 2864.59 | 2864.5 | Figure S7e | I/LSFQQVKEKVC(Acm)KVEAKI/LGKKI/LPFC(Acm) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biolchi, A.M.; de Oliveira, D.G.R.; Amaral, H.d.O.; Campos, G.A.A.; Gonçalves, J.C.; de Souza, A.C.B.; Lima, M.R.; Silva, L.P.; Mortari, M.R. Fraternine, a Novel Wasp Peptide, Protects against Motor Impairments in 6-OHDA Model of Parkinsonism. Toxins 2020, 12, 550. https://doi.org/10.3390/toxins12090550
Biolchi AM, de Oliveira DGR, Amaral HdO, Campos GAA, Gonçalves JC, de Souza ACB, Lima MR, Silva LP, Mortari MR. Fraternine, a Novel Wasp Peptide, Protects against Motor Impairments in 6-OHDA Model of Parkinsonism. Toxins. 2020; 12(9):550. https://doi.org/10.3390/toxins12090550
Chicago/Turabian StyleBiolchi, Andréia Mayer, Danilo Gustavo Rodrigues de Oliveira, Henrique de Oliveira Amaral, Gabriel Avohay Alves Campos, Jacqueline Coimbra Gonçalves, Adolfo Carlos Barros de Souza, Marcos Robalinho Lima, Luciano Paulino Silva, and Márcia Renata Mortari. 2020. "Fraternine, a Novel Wasp Peptide, Protects against Motor Impairments in 6-OHDA Model of Parkinsonism" Toxins 12, no. 9: 550. https://doi.org/10.3390/toxins12090550
APA StyleBiolchi, A. M., de Oliveira, D. G. R., Amaral, H. d. O., Campos, G. A. A., Gonçalves, J. C., de Souza, A. C. B., Lima, M. R., Silva, L. P., & Mortari, M. R. (2020). Fraternine, a Novel Wasp Peptide, Protects against Motor Impairments in 6-OHDA Model of Parkinsonism. Toxins, 12(9), 550. https://doi.org/10.3390/toxins12090550








