Jitter Evaluation in Distant and Adjacent Muscles after Botulinum Neurotoxin Type A Injection in 78 Cases
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. Single-Fiber Electromyography
2.3. Correlation between Jitter and Variables
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Patients
5.2. Inclusion Criteria
5.3. Exclusion Criteria
5.4. Single-Fiber Electromyography
5.5. Statistics
5.6. Ethics
Author Contributions
Funding
Conflicts of Interest
References
- Scott, A.B.; Rosenbaun, A.; Collins, C.C. Pharmacologic weakening of extraocular muscles. Investig. Ophthalmol. Vis. Sci. 1973, 12, 924–927. [Google Scholar]
- Rossetto, O.; Megighian, A.; Scorzeto, M.; Montecucco, C. Botulinum neurotoxins. Toxicon 2013, 67, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural, and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F. Conversion ratio between Botox®, Dysport®, and Xeomin® in clinical practice. Toxins (Basel) 2016, 8, 65. [Google Scholar] [CrossRef]
- Pantano, S.; Montecucco, C. The blockade of the neurotransmitter release apparatus by botulinum neurotoxins. Cell. Mol. Life Sci. 2014, 71, 793–811. [Google Scholar] [CrossRef]
- Hong, B.; Chen, M.; Hu, X. Influence of injection of chinese botulinum toxin type A on the histomorphology and myosin heavy chain composition of rat gastrocnemius muscles. J. Zhejiang Univ. Sci. B 2013, 14, 983–992. [Google Scholar] [CrossRef]
- Slater, C.R. ‘Fragmentation’ of NMJs: A sign of degeneration or regeneration? A long journey with many junctions. Neuroscience 2020, 439, 28–40. [Google Scholar] [CrossRef]
- Franz, C.K.; Puritz, A.; Jordan, L.A.; Chow, J.; Ortega, J.A.; Kiskinis, E.; Heckman, C.J. Botulinum toxin conditioning enhances motor axon regeneration in mouse and human preclinical models. Neurorehabil. Neural Repair 2018, 32, 735–745. [Google Scholar] [CrossRef]
- Schulte-Mattler, W.J. Use of botulinum toxin A in adult neurological disorders: Efficacy, tolerability, and safety. CNS Drugs 2008, 22, 725–738. [Google Scholar] [CrossRef]
- Josefsson, J.-O.; Thesleff, S. Electromyographic findings in experimental botulinum intoxication. Acta Physiol. Scand. 1961, 51, 163–168. [Google Scholar] [CrossRef]
- Sanders, D.B.; Massey, E.W.; Buckley, E.G. Botulinum toxin for blepharospasm: Single-fiber EMG studies. Neurology 1986, 36, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Bogucki, A. Serial SFEMG studies of orbicularis oculi muscle after the first administration of botulinum toxin. Eur. J. Neurol. 1999, 6, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Lispi, L.; Leonardi, L.; Petrucci, A. Longitudinal neurophysiological assessment of intramuscular type—A botulin toxin in healthy humans. Neurol. Sci. 2018, 39, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Eleopra, R.; Rinaldo, S.; Montecucco, C.; Rossetto, O.; Devigili, G. Clinical duration of action of different botulinum toxin types in humans. Toxicon 2020, 179, 84–91. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Andersson, M.; Punga, A.R. Correlation of botulinum toxin dose with neurophysiological parameters of efficacy and safety in the glabellar muscles: A double-blind, placebo-controlled, randomized study. Acta Derm. Venereol. 2014, 94, 32–37. [Google Scholar] [CrossRef]
- Lange, D.J.; Brin, M.F.; Warner, C.L.; Fahn, S.; Lovelace, R.E. Distant effects of local injection of botulinum toxin. Muscle Nerve 1987, 10, 552–555. [Google Scholar] [CrossRef]
- Olney, R.K.; Aminoff, M.J.; Gelb, D.J.; Lowenstein, D.H. Neuromuscular effects distant from the site of botulinum neurotoxin injection. Neurology 1988, 38, 1780–1783. [Google Scholar]
- Lange, D.J.; Rubin, M.; Greene, P.E.; Kang, U.J.; Moskowitz, C.B.; Brin, M.F.; Lovelace, R.E.; Fahn, S. Distant effects of locally injected botulinum toxin: A double-blind study of single fiber EMG changes. Muscle Nerve 1991, 14, 672–675. [Google Scholar] [CrossRef]
- Girlanda, P.; Vita, G.; Nicolosi, C.; Milone, S.; Messina, C. Botulinum toxin therapy: Distant effects on neuromuscular transmission and autonomic nervous system. J. Neurol. Neurosurg. Psychiatry 1992, 55, 844–845. [Google Scholar] [CrossRef]
- Garner, C.G.; Straube, A.; Witt, T.N.; Gasser, T.; Oertel, W.H. Time course of distant effects of local injections of botulinum toxin. Mov. Disord. 1993, 8, 33–37. [Google Scholar] [CrossRef]
- Wan, X.; Tang, X.; Cui, L. Remote effects of local injection of botulinum toxin type A. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 1999, 21, 362–367. [Google Scholar] [PubMed]
- Comella, C.L.; Pullman, S.L. Botulinum toxins in neurological disease. Muscle Nerve 2004, 29, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Roche, N.; Schnitzler, A.; Genêt, F.; Durand, M.-C.; Bensmail, D. Undesirable distant effects following botulinum toxin type a injection. Clin. Neuropharmacol. 2008, 31, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, A.; Genet, F.; Durand, M.-C.; Roche, N.; Bensmail, D.; Chartier-Kastler, E.; Denys, P. Pilot study evaluating the safety of intradetrusor injections of botulinum toxin type A: Investigation of generalized spread using single-fiber EMG. Neurourol. Urodyn. 2011, 30, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Simpson, D.M. Contralateral weakness following botulinum toxin for poststroke spasticity. Muscle Nerve 2012, 46, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Ruet, A.; Durand, M.-C.; Denys, P.; Lofaso, F.; Genet, F.; Schnitzler, A. Single-fiber electromyography analysis of botulinum toxin diffusion in patients with fatigue and pseudobotulism. Arch. Phys. Med. Rehabil. 2015, 96, 1103–1109. [Google Scholar] [CrossRef]
- Watts, J.; Brew, B.; Tisch, S. Myasthenia gravis exacerbation with low dose ocular botulinum toxin for epiphoria. J. Clin. Neurosci. 2015, 22, 1979–1981. [Google Scholar] [CrossRef]
- Timmermans, G.; Depierreux, F.; Wang, F.; Hansen, I.; Maquet, P. Cosmetic injection of botulinum toxin unmasking subclinical myasthenia gravis: A case report and literature review. Case Rep. Neurol. 2019, 11, 244–251. [Google Scholar] [CrossRef]
- Punga, A.R.; Liik, M. Botulinum toxin injections associated with suspected myasthenia gravis: An underappreciated cause of MG-like clinical presentation. Clin. Neurophysiol. Pract. 2020, 5, 46–49. [Google Scholar] [CrossRef]
- Osio, M.; Mailland, E.; Muscia, F.; Nascimbene, C.; Vanotti, A.; Bana, C.; Corsi, F.; Foschi, D.; Mariani, C. Botulinum neurotoxin-A does not spread to distant muscles after intragastric injection: A double-blind single-fiber electromyography study. Muscle Nerve 2010, 42, 165–169. [Google Scholar] [CrossRef]
- Ostergaard, L.; Fuglsang-Frederiksen, A.; Werdelin, L.; Sjö, O.; Winkel, H. Quantitative EMG in botulinum toxin treatment of cervical dystonia. A double-blind, placebo-controlled study. Electroencephalogr. Clin. Neurophysiol. 1994, 93, 434–439. [Google Scholar] [CrossRef]
- Odergren, T.; Tollbäck, A.; Borg, J. Electromyographic single motor unit potentials after repeated botulinum toxin treatments in cervical dystonia. Electroencephalogr. Clin. Neurophysiol. 1994, 93, 325–329. [Google Scholar] [CrossRef]
- Eleopra, R.; Tugnoli, V.; Caniatti, L.; De Grandis, D. Botulinum toxin treatment in the facial muscles of humans: Evidence of an action in untreated near muscles by peripheral local diffusion. Neurology 1996, 46, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Shaari, C.M.; George, E.; Wu, B.L.; Biller, H.F.; Sanders, I. Quantifying the spread of botulinum toxin through muscle fascia. Laryngoscope 1991, 101, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Stålberg, E.; Sanders, D.B.; Ali, S.; Cooray, G.; Leonardis, L.; Löseth, S.; Machado, F.; Maldonado, A.; Martinez-Aparicio, C.; Sandberg, A.; et al. Reference values for jitter recorded by concentric needle electrodes in healthy controls: A multicenter study. Muscle Nerve 2016, 53, 351–362. [Google Scholar] [CrossRef] [PubMed]
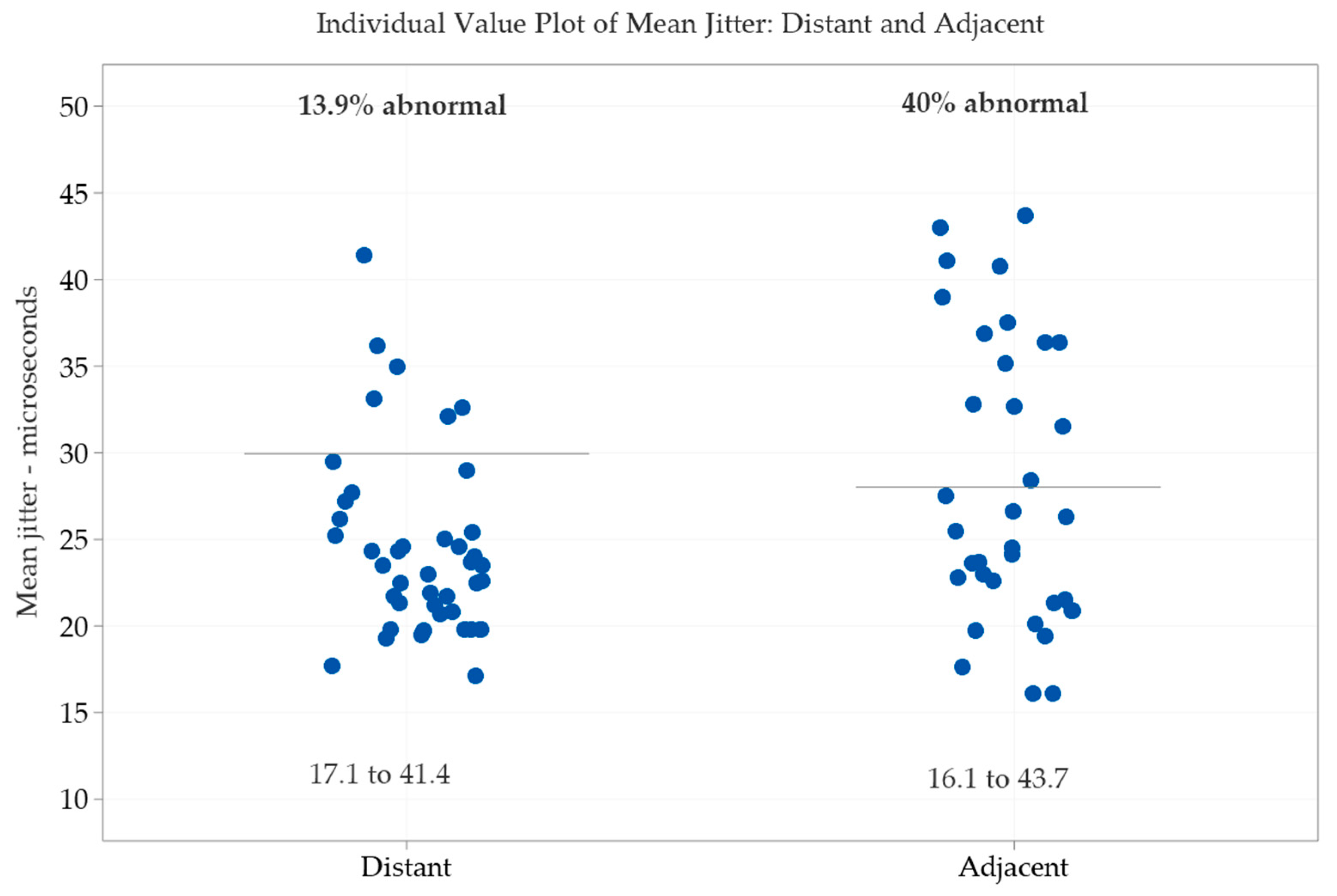
| Variables | Normality-Test | Mean | SD | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|---|---|
| Age | Yes | 65.12 | 10.47 | 35 | 83 | |||
| Mean jitter (µs) | No | 17.1 | 20.7 | 23.5 | 26.2 | 41.4 | ||
| Abnormal individual jitter values (%) | No | 0 | 0 | 5 | 5 | 30 | ||
| Blocking (%) | No | 0 | 0 | 0 | 0 | 5 | ||
| BoNT/A first (days) | No | 0 | 1099 | 1743 | 2331 | 6575 | ||
| BoNT/A last (days) | No | 5 | 64 | 85 | 138 | 229 | ||
| Number of injections | No | 1 | 9 | 13 | 18 | 30 | ||
| Units per injection | No | 30 | 60 | 60 | 80 | 600 | ||
| Units total since first | No | 60 | 660 | 880 | 1440 | 9600 | ||
| Number muscles injected | No | 1 | 2 | 3 | 3 | 5 |
| Variables | Normality-Test | Mean | SD | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|---|---|
| Age | Yes | 61.91 | 12.08 | 30 | 79 | |||
| Jitter (µs) | No | 16.1 | 21.3 | 25.5 | 36.4 | 43.7 | ||
| Abnormal individual jitter values (%) | No | 0 | 5 | 10 | 25 | 70 | ||
| Blocking (%) | No | 0 | 0 | 0 | 5 | 15 | ||
| BoNT/A first (days) | No | 238 | 1190 | 2050 | 3003 | 4365 | ||
| BoNT/A last (days) | No | 11 | 42 | 82 | 182 | 320 | ||
| Number of injections | Yes | 15.7 | 8.02 | 3 | 9 | 28 | ||
| Units per injection | No | 45 | 60 | 60 | 200 | 550 | ||
| Units total since first | No | 180 | 765 | 1200 | 1620 | |||
| Number muscles injected | No | 2 | 3 | 3 | 3 | 5 |
| Variables | Distant vs. Adjacent | t-Test | U-Test | Significance |
|---|---|---|---|---|
| Age | p = 0.2138 | Yes | No | |
| BoNT/A first (days) | p = 0.1689 | Yes | No | |
| BoNT/A last (days) | p = 0.9661 | Yes | No | |
| BoNT/A number of injections | p = 0.2785 | Yes | No | |
| BoNT/A Units per injection | p = 0.9633 | Yes | No | |
| BoNT/A Units total | p = 0.1153 | Yes | No | |
| Number of muscles injected | p = 0.0582 | Yes | No | |
| Mean jitter | p = 0.0723 | Yes | No | |
| Abnormal individual jitter values (%) | p = 0.0003 | Yes | Yes | |
| Impulse blocking (%) | p = 0.0383 | Yes | Yes |
| Variables | Distant Muscle | Adjacent Muscle | Power |
|---|---|---|---|
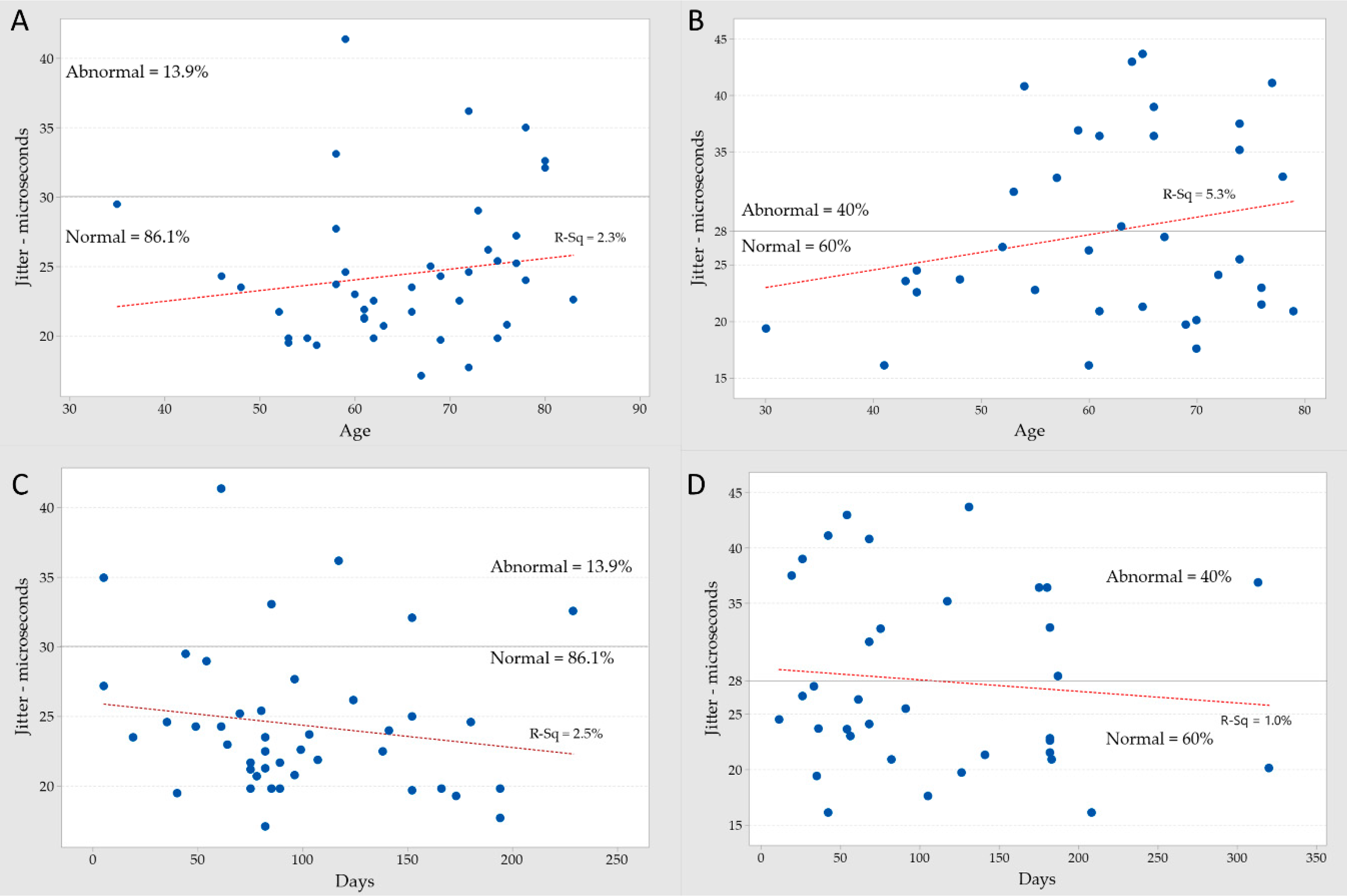
| Mean jitter vs. BoNT/A units per injection | 12.8% | 5.9% | weak |
| Mean jitter vs. total BoNT/A units summated | 11.4% | 3.3% | weak |
| Mean jitter vs. days after last BoNT/A injection | 2.5% | 1.0% | weak |
| Mean jitter vs. age in the last BoNT/A injection | 2.3% | 5.3% | weak |
| Mean jitter vs. MUAP amplitude | 1.9% | 0.0% | weak |
| Mean jitter vs. number of injected muscles | 1.4% | 4.6% | weak |
| Mean jitter vs. days after first BoNT/A injection | 0.8% | 0.0% | very weak |
| Variables | Distant | Distant | Adjacent | Adjacent |
|---|---|---|---|---|
| Lowest mean jittert | 17.1 µs | 16.1 µs | ||
| Highest mean jitter | 41.4 µs | 43.7 µs | ||
| BoNT/A last injection | 61 days | 82 days | 131 days | 42 days |
| Units | 600 U | 30 U | 90 U | 550 U |
| Age/Sex | 51 M | 67 F | 65 M | 60 F |
| Variables | Distant | Distant | Adjacent | Adjacent |
|---|---|---|---|---|
| Lowest mean jitter | 17.7 µs | 20.1 µs | ||
| Highest mean jitter | 32.6 µs | 36.9 µs | ||
| BoNT/A last injection | 229 days | 194 days | 313 days | 320 days |
| Units | 60 U | 60 U | 400 U | 45 U |
| Age/Sex | 80 F | 72 M | 59 M | 70 M |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouyoumdjian, J.A.; Graça, C.R.; Oliveira, F.N. Jitter Evaluation in Distant and Adjacent Muscles after Botulinum Neurotoxin Type A Injection in 78 Cases. Toxins 2020, 12, 549. https://doi.org/10.3390/toxins12090549
Kouyoumdjian JA, Graça CR, Oliveira FN. Jitter Evaluation in Distant and Adjacent Muscles after Botulinum Neurotoxin Type A Injection in 78 Cases. Toxins. 2020; 12(9):549. https://doi.org/10.3390/toxins12090549
Chicago/Turabian StyleKouyoumdjian, Joao Aris, Carla Renata Graça, and Fabio Nazare Oliveira. 2020. "Jitter Evaluation in Distant and Adjacent Muscles after Botulinum Neurotoxin Type A Injection in 78 Cases" Toxins 12, no. 9: 549. https://doi.org/10.3390/toxins12090549
APA StyleKouyoumdjian, J. A., Graça, C. R., & Oliveira, F. N. (2020). Jitter Evaluation in Distant and Adjacent Muscles after Botulinum Neurotoxin Type A Injection in 78 Cases. Toxins, 12(9), 549. https://doi.org/10.3390/toxins12090549





